UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________
FORM 20-F
(Mark One)
| REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
| Or | |||||
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended December 31 , 2023
Or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
| Or | |||||
| SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
Date of event requiring this shell company report
For the transition period from to
Commission File Number: 001-31368
_______________________________
(Exact name of registrant as specified in its charter)
N/A
(Translation of registrant’s name into English)
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
________________________
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
________________________
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class: | Trading Symbol | Name of each exchange on which registered: | ||||||
| * | ||||||||
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
The number of outstanding shares of each of the issuer’s classes of capital or common stock as of December 31, 2023 was:
Ordinary shares: 1,264,799,969
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐.
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or an emerging growth company. See definition of “large accelerated filer”, “accelerated filer” or “emerging growth company” in Rule 12b-2 of the Exchange Act.
| ☒ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Emerging growth company | ||||||||||||||||||
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards(1) provided pursuant to Section 13(a) of the Exchange Act. ☐
(1) The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP | ☐ | as issued by the International Accounting Standards Board | ☒ | Other | ☐ | ||||||||||||
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17. ☐ Item 18. ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒ .
*Not for trading but only in connection with the registration of American Depositary Shares representing such ordinary shares.
Presentation of financial
and other information
and other information
The consolidated financial statements contained in this annual report on Form 20-F have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB) and with IFRS as endorsed by the European Union, as of December 31, 2023.
Unless otherwise indicated or the context requires otherwise, the terms “Sanofi,” the “Company,” the “Group,” “we,” “our,” or “us” refer to Sanofi and its consolidated subsidiaries.
All references herein to “United States” or “US” are to the United States of America, references to “dollars” or “$” are to the currency of the United States, references to “France” are to the Republic of France, and references to “euro” and “€” are to the currency of the European Union member states (including France) participating in the European Monetary Union.
As of the date of this report on Form 20-F, all commercial trademarks mentioned here are protected, and are trademarks of Sanofi and/or its subsidiaries, with the exception of:
•trademarks used or that may be or have been used under license by Sanofi and/or its affiliates, such as ALDURAZYME, a trademark of the Biomarin/Genzyme LLC Joint Venture; ALPROLIX, a trademark of Swedish Orphan Biovitrum AB in Europe; ALTUVIIIO, a trademark of Sobi in Europe and in Africa; ANKET, a trademark of Innate Pharma; ATOMNET, a trademark of Atomwise, Inc.; CIALIS, a trademark of Eli Lilly; ELOCTATE, a trademark of Swedish Orphan Biovitrum AB in Europe; STAMARIL, a trademark of the Institut Pasteur; TAMIFLU, a trademark of Hoffmann-La Roche; VAXELIS, a trademark of MSP Vaccine Company (US) and MCM Vaccine B.V. (Netherlands); ZALTRAP, a trademark of Regeneron in the United States;
•trademarks sold by Sanofi and/or its affiliates to a third party, such as ALTACE, a trademark of King Pharmaceuticals in the United States; HYALGAN, a trademark of Fidia Farmaceutici S.p.A.; LIBTAYO, a trademark of Regeneron; PRALUENT, a trademark of Regeneron in the United States; SEPRAFILM, a trademark of Baxter International Inc.; and
•other third party trademarks such as PLAN BEE, a trademark of Amélie Perennou in France; STOXX, a trademark of STOXX Ltd; UNISOM, a trademark of J&J on certain geographic areas and Paladin Labs Inc. in Canada; and ZANTAC, a trademark of Glaxo Group Limited (except in the US and Canada).
Not all trademarks related to products under development have been authorized as of the date of this annual report by the relevant health authorities.
The data relating to market shares and ranking information for pharmaceutical products, in particular as presented in “Item 4. Information on the Company — B. Business Overview — B.5. Markets — B.5.1. Marketing and distribution,” are based primarily on sales data excluding vaccines and in constant euros (unless otherwise indicated) on a September 2023 MAT (Moving Annual Total) basis. The data are primarily from a IQVIA local sales audit, supplemented by country-specific sources.
Product indications described in this annual report are composite summaries of the major indications approved in the product’s principal markets. Not all indications are necessarily available in each of the markets in which the products are approved. The summaries presented herein for the purpose of financial reporting do not substitute for careful consideration of the full labeling approved in each market.
Cautionary statement regarding
forward-looking statements
forward-looking statements
This annual report contains certain forward-looking statements within the meaning of applicable federal securities law, including the Private Securities Litigation Reform Act of 1995, as amended. We may also make written or oral forward-looking statements in our periodic reports to the Securities and Exchange Commission on Form 6-K, in our annual report to shareholders, in our offering circulars and prospectuses, in press releases and other written materials and in oral statements made by our officers, directors or employees to third parties. Examples of such forward-looking statements include:
•projections of operating revenues, net income, business net income, earnings per share, business earnings per share, capital expenditures, cost savings, restructuring costs, positive or negative synergies, dividends, capital structure or other financial items or ratios;
•statements of our profit forecasts, trends, plans, objectives or goals, including those relating to products, clinical trials, regulatory approvals and competition; and
•statements about our future events and economic performance or that of France, the United States or any other countries in which we operate.
Words such as “believe,” “anticipate,” “can,” “contemplate,” “could,” “plan,” “expect,” “intend,” “is designed to,” “may,” “might,” “plan,” “potential,” “objective,” “target,” “estimate,” “project,” “predict,” “forecast,” “ambition,” “guideline,” “should,” “will,” "goal," or the negative of these and similar expressions are intended to identify forward-looking statements but are not the exclusive means of identifying such statements.
Forward-looking statements involve inherent, known and unknown risks and uncertainties associated with the regulatory, economic, financial and competitive environment, and other factors that could cause actual future results to differ materially from those expressed or implied in the forward-looking statements.
These risks and uncertainties include risk factors, which could affect future results and cause actual results to differ materially from those contained in any forward-looking statements, and which include those discussed under “Item 3. Key Information — D. Risk Factors.” Additional risks, not currently known or that are currently considered immaterial by the Group, may have the same unfavorable effect and investors may lose all or part of their investment.
As a result of these factors, we cannot assure you that the forward-looking statements in this annual report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. Moreover, forward-looking statements speak only as of the date they are made. Other than required by law, we do not undertake any obligation to update them in light of new information, future developments or otherwise, except as required by law. These forward-looking statements are based upon information, assumptions and estimates available to us as of the date of this annual report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information.
You should read this annual report and the documents that we reference in this annual report and have filed as exhibits completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these statements.
Abbreviations
Principal abbreviations used in the Annual Report on Form 20-F
| ADR | American Depositary Receipt | ||||
| ADS | American Depositary Share | ||||
| AFEP | Association française des entreprises privées (French Association of Large Companies) | ||||
| AMF | Autorité des marchés financiers (the French market regulator) | ||||
| ANDA | Abbreviated New Drug Application | ||||
| BLA | Biologic License Application | ||||
| BMS | Bristol-Myers Squibb | ||||
| CEO | Chief Executive Officer | ||||
| CER | Constant exchange rates | ||||
| CGU | Cash generating unit | ||||
| CHC | Consumer Healthcare | ||||
| CHMP | Committee for Medicinal Products for Human Use | ||||
| COVALIS | Sanofi committee for internal occupational exposure limits (Comité des Valeurs Limites Internes Sanofi) | ||||
CSR | Corporate Social Responsibility | ||||
| CVR | Contingent value right | ||||
| EFPIA | European Federation of Pharmaceutical Industries and Associations | ||||
| EMA | European Medicines Agency | ||||
| EU | European Union | ||||
| FCF | Free cash flow | ||||
| FDA | US Food and Drug Administration | ||||
| GAVI | Global Alliance for Vaccines and Immunisation | ||||
| GBU | Global Business Unit | ||||
| GERS | Groupement pour l'Élaboration et la Réalisation de Statistiques (French pharmaceutical industry statistics partnership) | ||||
| GCP | Good clinical practices | ||||
| GDP | Good distribution practices | ||||
| GLP | Good laboratory practices | ||||
| GLP-1 | Glucagon-like peptide-1 | ||||
| GMP | Good manufacturing practices | ||||
| GRI | Global Reporting Initiative | ||||
| Hib | Haemophilus influenzae type b | ||||
| HSE | Health, Safety and Environment | ||||
| IASB | International Accounting Standards Board | ||||
| ICH | International Council for Harmonization | ||||
IFPMA | International Federation of Pharmaceutical Manufacturers & Associations | ||||
| IFRIC | International Financial Reporting Interpretations Committee | ||||
| IFRS | International Financial Reporting Standards | ||||
| IPV | Inactivated polio vaccine | ||||
| ISIN | International Securities Identification Number | ||||
| J-MHLW | Japanese Ministry of Health, Labor and Welfare | ||||
LoE | Loss of Exclusivity | ||||
| LSD | Lysosomal storage disorder | ||||
| MEDEF | Mouvement des entreprises de France (French business confederation) | ||||
mRNA | messenger RNA | ||||
| MS | Multiple sclerosis | ||||
| NASDAQ | National Association of Securities Dealers Automated Quotations | ||||
| NDA | New Drug Application | ||||
| NHI | National Health Insurance (Japan) | ||||
| NYSE | New York Stock Exchange | ||||
| OECD | Organisation for Economic Co-operation and Development | ||||
| OPV | Oral polio vaccine | ||||
| OTC | Over the counter | ||||
| PhRMA | Pharmaceutical Research and Manufacturers of America | ||||
| PMDA | Pharmaceuticals and Medical Devices Agency (Japan) | ||||
| PRV | Priority Review Voucher | ||||
| PTE | Patent Term Extension | ||||
| QIV | Quadrivalent influenza vaccine | ||||
| R&D | Research and development | ||||
| ROA | Return on assets | ||||
| SA | Société anonyme (French public limited corporation) | ||||
| SEC | US Securities and Exchange Commission | ||||
| SPC | Supplementary Protection Certificate | ||||
| TRIBIO | Sanofi Committee for Biological Risk Prevention (Biosafety, Biosecurity, Biosurveillance) | ||||
| TSR | Total shareholder return | ||||
| UNICEF | United Nations Children’s Emergency Fund | ||||
| US | United States of America | ||||
| WHO | World Health Organization | ||||
| TABLE OF CONTENTS | |||||
| Item 1. | ||||||||||||||
| Item 2. | ||||||||||||||
| Item 3. | ||||||||||||||
| Item 4. | ||||||||||||||
| Item 4.A | ||||||||||||||
| Item 5. | ||||||||||||||
| Item 6. | ||||||||||||||
| Item 7. | ||||||||||||||
| Item 8. | ||||||||||||||
| Item 9. | ||||||||||||||
| Item 10. | |||||||||||
| Item 11. | |||||||||||
| Item 12. | |||||||||||
| Item 13. | |||||||||||
| Item 14. | |||||||||||
| Item 15. | |||||||||||
| Item 16A. | |||||||||||
| Item 16B. | |||||||||||
| Item 16C. | |||||||||||
| Item 16D. | |||||||||||
| Item 16E. | |||||||||||
| Item 16F. | |||||||||||
| Item 16G. | |||||||||||
| Item 16H. | |||||||||||
| Item 16I. | |||||||||||
Item 16J. | |||||||||||
Item 16K. | |||||||||||
| Item 17. | |||||||||||
| Item 18. | |||||||||||
| Item 19. | |||||||||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| [CETTE PAGE EST LAISSÉE EN BLANC VOLONTAIREMENT] | ||
| PART I | ||
| ITEM 1. Identity of Directors, Senior Management and Advisers | ||
Part I
Item 1. Identity of Directors, Senior Management and Advisers
N/A
Item 2. Offer Statistics and Expected Timetable
N/A
Item 3. Key Information
A. Selected financial data
N/A
B. Capitalization and indebtedness
N/A
C. Reasons for offer and use of proceeds
N/A
D. Risk factors
Important factors that could cause actual financial, business, research, or operating results to differ materially from expectations are disclosed in this annual report, including without limitation the following risk factors. Investors should carefully consider all the information set forth in the following risk factors and elsewhere in this document before deciding to invest in any of the Company’s securities. In addition to the risks listed below, we may be subject to other material risks that as of the date of this report are not currently known to us or that we deem immaterial at this time.
Risks relating to legal and regulatory matters
Product liability claims could adversely affect our business, results of operations and financial condition
Product liability is a significant risk for any pharmaceutical company, given that liability claims relating to our industry are unforeseeable by nature. The evolving regulatory environment worldwide (the ever-more stringent regulatory requirements applicable to the pharmaceutical industry, plus more stringent data, quality and supply obligations) clearly impacts our potential liability, and we may incur different liability claims to what we have handled in the past, with regard to their nature, scope and level. For a detailed analysis of the regulatory environment in which we operate, refer to “Item 4. Information on the Company - B. Business Overview - B.5.3 Regulatory framework.” Substantial damages have been awarded by some jurisdictions and/or settlements agreed – notably in the United States and other common law jurisdictions – against pharmaceutical companies based on claims for injuries allegedly caused by the use of their products. Such claims can also lead to product recalls, withdrawals, or declining sales, and/or be accompanied by consumer fraud claims by customers, third-party payers seeking reimbursement of the cost of the product and/or other claims, including potential civil or criminal governmental actions.
We are currently defending a number of product liability claims (see Note D.22.a. to the consolidated financial statements included at Item 18. of this annual report) notably with respect to TAXOTERE, ZANTAC, DEPAKINE and GOLD BOND, and there can be no assurance that we will be successful in defending these claims, or that we will not face additional claims in the future.
Establishing the full side effect profile of a pharmaceutical drug goes beyond data derived from preapproval clinical studies which may only involve several hundred to several thousand patients. Routine review and analysis of the continually growing body of post-marketing safety data and clinical trials provide additional information – for example, potential evidence of rare, population-specific or long-term adverse events or of drug interactions that were not observed in preapproval clinical studies. This causes product labeling to evolve over time following interactions with regulatory authorities, which may include restrictions of therapeutic indications, new contraindications, warnings or precautions and occasionally even the suspension or withdrawal of a product marketing authorization. Following any of these events, pharmaceutical companies can face significant product liability claims (see Note D.22.a. to the consolidated financial statements included at Item 18. of this annual report).
Furthermore, we commercialize several devices (some of which use new technologies) which, if they malfunction, could cause unexpected damage and lead to product liability claims (see “Breaches of data security, disruptions of information technology systems and cyber threats could result in financial, legal, business or reputational harm” below).
SANOFI FORM 20-F 2023 | 1 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Although we continue to insure a portion of our product liability with third-party carriers, product liability coverage is increasingly difficult and costly to obtain, particularly in the United States. In the future, it is possible that self-insurance may become the sole commercially reasonable means available for managing the financial risk associated with product liability in our pharmaceuticals and vaccines businesses (see “Item 4. Information on the Company — B. Business Overview — B.8. Insurance and Risk Coverage”). In cases where we self-insure, the legal costs that we would bear for handling such claims, and potential damage awards to be paid to claimants, could have a negative impact on our financial condition. Due to insurance conditions, even when we have insurance coverage, recoveries from insurers may not be totally successful due to market-driven insurance limitations and exclusions. Moreover, insolvency of an insurer could affect our ability to recover claims on policies for which we have already paid a premium.
Product liability claims, regardless of their merits or the ultimate success of our defense, are costly, divert management’s attention, may harm our reputation, and can impact the demand for our products and generate speculative news flows and/or rumors relating to such claims. Substantial product liability claims could materially adversely affect our business, results of operations and financial condition, and/or may have an impact on market perception of our company and negatively affect our stock price.
Claims and investigations relating to ethics and business integrity, competition law, marketing practices, pricing, human rights of workers and other legal matters could adversely affect our business, results of operations and financial condition
Our industry is heavily regulated and legal requirements vary from country to country, and new requirements are imposed on our industry from time to time. Governments and regulatory authorities around the world have been strengthening implementation and enforcement activities in recent years, including in relation to anti-bribery, anti-corruption and ethical requirements with respect to medical and scientific research, interactions with healthcare professionals and payers, and respect for the human rights of workers.
We have adopted a Code of Conduct that requires employees to comply with applicable laws and regulations, as well as the specific principles and rules of conduct set forth in the Code. We also have policies and procedures designed to help ensure that we, our officers, employees, agents, intermediaries and other third parties comply with applicable laws and regulations (including but not limited to the US Foreign Corrupt Practices Act (FCPA), the UK Bribery Act, the OECD Anti-Bribery Convention, the French Anti-Corruption measures law (Sapin II), the French duty of vigilance law and other anti-bribery laws and regulations).
Notwithstanding these efforts, failure to comply with laws and regulations (including as a result of a business partner’s breach) may occur and could result in liabilities for us and/or our management.
Sanofi and certain of its subsidiaries could become the subject of investigations or proceedings by various government entities or could face audits and/or litigation, including allegations of corruption, claims related to employment matters, patent and intellectual property disputes, consumer law claims, competition law and tax audits. We are currently the target of a number of lawsuits relating to pricing and marketing practices (including, for example, “whistleblower” litigation in the United States), which we are vigorously defending. With respect to tax issues, the complexity of the fiscal environment is such that the ultimate resolution of any tax matter may result in payments that are greater or less than the amounts we have accrued. See “Item 8. Financial Information — A. Consolidated Financial Statements and Other Financial Information — Information on Legal or Arbitration Proceedings” and Note D.22. to our consolidated financial statements included at Item 18. of this annual report. In addition, responding to such investigations is costly and may divert management’s attention from our business.
Unfavorable outcomes in any of these matters, or in similar matters that may arise in the future, could preclude the commercialization of our products, harm our reputation, negatively affect the profitability of existing products and subject us to substantial fines, punitive damages, penalties and injunctive or administrative remedies, potentially leading to the imposition of additional regulatory controls, monitoring or self-reporting obligations, or exclusion from government reimbursement programs or markets, all of which could have a material adverse effect on our business, results of operations or financial condition.
The unpredictability of these proceedings could lead Sanofi, after consideration of all relevant factors, to enter into settlement agreements to settle certain claims. Such settlements may involve significant monetary payments and/or potential criminal penalties, may include admissions of wrongdoing and may require entering into a Corporate Integrity Agreement (CIA) or a Deferred Prosecution Agreement (in the United States), which is intended to regulate company behavior for a specified number of years. For example, on February 28, 2020, Sanofi US entered into a civil settlement with the United States Department of Justice and agreed to pay approximately $11.85 million to resolve allegations regarding certain charitable donations Sanofi US made to an independent patient assistance foundation that assisted patients being treated for multiple sclerosis. In connection with this settlement, Sanofi US also entered into a CIA with the Office of the Inspector General for the United States Department of Health and Human Services effective the same day, which will require us to meet and maintain certain compliance requirements in the United States.
Our activities (including our products and manufacturing activities) are subject to significant government regulations and regulatory approvals, which are often costly and could result in adverse consequences to our business if we fail to anticipate the regulations, comply with them, maintain the required approvals, and/or adapt to changes in applicable regulations
Obtaining a marketing authorization for a product is a long and highly regulated process requiring us to present extensive documentation and data to the relevant regulatory authorities either at the time of the filing of the application for a marketing authorization or later during its review. Each regulatory authority may impose its own requirements which can evolve over time.
2 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Each regulatory authority may also delay or refuse to grant approval even though a product has already been approved in another country. Regulatory authorities are increasingly strengthening their requirements on product safety and risk/benefit profiles. All of these requirements, including post-marketing requirements, have increased the costs associated with maintaining marketing authorizations (see “Item 4. Information on the Company — B. Business Overview — B.5. Markets — B.5.3. Regulatory framework”).
Moreover, to monitor our compliance with applicable regulations, the FDA, EMA, WHO and comparable national agencies in other jurisdictions routinely conduct regulatory inspections of our facilities, distribution centers, commercial activities and development centers (including hospitals), and may identify potential deficiencies which we must adequately address. For example, in November 2020, the FDA issued a Complete Response Letter (CRL) regarding the Biologics License Application (BLA) for sutimlimab, a monoclonal antibody for the treatment of hemolysis in adults with cold agglutinin disease (which has been approved in the meantime), referring to certain deficiencies identified by the agency during a pre-license inspection of a third-party facility responsible for manufacturing. More generally, if we fail to adequately respond to regulatory inspection observations identified during an inspection or fail to comply with applicable regulatory requirements at all or within the targeted timeline, we could be subject to enforcement, remedial and/or punitive actions by the FDA (such as a Warning Letter, injunction, seizure or cease and desist order), the EMA or other regulatory authorities. In addition, we have an obligation to monitor and report adverse events and safety signals. In order to comply with these duties, we must regularly train our employees and certain third parties (such as external sales forces and distributor employees) on regulatory matters, including on pharmacovigilance. If we fail to train these people, or fail to train them appropriately, or if they do not comply with contractual requirements, we may be exposed to the risk that safety events are not reported or not reported in a timely manner in breach of our reporting obligations.
Due to regulatory or geopolitical constraints, we may face delays in our clinical trials due, for example, to the new EU Clinical Trials Regulation review process for approvals of new trials or for the transition of ongoing trials under such new regulation, and/or restrictions imposed on clinical trial sites, and/or delays in the supply chain for investigational products and/or the initiation and enrollment of patients in our clinical trials, and/or disruptions related to regulatory approvals, for instance due to the inability of health authorities to perform inspections in other countries and/or delays in label expansions for existing products. We may not be able to fully mitigate these delays, which could negatively impact the timing of our pipeline development programs and may have a negative impact on our product development and launches and hence on future product sales, business and results of operations.
In addition, all aspects of our business, including research and development, manufacturing, marketing, reimbursement, pricing and sales, are subject to extensive legislation and governmental regulation. Changes in applicable laws and the costs of compliance with such laws and regulations could have an adverse effect on our business.
For example, in response to the European Union regulations for Medical Devices (EU MDR), which entered into force in May 2021, Sanofi created the EU MDR task force. This task force was commissioned to address the risk of potential delays in approvals (for new drug-device combination products, for substantial changes to the design or intended purpose of the device component of already approved drug-device combination products, and for medical devices) and of product discontinuation (for some legacy medical devices), as well as compliance risks for existing products due to increased requirements for post-marketing surveillance, clinical evaluations, traceability and transparency. A similar task force was set up in the first quarter of 2021 to examine risks related to the new regulations for In-Vitro Diagnostic Devices (IVDR) implemented in May 2022.
For information about risks related to changes:
•in proprietary rights rules and regulations, see “– We rely on our patents and other proprietary rights to provide exclusive rights to market certain of our products. If such patents and other rights were limited, invalidated or circumvented, our financial results could be adversely affected” below; and
•in environmental rules and regulations, see “– Management of the historical contamination related to our past industrial activities could adversely impact our results of operations and reputation” below.
In addition, changes in tax laws or regulations or their interpretation or exposures to additional tax liabilities around the world could negatively impact our operating results. Changes to tax laws or regulations may occur at any time, and any related expense or benefit recorded may be material to the fiscal quarter and year in which the law change is enacted.
Furthermore, most of the jurisdictions in which we operate have double tax treaties with other foreign jurisdictions, which provide a framework for mitigating the impact of double taxation on our revenues and capital gains. However, the outcome of those mechanisms developed to resolve such conflicting claims can be uncertain and can be expected to be very lengthy. Accruals for tax contingencies are made based on experience, interpretations of tax law, and judgments about potential actions by tax authorities. However, due to the complexity of tax contingencies, the ultimate resolution of any tax matter may result in payments materially different from the amounts accrued.
We rely on our patents and other proprietary rights to provide exclusive rights to market certain of our products. If such patents and other rights were limited, invalidated or circumvented, our financial results could be adversely affected
Through patent and other proprietary rights, such as data exclusivity or supplementary protection certificates in Europe, we hold exclusivity rights for a number of our research-based products. However, the protection that we are able to obtain varies in its duration and scope. Furthermore, patents and other proprietary rights do not always provide effective protection for our products. We cannot be certain that we will obtain adequate patent protection for new products and technologies in important markets or that such protections, once granted, will last as long as originally anticipated.
SANOFI FORM 20-F 2023 | 3 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
For example, governmental authorities are increasingly looking to facilitate generic and biosimilar competition for existing products through new regulatory proposals intended to achieve, or resulting in, changes to the scope of patent or data exclusivity rights and through the use of accelerated regulatory pathways for generic and biosimilar drug approvals. At the EU level, the proposed wide-ranging revision of the general pharmaceutical legislation may pose downside risks to innovation and competitiveness in Europe, primarily due to the reduction of intellectual property (IP) protections and a stricter incentives framework for orphan medicinal products (OMPs). Such regulatory proposals could make patent prosecution for new products more difficult and time consuming or could adversely affect the exclusivity period for our products.
Moreover, manufacturers of generic products or biosimilars are increasingly seeking to challenge patent validity or coverage before the patents expire, and manufacturers of biosimilars or interchangeable versions of the products are seeking to have their version of the product approved before the exclusivity period ends. Furthermore, in an infringement suit against a third party, we may not prevail and the decision rendered may not conclude that our patent or other proprietary rights are valid, enforceable or infringed. Our competitors may also successfully avoid our patents. Even in cases where we ultimately prevail in an infringement claim, legal remedies available for harm caused to us by infringing products may be inadequate to make us whole. Moreover, a successful result against a competing product for a given patent or in a specific country is not necessarily predictive of our future success against another competing product or in another country because of local variations in the patents and patent laws.
In addition, if we lose patent protection as a result of an adverse court decision or a settlement, we face the risk that government and private third-party payers and purchasers of pharmaceutical products may claim damages alleging they have over-reimbursed or overpaid for a drug. For example, in 2009, in Australia, our patent on clopidogrel was ultimately held invalid. Following this decision, the Australian Government sought damages for its alleged over-reimbursement of clopidogrel drugs due to the preliminary injunction we had secured against the sale of generic clopidogrel during the course of the litigation. The Australian Government’s claim was dismissed following two decisions, one of the Federal Court of Australia in April 2020 and one of the Full Court of the Federal Court of Australia in June 2023. On December 18, 2023, the Australian Government was granted a special leave to appeal to the High Court of Australia.
We also rely on unpatented proprietary technology, know-how, trade secrets and other confidential information, which we seek to protect through various measures, including confidentiality agreements with licensees, employees, third-party collaborators, and consultants who may have access to such information. If these agreements are breached or our other protective measures should fail, then our contractual or other remedies may not be adequate to cover our losses.
In certain cases, to terminate or avoid patent litigation we or our collaboration partners may be required to obtain licenses from the holders of third-party intellectual property rights. Any payments under these licenses may reduce our profits from such products and we may not be able to obtain these licenses on favorable terms or at all.
Third parties may also request a preliminary or permanent injunction in a country from a court of law to prevent us from marketing a product if they consider that we infringe their patent rights in that country. If third parties obtain a preliminary or permanent injunction or if we fail to obtain a required license for a country where valid third-party intellectual property rights as confirmed by a court of law exist, or if we are unable to alter the design of our technology to fall outside the scope of third-party intellectual property rights, we may be unable to market some of our products in certain countries, which may limit our profitability.
In addition, the pursuit of valid business opportunities may require us to challenge intellectual property rights held by others that we believe were improperly granted, including through negotiation and litigation, and such challenges may not always be successful. Third parties may claim that our products infringe one or more patents owned or controlled by them. Claims of intellectual property infringement can be costly and time-consuming to resolve, may delay or prevent product launches, and may result in significant royalty payments or damages.
Furthermore, some countries may consider granting a compulsory license to a third party to use patents protecting an innovator’s product, which limits the value of the patent protection granted to such products.
We have increased the proportion of biological therapeutics in our pipeline relative to traditional small molecule pharmaceutical products. Typically, the development, manufacture, sale and distribution of biological therapeutics is complicated by third-party intellectual property rights (otherwise known as freedom to operate (FTO) issues), to a greater extent than for the small molecule therapeutics, because of the types of patents allowed by national patent offices. Further, our ability to successfully challenge third-party patent rights is dependent on the legal interpretation and case law of national courts. In addition, we expect to face increasing competition from biosimilars in the future. With the accelerated regulatory pathways provided in the United States and Europe for biosimilar drug approval, biosimilars can be a threat to the exclusivity of any biological therapeutics we sell or may market in the future and can pose the same issues as the small molecule generic threat described above. If a biosimilar version of one of our products were to be approved, it could reduce our sales and/or profitability of that product.
We currently hold trademark registrations and have trademark applications pending in many jurisdictions, any of which may be the subject of a governmental or third-party objection, which could prevent the maintenance or issuance of the trademark. As our products mature, our reliance on our trademarks and trade dress to differentiate us from our competitors increases and, as a result, our business could be adversely affected if we are unable to prevent third parties from adopting, registering or using trademarks and trade dress that infringe, dilute or otherwise violate our rights.
If our patents and/or proprietary rights to our products were limited or circumvented, our financial results could be adversely affected.
4 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Failure to comply with data ethics and privacy regulations could adversely affect our business and reputation
We operate in an environment that relies on the collection, processing, analysis and interpretation of large sets of patients’ and other individuals’ personal data, and the operation of our business requires data to flow freely across borders of numerous countries.
The legal and regulatory environment of data privacy is diversified, with regional legislation such as the General Data Protection Regulation (GDPR) in Europe, the Personal Information Protection Law (PIPL) enacted in 2021 in China, and other significant privacy legislation, including the California Consumer Privacy Act (CCPA) in the United States. As the framework continues to evolve, some uncertainty remains with respect to absence of clear guidance or case law.
Such uncertainty could result in an operational risk limiting or preventing the transfer of data across borders, which may have an impact on our activities (e.g., on clinical trials). Breach of the regulations described above could also carry financial sanctions and may harm our reputation and those of our activities that rely on personal data processing.
Furthermore, the increasing volume of data processed and advances in new technologies, such as artificial intelligence, have resulted in a greater focus on data governance and the ethical use of personal data. Failure in our data governance and ethical use of personal data could affect our business and reputation.
Risks relating to our business
The pricing and reimbursement of our products is negatively affected by increasing cost containment pressures and decisions of governmental authorities and other third parties
The commercial success of our existing products and our product candidates depends in part on their pricing and reimbursement conditions. Our products are negatively affected by continued downward pricing pressure and scrutiny due, inter alia, to:
•stricter price and access controls imposed by governments and other payers around the world:
–requirements for greater transparency around drug pricing and drug development costs,
–widespread use of international reference pricing and therapeutic reference pricing, among other pricing methodologies and caps,
–mandatory price cuts, renegotiations, industry payback and rebates,
–delisting from reimbursement and restrictions on the label population,
–access restrictions for high-priced innovative medicines,
–prescribing guidelines and binding medicine utilization controls,
–Medicare drug price negotiations under the US Inflation Reduction Act (IRA),
–greater use of tendering and centralized procurement (national/regional/class-wide level),
–cross-country cooperation in price negotiations, contracting or procurement, which is already occurring to some extent, such as the Vaccine Alliance (GAVI), the COVID-19 Vaccines Global Access (COVAX) initiative, the BeNeLuxA alliance in Europe, and the Pan American Health Organization (PAHO),
–shifting of the payment burden to US patients and access disruptions through co-pay accumulator and maximizer programs as well as alternative funding programs,
–more aggressive formulary utilization management controls (including stepped therapy, strict prior authorization criteria, formulary exclusions) by US insurers and pharmacy benefits managers (PBMs), and
–discriminatory and non-transparent pricing and procurement policies (e.g. government procurement restrictions, import bans) in favor of domestic pharmaceutical companies;
•widespread use of health technology assessment (HTA) to inform coverage and reimbursement decisions:
–more stringent evidence and value requirements (e.g. comparative effectiveness, patient preferences, real-word evidence, health economic modelling) by payers and HTA authorities, raising the bar for market entry,
–unreasonable thresholds for cost-effectiveness, and
–increasingly restrictive HTA decisions with significant variation across markets;
•increased generic and biosimilar competition, accelerating price erosion:
–next generation biosimilars coming to the market across major therapeutic areas,
–potential savings from increased biosimilar use, expected to be a cumulative $285 billion globally through 2025 according to the IQVIA Institute, and
–evolving regulatory landscapes to support interchangeability (e.g., in the US and EU) and pharmacy substitution (e.g. in the EU Nordic countries, Germany and France).
In the United States, which accounted for 43.0% of our net sales in 2023, the IRA was enacted in August 2022. The law includes three core drug pricing provisions (Medicare negotiation, Part D redesign, and Medicare inflation penalties) with effective dates ranging from 2022 through 2026. The IRA legislation is likely to have a negative impact on industry revenue growth and future innovation. While significant uncertainties remain on the process and methods of Medicare negotiation, we anticipate exposure to the negotiation provision in the latter part of the decade, and in the case of DUPIXENT not before 2031.
SANOFI FORM 20-F 2023 | 5 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Furthermore, we face increasing pricing pressure and gross-to-net (GTN) erosion from continuing vertical integration and consolidation of the US health insurance market. With the three largest pharmacy benefit manager group purchasing organizations (PBM GPOs) (i.e. Ascent, Zinc and Emisar) now covering over 85% of prescription drug claims, consolidation has led to increased utilization management and restrictive formularies, resulting in strong bargaining power to negotiate discounted prices, thereby adversely impacting our sales.
There is also uncertainty about the potential impact of the continued growth of the federal 340B drug pricing program, among other risks.
In China, pricing pressure is likely to intensify as a growing number of our products are subject to national reimbursement drug list (NRDL) price negotiations and national volume-based procurement (VBP) tenders, with the lowest prices prevailing to compete with local champions. At market entry, new drugs listed on the NRDL had an average price cut of 60% over the past five years. Further expansion of the VBP policy to biologics and biosimilars also poses a growing threat to our key established products, with over 500 drugs targeted for inclusion by 2025.
In Europe, budget pressure remains high in the wake of Covid-19, translating into an acceleration of cost-containment policies in major EU markets.
The industry is also facing high uncertainty in the context of the new pharmaceutical strategy in Europe, including numerous policy changes to the EU pharmaceutical legislation, HTA and joint procurement, among others. If passed, these reforms will likely create new challenges and risks for access, innovation and competitiveness in Europe, primarily because of the reductions in regulatory data protection (from eight to six years for all new drugs) and market exclusivity for orphan medicines.
Our research and development efforts may not succeed in adequately renewing our product portfolio
Discovering and developing a new product is a costly, lengthy and uncertain process. To be successful in the highly competitive pharmaceutical industry, we must commit substantial resources each year to research and development in order to develop new products to compensate for decreasing sales of products facing patent expiration and termination of regulatory data exclusivity, introduction of lower-priced generics, or competition from new products of competitors that are perceived as being superior or equivalent to our products. We must pursue both early-stage research and early and late development stages in order to propose a sustainable and well-balanced portfolio of products. In 2023, we spent €6,728 million on research and development, amounting to 15.6% of our net sales. As part of an update on our strategy, we announced in October 2023 our intent to increase our research and development investments. Failure to invest in the right technology platforms, therapeutic areas, product classes, geographic markets and/or licensing opportunities could adversely impact the productivity of our pipeline.
We are pursuing our strategic transformation through R&D, including potential multi-indication assets such as amlitelimab, frexalimab and the oral TNFR1si, intended to address unmet patient needs in markets with low penetration of advanced therapies. We focus our R&D strategy on oncology, immunology and inflammation, multiple sclerosis, neurology, rare diseases and rare blood disorders. In 2021, Sanofi acquired Translate Bio to accelerate the deployment of mRNA technology for the development of new vaccines, including for seasonal influenza, and beyond vaccines, therapeutics where there is a strong unmet medical need. However, mRNA technology is still in its early days and the ability of this technology to produce strong results and safety still remains to be fully asserted. We may also fail to improve our development productivity sufficiently to sustain our pipeline (see also “— We may fail to successfully identify external business opportunities or realize the anticipated benefits from our strategic investments or divestments” below). Other new innovations under investigation, such as natural killer (NK) cells and conditionally activated biologics, raise similar uncertainties.
In addition, the competitive landscape includes a high level of uncertainty as numerous companies are working on or may be evaluating similar targets and a product considered as promising at the beginning of its development may become less attractive if a competitor addressing the same unmet need reaches the market earlier. There can be no assurance that any of our product candidates will be proven safe or effective (see “Item 4. Information on the Company — B. Business Overview — B.4. Global research & development”). Over these research and development cycles, usually spanning several years, there is a substantial risk at each stage of development – including pre-clinical activities and clinical trials – that we will not achieve our goals of safety and/or efficacy and that we will have to abandon a product in which we have invested substantial amounts of money and human resources. For instance, in 2022 patient enrollment of Phase 3 tolebrutinib trials paused globally after a decision of the FDA regarding potential side effects. As another example, the global clinical development program of amcenestrant (breast cancer) was discontinued in August 2022 following the outcome of the prespecified interim analysis of a Phase 3 trial. More and more trials are designed with clinical endpoints of superiority; failure to achieve those endpoints could damage the product’s outlook and our overall development program.
Decisions concerning the studies to be carried out can have a significant impact on the marketing strategy for a given product. Multiple in-depth studies can demonstrate that a product has additional benefits, facilitating the product’s marketing, but such studies are expensive and time consuming and may delay the product’s submission to regulatory authorities for approval.
In addition, following (or in some cases contemporaneously with) the marketing authorization, the dossier is also submitted to governmental agencies and/or national or regional third-party payers (HTA bodies) for review. These HTA bodies evaluate evidence on the value of the new product, assess the medical need it serves, and provide recommendations on the corresponding reimbursement. Such analyses may require additional studies, including comparative studies, which may effectively delay marketing, change the population which the new product treats, and add costs to its development. Our continuous investments in research and development for future products and for the launches of newly registered molecules could therefore result in increased costs without a proportionate increase in revenues, which would negatively affect our operating results and profitability.
6 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Lastly, there can be no assurance that all the products approved or launched will achieve commercial success.
In addition, even after a product reaches the market, certain developments following regulatory approval may decrease demand for our products. Clinical trials and post-marketing surveillance of certain marketed drugs have the potential to raise concerns among some prescribers and patients relating to the safety or efficacy of pharmaceutical products in general, which could negatively affect the sales of such products or lead to increased volatility in market reaction.
Breaches of data security, disruptions of information technology systems and cyber threats could result in financial, legal, competitive, operational, business or reputational harm
Our business depends heavily on the use of interdependent information technology systems, including Internet-based systems and digital tools. Certain key areas such as research and development, production and sales are to a large extent dependent on our information systems (including cloud-based computing) or those of third-party providers (including for the storage and transfer of critical, confidential, sensitive or personal information regarding our patients, clinical trials, vendors, customers, employees, collaborators and others). We are therefore vulnerable to cybersecurity attacks and incidents and misuse or manipulation of any of these IT systems could result in exposure of confidential information or the modification of critical data.
We and our third-party service providers, suppliers, contract manufacturers, distributors or other contracting third parties use, to the best of our ability, secure information technology systems for the protection of data and threat detection. Like many companies, we may experience certain of the following events which pose a risk to the security and availability of these systems and networks, and the confidentiality, integrity, and availability of our sensitive data: breakdown, outages, service disruption or impairment, data loss or deterioration in the event of a system malfunction or increasing threat of data theft or corruption in the event of a cyber-attack, security breach, industrial espionage attacks, insider threat attacks, cybercrimes, including state-sponsored cybercrimes, malware, misplaced or lost data, programming or human errors or other similar events. Also, in the event of an attack, US and European legislation related to the financing of terrorism imposes increasing restrictions on payments of ransom. As a result, our ability to recover the data might be limited. Therefore, our business continuity could be at risk if we are unable to recover data through back-ups and restorations.
We are increasingly using generative artificial intelligence to enhance our business processes. Although we have set up a governance body to control the artificial intelligence initiatives taken on a company-wide scale and have made a generative artificial intelligence charter available to all our employees, this new technology, like other artificial intelligence technology, entails risks linked to transparency and explainability, privacy and confidentiality, eco-responsibility, and cybersecurity. Those risks could lead to, among other things, unethical practices, business and reputational harm, cyber-attacks and security breaches (see "— We may fail to develop or take advantage of digitalization and prioritizing data as an organizational asset" below).
Each of these events could negatively impact important processes, such as scientific research and clinical trials, the submission of outcomes to health authorities for marketing authorizations, the functioning of production processes and the supply chain, compliance with legal requirements, trade secrets, security strategies and other key activities, including Sanofi’s employees’ ability to communicate between themselves as well as with third parties (see also “— Product liability claims could adversely affect our business, results of operations and financial condition” above). This could result in material financial, legal, competitive, operational, business or reputational harm.
Although we maintain relevant insurance coverage, this insurance may not be sufficiently available in the future to cover the financial, business or reputational losses that may result from an interruption or breach of our systems. For example, certain types of cyber-attacks could be considered as an Act of War subject to insurance exclusion.
The manufacture of our products is technically complex, and supply interruptions, product recalls or inventory losses caused by unforeseen events may reduce sales, adversely affect our operating results and financial condition, delay the launch of new products and negatively impact our image
Many of our products are manufactured using technically complex processes with production constraints, including the need for specialized facilities, trained and certified employees, and highly specific raw materials. We must ensure that all manufacturing processes comply with (i) current Good Manufacturing Practices (cGMP), (ii) other applicable regulations issued by governmental health authorities around the world, as well as (iii) our own quality standards. Third parties supply us with a portion of our raw materials, active ingredients and medical devices, which exposes us to the risk of a supply shortage or interruption especially in the event that these suppliers are unable to manufacture our products in line with quality standards or if they experience financial difficulties.
Epidemics and other public health crises, such as the COVID-19 pandemic, expose us to risks of a slowdown or temporary suspension in the production of our APIs, raw materials, and some of our products. Any prolonged restrictive measures put in place in order to control an outbreak of contagious disease or other adverse public health development, in any of our principal production sites, may have a material and adverse effect on our manufacturing operations. Any of these factors could adversely affect our business, operating results or financial condition (see “Item 4. Information on the Company — B. Business Overview — B.7. Production and raw materials” for a description of these outsourcing arrangements and “A failure in our crisis and business continuity management processes in case of unpredictable events could have negative consequences for our business, operations and reputation” below).
Our business may require the transformation and adaptation of our plants in order to ensure the continuity of production of our products in sufficient quantities to satisfy demand. This may be necessary to meet the need for the production of new products, including biologics, or to ensure the scaling up production of products under development once approved. This need may also result from new regulatory requirements. Furthermore, our biological products, in particular, are subject to the risk of
SANOFI FORM 20-F 2023 | 7 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
manufacturing stoppages or the risk of loss of inventory because of the difficulties inherent in the processing of biological materials and the potential difficulties in accessing adequate amounts of raw materials meeting required standards. In addition, specific storage and distribution conditions are required for many biological products (for example, cold storage is required for certain vaccines, insulin-based products and some hemophilia products). These production difficulties may also be encountered during testing, which is a mandatory requirement prior to drug products being released.
The complexity of our production processes, as well as standards required for the manufacture of our products, subject us to risks because the investigation and remediation of any identified or suspected problems can cause production delays, substantial expense, product recalls or lost sales and inventories, and delay the launch of new products; this could adversely affect our operating results and financial condition, and cause reputational damage and the risk of product liability (see “— Product liability claims could adversely affect our business, results of operations and financial condition” above). In addition, some of our production sites, and some of our suppliers’ and/or contractors’ sites, are located in areas exposed to natural disasters such as floods, earthquakes and hurricanes. Such disasters could be exacerbated by climate change. In the event of a major disaster, we could experience severe destruction or interruption of our operations and production capacity at these sites.
When manufacturing disruptions occur, we may not have alternate manufacturing capacity, particularly for certain biologics. In the event of manufacturing disruptions, our ability to use backup facilities or set up new facilities is more limited because biologics are more complex to manufacture and generally require dedicated facilities. Even though we aim to have backup sources of supply whenever possible, including by manufacturing backup supplies of our principal active ingredients at additional facilities when practicable, we cannot be certain they will be sufficient if our principal sources become unavailable. Switching sources and manufacturing facilities requires significant time and prior approval by health authorities.
Supply shortages generate even greater negative reactions when they occur with respect to life saving medicines with limited or no viable therapeutic alternatives. Shortages of specific products can have a negative impact on the confidence of patients, customers and professional healthcare providers and the image of Sanofi and may lead to lower product revenues.
A substantial share of the revenue and income of Sanofi depends on the performance of certain flagship products
As part of the presentation of our strategy in December 2019, we announced our intent to prioritize our activities on growth drivers including DUPIXENT and our Vaccines operations, which we have identified as key growth drivers. Nevertheless market expansion and new launches of medicines and vaccines may not deliver the expected benefits. We may also encounter failures or delays in our launch strategy (in terms of timing, pricing, market access, marketing efforts and dedicated sales forces), such that our products that may not deliver the expected benefits. The competitive environment for a given product may also have changed by the time of the actual launch, modifying our initial expectations. The need to prioritize the allocation of resources may also cause delays in or hamper the launch or expansion of some of our products.
Also, we currently generate a substantial share of our net sales from certain key products (see “Item 5. Operating and Financial Review and Prospects — A.2. Results of Operations — Year ended December 31, 2023 compared with year ended December 31, 2022 — A.2.1.3/Net Sales — Biopharma segment”). For example, DUPIXENT generated net sales of €10,715 million in 2023 representing 24.9% of our net sales for the year and is Sanofi’s biggest product in terms of sales.
Among our flagship products, LANTUS, LOVENOX, PLAVIX and JEVTANA already face generic competition on the market. LANTUS is particularly important; it was one of Sanofi’s leading products in 2023 with net sales of €1,420 million. AUBAGIO, another leading product, has faced generic competition in the US since March 2023, following a settlement agreement entered into in 2017 and in Europe since the fourth quarter of 2023.
More generally, an expiration of effective intellectual property protections for our products typically results in the market entry of one or more lower-priced generic competitors, often leading to a rapid and significant decline in revenues on those products (for information regarding ongoing patent litigation see Note D.22.b. to the consolidated financial statements included at Item 18. of this annual report).
The introduction of a generic product results in adverse price and volume effects for our branded or genericized products. For example, although we do not believe it is possible to state with certainty what level of net sales would have been achieved in the absence of generic competition, a comparison of our consolidated net sales for 2023 and 2022 for the main products affected by generic and biosimilar competition shows a loss of €651 million of net sales on a reported basis (see “Item 5. Operating and Financial Review and Prospects — A.1.2. Impacts of Competition from generics and biosimilars”). However, other parameters may have contributed to the loss of sales, such as a fall in the average price of certain products (e.g., LANTUS).
Furthermore, in general, if one or more of our flagship products were to encounter problems (such as material product liability litigation, unexpected side effects, product recalls, non-approval by the health authorities of a new indication for a marketed product, pricing pressure and manufacturing or supply issues), the adverse impact on our business, results of operations and financial condition could be significant.
We rely on third parties for the discovery, manufacture, marketing and distribution of some of our products
Our industry is both highly collaborative and competitive, whether in the discovery and development of new products, in-licensing, the marketing and distribution of approved products, or manufacturing activities. We expect that we will continue to rely on third parties for key aspects of our business and we need to ensure our attractiveness as a potential partner.
8 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
We conduct a number of significant research and development programs and market some of our products in collaboration with other biotechnology and pharmaceutical companies. For example, we currently have a global strategic collaboration with Regeneron on monoclonal antibodies for the development and commercialization of DUPIXENT, KEVZARA (sarilumab) and SAR440340 (REGN3500- itepekimab) (see “Item 5. Operating and Financial Review and Prospects — A.1.7. Financial Presentation of Alliances — A.1.7.1/ Alliance Arrangements with Regeneron Pharmaceuticals Inc.”). We rely upon Regeneron to successfully carry out their responsibilities with regard to the manufacture and supply of these collaboration antibodies. (see “Item 4. Information on the Company — B. Business Overview”). We may rely on partners to design and manufacture medical devices as well, notably for the administration of our products. Finally, we may also rely on partners for the development and commercialization of in-vitro diagnostic tests used in clinical trials and in-vitro diagnostic tests which are specified in the labeling of our products as necessary or useful for the management of patients taking our products.
As regards products recently launched or under development for which we have a collaboration agreement with partners, the terms of the applicable alliance agreement may require us to share profits and losses arising from commercialization of such products with our partners. This differs from the treatment of revenue and costs generated by other products for which we have no alliance agreement, and such profit sharing may deliver a lower contribution to our financial results.
We could also be subject to the risk that we may not properly manage the decision-making process with our partners. Decisions may be controlled by, or subject to the approval of our collaboration partners, who may have views that differ from ours. We are also subject to the risk that our partners may not perform effectively, which could have a detrimental effect when our collaboration partners are responsible for the performance of certain key tasks or functions, for example related to manufacturing or distribution. This risk is further increased by the growing number of distribution centers divested by Sanofi as part of its global strategy and by the resulting growing externalization of distribution tasks and functions.
Any failures in the development process or differing priorities may adversely affect our business, including the activities conducted through our collaboration arrangements. We also cannot guarantee that third-party manufacturers will be able to meet our near-term or long-term manufacturing requirements, for internal reasons (e.g., in case of financial difficulties), reasons directly related to their contractual relationship with Sanofi, or external reasons (e.g., in the event of a health crisis). Thereby, following the completion of the spin-off of EUROAPI in May 2022, EUROAPI became a third-party manufacturer and continues to manufacture a certain number of APIs for Sanofi. We are also subject to the risk that contract research organizations or other vendors (for instance regarding digital activities) retained by us or our collaboration partners may not perform effectively.
Any conflicts or difficulties with our partners during the course of these agreements or at the time of their renewal or renegotiation, or any disruption in the relationships with our partners, may affect the development, manufacturing, launch and/or marketing of certain of our products or product candidates and may cause a decline in our revenues or otherwise negatively affect our results of operations.
We are subject to the risk of non-payment by our customers(1)
We run the risk of delayed payments or even non-payment by our customers, which consist principally of wholesalers, distributors, pharmacies, hospitals, clinics and government agencies. This risk is accentuated by recent concentrations among distributors and retailers, as well as by uncertainties around global credit and economic conditions, in particular in emerging markets. As a result, we may be affected by fluctuations in the buying patterns of such customers. The United States poses particular customer credit risk issues because of the concentrated distribution system: our three main customers represented respectively 11%, 9% and 7% of our consolidated net sales in 2023. We are also exposed to large wholesalers in other markets, particularly in Europe. An inability of one or more of these wholesalers to honor their debts to us could adversely affect our financial condition (see Note D.34. to our consolidated financial statements included at Item 18. of this annual report).
In some countries, some customers are public or subsidized health systems. The economic and credit conditions in these countries may lead to an increase in the average length of time needed to collect on accounts receivable.
Global economic conditions and an unfavorable financial environment could have negative consequences for our business(2)
Over the past several years, growth of the global pharmaceutical market has become increasingly tied to global economic growth. In this context, a substantial and lasting slowdown of the global economy, major national economies or emerging markets could negatively affect growth in the global pharmaceutical market and, as a result, adversely affect our business. For example, unpredictable political conditions that currently exist in various parts of the world could have a material negative impact on our business, in particular armed conflicts between Russia and Ukraine since 2022, and in Gaza following the terrorist attacks that occurred in Israel in October 2023. The consequences of those conflicts are difficult to predict and will depend on developments outside Sanofi’s control, including, but not limited to the duration and severity of the conflicts, and the consequences of the ongoing and additional financial and economic sanctions imposed by governments in response. Other related issues have arisen or are arising such as regional instability; geopolitical uncertainties; adverse effects on fuel and energy costs, supply chains, macroeconomic conditions, inflation, and currency exchange rates in various regions of the world; and exposure of third parties to gas shortages. Collectively, such unstable conditions could, among other things, disturb the international flow of goods and increase the costs and difficulties associated with international transactions.
(1) The information in this section supplements the disclosures required under IFRS 7 as presented in Notes B.8.7., D.10. and D.34. to our consolidated financial statements, provided at Item 18. of this annual report.
(2) The information in this section supplements the disclosures required under IFRS 7 as presented in Note B.8.7. to our consolidated financial statements, provided at Item 18. of this annual report.
SANOFI FORM 20-F 2023 | 9 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Unfavorable economic conditions have reduced the sources of funding for national social security systems, leading to austerity measures including heightened pressure on drug prices, increased substitution of generic drugs, and the exclusion of certain products from formularies among others (see “— The pricing and reimbursement of our products is negatively affected by increasing cost containment pressures and decisions of governments and other third parties” above).
Further, our net sales may be negatively impacted by the continuing challenging global economic environment, as high unemployment, increases in cost-sharing, and lack of developed third-party payer systems in certain regions may lead some patients to switch to generic products, delay treatments, skip doses or use other treatments to reduce their costs. In the United States there has been a significant increase in the number of beneficiaries in the Medicaid program, under which sales of pharmaceuticals are subject to substantial rebates and, in many US states, to formulary restrictions limiting access to brand-name drugs, including ours. Also, employers may seek to transfer a greater portion of healthcare costs to their employees due to rising costs, which could lead to further downward price pressure and/or lower demand.
Our Consumer Healthcare (CHC) business could also be adversely impacted by difficult economic conditions, as the financial resources of our customers may be reduced as a result of the economic situation.
If economic conditions worsen, or in the event of default or failure of major players including wholesalers or public sector buyers financed by insolvent states, our financial situation, the profitability and results of our operations and the distribution channels of our products may be adversely affected. See also “— We are subject to the risk of non-payment by our customers” above.
A failure in our crisis and business continuity management processes in case of unpredictable events could have negative consequences for our business, operations and reputation
Despite enhanced crisis preparedness and response, due in particular to recent crises such as the COVID-19 pandemic and the war in Ukraine since February 2022, unpredictable and extraordinary internal or external events, or a combination of escalating events that may occur as a result of a large scale cyber-attack (see also “— Breaches of data security, disruptions of information technology systems and cyber threats could result in financial, legal, competitive, operational, business or reputational harm” above), a pandemic or natural disasters, which could result in the failure of critical processes within Sanofi or a third party on whom we rely. Such failure may adversely impact our business, operations and reputation.
The occurrence of these unforeseen events may also heighten other risks such as a disruption or temporary suspension in production of APIs, raw materials and some of other products and/or lead to manufacturing delays or disruptions and supply chain interruptions (including to the extent those measures apply to our third-party suppliers) and may have an adverse effect on our business (see “— The manufacture of our products is technically complex, and supply interruptions, product recalls or inventory losses caused by unforeseen events may reduce sales, adversely affect our operating results and financial condition, delay the launch of new products and negatively impact our image” above). Also, a sudden increase in demand for selected medicinal products in the event of a crisis can result in short-term unavailability or shortages of raw materials.
Climate change or legal, regulatory or market measures to address climate change may negatively affect our business and results of operations
Climate change resulting from increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere could present risks to our operations, including an adverse impact on global temperatures, weather patterns and the frequency and severity of extreme weather and natural disasters. Natural disasters and extreme weather conditions, such as a hurricane, tornado, earthquake, wildfire or flooding, may pose physical risks to our facilities and disrupt the operation of our supply chain. The impacts of the changing climate on water resources may result in water scarcity, limiting our ability to access sufficient high-quality water in certain locations, which may increase operational costs. For example, some of our facilities are located in certain areas in France where specific administrative prefectural orders have been issued to regulate the water consumption (e.g. prefectural order issued by the Préfet de la Gironde in April 2023 regarding requirements imposed on Sanofi for the operation of a drug manufacturing facility located in the city of Ambarès-et-Lagrave (i.e. implementation of specific water saving measures in the event of a period of drought)).
Concern over climate change may also result in new or additional legal or regulatory requirements, designed to reduce greenhouse gas emissions and/or mitigate the effects of climate change on the environment. If such laws or regulations are more stringent than current legal or regulatory obligations (e.g. increased carbon taxation risk), we may experience disruption in, or an increase in the costs associated with sourcing, manufacturing and distribution of our products, which may adversely affect our business, results of operations or financial condition.
The increasing use of social media platforms and new technologies present risks and challenges for our business and reputation
We increasingly rely on social media, new technologies and digital tools to communicate about our products and about diseases or to provide health services. The use of these media requires specific attention, monitoring programs and moderation of comments. Political and market pressures may be generated by social media because of rapid news cycles. This may result in commercial harm, overly restrictive regulatory actions and erratic share price performance. In addition, unauthorized communications, such as press releases or posts on social media, purported to be issued by Sanofi, may contain information that is false or otherwise damaging and could have an adverse impact on our image and reputation and on our stock price. Negative or inaccurate posts or comments about Sanofi, our business, directors or officers on any social networking website could seriously damage our reputation. In addition, our employees and partners may use social media and mobile technologies inappropriately, which may give rise to liability for Sanofi, or which could lead to breaches of data security, loss of trade secrets or other intellectual property or public disclosure of sensitive information. Such uses of social media and mobile technologies could have an adverse effect on our reputation, business, financial condition and results of operations.
10 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Risks relating to Sanofi’s structure and strategy
We may fail to successfully identify external business opportunities or realize
the anticipated benefits from our strategic investments or divestments
the anticipated benefits from our strategic investments or divestments
We pursue a strategy of selective acquisitions, in-licensing and collaborations in order to reinforce our pipeline and portfolio. We are also proceeding to selective divestments to focus on key business areas. The implementation of this strategy depends on our ability to identify transaction opportunities, mobilize the appropriate resources in order to enter into agreements in a timely manner, and execute these transactions on acceptable economic terms. Moreover, entering into in-licensing or collaboration agreements generally requires the payment of significant “milestones” well before the relevant products reach the market, without any assurance that such investments will ultimately become profitable in the long term (see Note D.21.1. to the consolidated financial statements included at Item 18. of this annual report and also “— We rely on third parties for the discovery, manufacture, marketing and distribution of some of our products” above). Once a strategic transaction is agreed upon with a third party, we may not be able to complete the transaction in a timely manner or at all.
For newly acquired activities or businesses, our growth objectives could be delayed or ultimately not realized, and expected synergies could be adversely impacted if, for example:
•we are unable to integrate those activities or businesses quickly or efficiently;
•key employees leave; or
•we have higher than anticipated integration costs.
The Translate Bio acquisition (see in “— Our research and development efforts may not succeed in adequately renewing our product portfolio” above) which was completed in 2021 may not generate the expected results in terms of developing new mRNA-based products to meet existing or future needs, and the potential of Translate Bio’s mRNA platform may not be realized to its full extent or because of the difficulty of integrating the activity quickly and efficiently into the Group.
We may also miscalculate the risks associated with business development transactions at the time they are made or may lack the resources or ability to access all the relevant information to evaluate such risks properly, including regarding the potential of research and development pipelines, manufacturing issues, tax or accounting issues, compliance issues, or the outcome of ongoing legal and other proceedings. It may also take a considerable amount of time and be difficult to implement a risk analysis and risk mitigation plan after the acquisition of an activity or business is completed due to lack of historical data. Acquired businesses may not always be in full compliance with legal, regulatory or Sanofi standards, including, for example, current Good Manufacturing Practices (cGMP), which can be costly and time consuming to remedy. As a result, risk management and coverage of such risks, particularly through insurance policies, may prove to be insufficient or ill-adapted.
With respect to divestments, their financial benefit could be impacted if we face significant financial claims or significant post-closing price adjustments. Furthermore, the value of the assets to be divested may deteriorate while we are in the process of executing our divestment strategy, with the risk that we do not realize the anticipated benefits.
Because of the active competition among pharmaceutical groups for business development opportunities, there can be no assurance of our success in completing these transactions when such opportunities are identified.
The globalization of our business exposes us to increased risks in specific areas
As part of the presentation of our strategy in December 2019, we identified our strong presence in China among our core drivers, with revenue amounting to 6.8% of our net sales in 2023.
Nevertheless, the difficulties in operating in emerging markets, a significant decline in the anticipated growth rate or an unfavorable movement of the exchange rates of currencies against the euro could impair our ability to take advantage of growth opportunities and could adversely affect our business, results of operations or financial condition. For instance, if a long-lasting epidemic and prolonged or repeated restrictive measures to control the outbreak were to result in an economic slowdown in any of our targeted markets, it would reduce our sales due to lower healthcare spending on other diseases and fewer promotional activities, and could significantly impact our business operations. Furthermore, it is not possible to predict if or how the current health crisis will impact any particular affected jurisdiction, or to what extent (see also “— Global economic conditions and an unfavorable financial environment could have negative consequences for our business” above).
Emerging markets also expose us to more volatile economic conditions, political instability (including a backlash in certain areas against free trade), competition from multinational or locally based companies that are already well established in these markets, the inability to adequately respond to the unique characteristics of emerging markets (particularly with respect to their underdeveloped judicial systems and regulatory frameworks), difficulties in recruiting qualified personnel or maintaining the necessary internal control systems, potential exchange controls, weaker intellectual property protection, higher crime levels (particularly with respect to counterfeit products), and compliance issues including corruption and fraud (see particularly “— Claims and investigations relating to ethics and business integrity, competition law, marketing practices, pricing, human rights of workers, data protection and other legal matters could adversely affect our business, results of operations and financial condition” above).
SANOFI FORM 20-F 2023 | 11 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
We may fail to develop or take advantage of digitalization and prioritizing data as an organizational asset
We have undertaken several digital initiatives, such as the implementation of artificial intelligence across the business. For example, in research and development, we have built multiple artificial intelligence programs to reduce research times through improved predictive modelling and we seek to automate time-consuming activities, enabling research and development teams to scale and accelerate research processes and improve potential target identification in therapeutic areas such as immunology, oncology and neurology. In manufacturing and supply, we have developed an in-house artificial intelligence-enabled yield optimization solution, which enables consistently higher yield levels and optimizes usage of raw materials, contributing to our environmental objectives and supporting improved cost efficiency. Other examples include the opening in June 2022 of the Artificial Intelligence Centre of Excellence in Toronto, joining our global network of existing digital hubs in Paris, Boston, New York and Barcelona, which focuses on using leading technologies to develop world-class data and artificial intelligence products that accelerate research and development; the opening in March 2022 of our Accelerator, an internal unit that functions like a startup business within Sanofi to develop products and solutions that will support our mission to transform the practice of medicine with the use of digital, data and artificial intelligence; the Future4Care initiative of June 2021, where we joined a startup incubator along with other CAC40 companies to help nurture emerging leaders in health tech; or our enabled manufacturing facility in the US.
Our success in these efforts will depend on many factors including data availability; technology architecture; entering into successful partnerships and alliances with technology companies; a profound transformation of the organization, including a cultural change among our employees and the development of relevant skills; attracting and retaining employees with appropriate skills and mindsets in a tight labor market; and successfully innovating across a variety of technology fields. The COVID-19 pandemic has accelerated our digital transformation, including in the ways we engage and interact with our stakeholders. However, there is no guarantee that our efforts toward a digital transformation will succeed. More generally, we may fail to capture the benefits of digitalization and valuing data as an enterprise asset at an appropriate cost and/or in a timely manner, and/or enter into appropriate partnerships. Competitors, including new entrants such as tech companies, may outpace us in this fast-moving area. If we fail to adequately integrate digital capabilities into our organization and business model, we could lose patients and market share. This could have an adverse impact on our business, prospects and results of operations.
The success of digital initiatives will also depend on our ability to shift our culture to a data-driven culture. This calls for clear accountability for data and a collectively owned operating model with supporting tools and competencies to manage data as an asset, and for the definition of a robust life-cycle management process for data that is applied consistently across Sanofi. Good overall management of data supports our overall operations and the delivery of exponential capabilities, including artificial intelligence and machine learning to fulfil our innovation and efficiency ambitions. Failure to do so could result in business, operational and financial harm.
We may fail to accelerate our operational efficiency and perform our transformation program
As part of the presentation of the next chapter of our Play to Win strategy in October 2023, we announced our intent to improve our operating efficiencies to fund growth. We also announced savings of a total of up to €2 billion from 2024 to the end of 2025, most of which will be reallocated to fund innovation and growth drivers. We further announced our intent to separate the CHC business in the fourth quarter of 2024 at the earliest, most likely via the creation of a publicly listed entity headquartered in France. To deploy our strategy, we must also disrupt our normal course of business and transform our operations. Nevertheless, we may not succeed in federating employees behind the transformation program, which may hamper our ability to execute such organizational changes. Besides, there is no guarantee that we will be able to fully deliver these operating efficiencies or separate the CHC business within the targeted timeline, or at all, or generate the expected benefits.
Unsuccessful management of sustainability (environmental, social and governance) matters could adversely affect our reputation and we may experience difficulties to meet the expectations of our stakeholders
Companies are increasingly expected to behave in a responsible manner on a variety of sustainability matters, by governmental and regulatory authorities, counterparties such as vendors and suppliers, customers, investors, the public at large and others. This context, driven in part by a rapidly changing regulatory framework in the US and in Europe, is raising new challenges and influencing strategic decisions that companies must take if they wish to optimize their positive impact and mitigate their negative impact on sustainability matters.
We have adopted a sustainability strategy that aims at ensuring global access and affordability, addressing unmet needs with transformative therapies, and minimizing the impact of our activities and products on the climate and the environment. The strategy includes leveraging our personnel’s experience and making societal impact a key driver of our employees’ engagement. However, despite our strong commitment we could be unable to meet our sustainability or other strategic objectives in an efficient and timely manner, or at all.
We may also be unable to meet the ever more demanding criteria used by rating agencies in their sustainability assessments process, leading to a downgrading in our rating. Financial investments in companies which perform well in sustainability assessments are increasingly popular, and major institutional investors have made known their interest in investing in such companies. Depending on sustainability assessments and on the rapidly changing views on acceptable levels of action across a range of sustainability topics from investors, we may be unable to meet society’s or investors’ expectations, our reputation may be harmed, we may face increased compliance or other costs and demand for securities issued by us and our ability to participate in the debt and equity markets may decrease.
12 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Our success depends in part on our senior management team and other key employees and our ability to attract, integrate and retain key personnel and qualified individuals in the face of intense competition
Our success depends on the expertise of our senior management team and other key employees. In 2023, there were 2,264 “Senior Leaders” within Sanofi. In addition, we rely heavily on recruiting and retaining talented people to help us meet our strategic objectives. We face intense competition for qualified individuals for senior management positions, or in specific geographic regions or in specialized fields such as clinical development, biosciences and devices, or digital and artificial intelligence. Our ability to hire qualified personnel also depends in part on our ability to reward performance, incentivize our employees and pay competitive compensation. The inability to attract, integrate and/or retain highly skilled personnel, in particular those in leadership positions, may weaken our succession plans, may materially adversely affect the implementation of our strategy and our ability to meet our strategic objectives, and could ultimately adversely impact our business or results of operations.
Environmental and safety risks of our industrial activities
Risks from manufacturing activities and the handling of hazardous materials could adversely affect our results of operations and reputation
Manufacturing activities, such as the chemical manufacturing of the active ingredients in our products and the related storage and transportation of raw materials, products and waste, expose us to risks of industrial accidents that may lead to discharges or releases of toxic or pathogenic substances or other events that can cause personal injury, property damage and environmental contamination, and may result in additional operational constraints, including the shutdown of affected facilities and/or the imposition of civil, administrative, criminal penalties and/or civil damages, and affect Sanofi’s reputation.
The occurrence of an industrial accident may significantly reduce the productivity and profitability of a particular manufacturing facility and adversely affect our operating results and reputation. Although we maintain property damage, business interruption and casualty insurance that we believe is in accordance with customary industry practices, this insurance may not be adequate to fully cover all potential hazards incidental to our business.
Management of the historical contamination related to our past industrial activities could adversely impact our results of operations and reputation
The environmental laws of various jurisdictions impose actual and potential obligations on our Company to manage and/or remediate contaminated sites. These obligations may relate to sites:
•that we currently own or operate;
•that we formerly owned or operated; or
•where waste from our operations was disposed.
These environmental remediation obligations could reduce our operating results. Sanofi accrues provisions for remediation when our management believes the need is probable and that it is reasonably possible to estimate the cost. See “Item 4. Information on the Company — B. Business Overview — B.9. Health, Safety and Environment” for additional information regarding our environmental policies. In particular, our provisions for these obligations may be insufficient if the assumptions underlying these provisions prove incorrect or if we are held responsible for additional, currently undiscovered contamination. These judgments and estimates may later prove inaccurate, and any shortfalls could have an adverse effect on our results of operations and financial condition. For more detailed information on environmental issues, see “Item 4. Information on the Company — B. Business Overview — B.9. Health, Safety and Environment and Notes B.12. and D.19.3. to the consolidated financial statements”.
We are or may become involved in claims, lawsuits and administrative proceedings relating to environmental matters. Some current and former Sanofi subsidiaries have been named as “potentially responsible parties” or the equivalent under the US Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (CERCLA, also known as “Superfund”), and similar statutes or obligations in France, Germany, Italy, Brazil and elsewhere. As a matter of statutory or contractual obligations, we and/or our subsidiaries may retain responsibility for environmental liabilities at some of the sites of our predecessor companies, or of subsidiaries that we demerged, divested or may divest. We have disputes outstanding regarding certain sites no longer owned or operated by the Company. An adverse outcome in such disputes might have an adverse effect on our operating results. See Note D.22.d to the consolidated financial statements included at Item 18. of this annual report and “Item 8. Financial Information — A. Consolidated Financial Statements and Other Financial Information — Information on Legal or Arbitration Proceedings”.
Environmental regulations are evolving. For example, in Europe, new or evolving regulatory regimes include the Registration, Evaluation, Authorization and Restriction of Chemicals Regulation (which would include, if passed, the restriction proposal on per- and polyfluoroalkyl substances (PFAS) recently released by the European Chemicals Agency (ECHA)); the Classification and Labelling regulations applicable to hazardous chemicals; directives related to the control of major-accident hazards (the “Seveso” directives); the Industrial Emission regulations; the Waste Framework Directive; the Emission Trading Scheme Directive; the Water Framework Directive; the Directive on Taxation of Energy Products and Electricity; as well as other regulations aimed at preventing climate change. Stricter environmental, safety and health laws and enforcement policies could result in substantial costs and liabilities to our Company and could subject our handling, manufacture, use, reuse or disposal of substances or pollutants, site restoration and compliance to more rigorous scrutiny than is currently the case. Consequently, compliance with these laws could result in capital expenditures as well as other costs and liabilities, thereby adversely affecting our business, results of operations or financial condition.
SANOFI FORM 20-F 2023 | 13 | ||||
| PART I | ||
| ITEM 3. Key Information | ||
Risks related to financial markets(3)
Fluctuations in currency exchange rates could adversely affect our results of operations and financial condition
Because we sell our products in numerous countries, our results of operations and financial condition could be adversely affected by fluctuations in currency exchange rates. We are particularly sensitive to movements in exchange rates between the euro and the US dollar, the Japanese yen, the Chinese yuan, and currencies in emerging markets. In 2023, 43.0% of our net sales were generated in the United States, 24.1% in Europe, and 32.9% in the Rest of the World region (see the definition in “Item 5. Operating and Financial Review and Prospects — A/ Operating results”), including countries that are, or may in future become, subject to exchange controls (including 6.8% in China and 3.7% in Japan). While we incur expenses in those currencies, the impact of currency exchange rates on these expenses does not fully offset the impact of currency exchange rates on our revenues. As a result, currency exchange rate movements can have a considerable impact on our earnings. When deemed appropriate and when technically feasible, we enter into transactions to hedge our exposure to foreign exchange risks. These efforts, when undertaken, may fail to offset the effect of adverse currency exchange rate fluctuations on our results of operations or financial condition. For more information concerning our exchange rate exposure, see “Item 11. Quantitative and Qualitative Disclosures about Market Risk.”
Risks relating to an investment in our shares or ADSs
Foreign exchange fluctuations may adversely affect the US dollar value of our ADSs and dividends (if any) regardless of our operating performance
Holders of ADSs face exchange rate risks. Our ADSs trade in US dollars and our shares trade in euros. The value of the ADSs and our shares could fluctuate substantially as the exchange rates between these currencies fluctuate. If and when we pay dividends, they would be denominated in euros. Fluctuations in the exchange rate between the euro and the US dollar will affect the US dollar amounts received by owners of ADSs upon conversion by the depositary of cash dividends, if any. Moreover, these fluctuations may affect the US dollar price of the ADSs on the NASDAQ Global Select Market (NASDAQ) whether or not we pay dividends, in addition to any amounts that a holder would receive upon our liquidation or in the event of a sale of assets, merger, tender offer or similar transaction denominated in euros or any foreign currency other than US dollars.
Persons holding ADSs rather than shares may have difficulty exercising certain rights as a shareholder
Holders of ADSs may have more difficulty exercising their rights as a shareholder than if they directly held shares. For example, if we issue new shares and existing shareholders have the right to subscribe for a pro rata portion of the new issuance, the depositary is allowed, at its own discretion, to sell this right to subscribe for new shares for the benefit of the ADS holders instead of making that right available to such holders. In that case, ADS holders could be substantially diluted. Holders of ADSs must also instruct the depositary how to vote their shares. Because of this additional procedural step involving the depositary, the process for exercising voting rights will take longer for holders of ADSs than for holders of shares. ADSs for which the depositary does not receive timely voting instructions will not be voted at any meeting. US investors may have difficulty in serving process or enforcing a judgment against us or our directors or executive officers.
Sales of our shares may cause the market price of our shares or ADSs to decline
Sales of large numbers of our shares, or a perception that such sales may occur, could adversely affect the market price for our shares and ADSs. To our knowledge, L’Oréal, our largest shareholder, is not subject to any contractual restrictions on the sale of the shares it holds in our Company. L’Oréal does not consider its stake in our Company as strategic.
Our largest shareholder owns a significant percentage of the share capital and voting rights of Sanofi
As of December 31, 2023, L’Oréal held approximately 9.35% of our issued share capital, accounting for approximately 16.77% of the voting rights (excluding treasury shares) of Sanofi. See “Item 7. Major Shareholders and Related Party Transactions — A. Major Shareholders”. Affiliates of L’Oréal currently serve on our Board of Directors. To the extent L’Oréal continues to hold a large percentage of our share capital and voting rights, it will remain in a position to exert greater influence in the appointment of the directors and officers of Sanofi and in other corporate actions that require shareholders’ approval.
(3) The information in this section supplements the disclosures required under IFRS 7 as presented in Note B.8.7. to our consolidated financial statements, provided at Item 18. of this annual report.
14 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Item 4. Information on the Company
Introduction
Sanofi is a leading global healthcare company, focused on patient needs and engaged in the research, development, manufacture and marketing of therapeutic solutions.
In the remainder of this section, a product is referred to either by its international non-proprietary name (INN) or its brand name, which is generally exclusive to the company that markets it. In most cases, the brand names of our products, which may vary from country to country, are protected by specific registrations. In this document, products are identified by their brand names used in France and/or in the US.
In 2022, Sanofi reported three operating segments: Pharmaceuticals, Vaccines and Consumer Healthcare. As a consequence of (i) progress on the “Play to Win” strategy leading to the creation of the standalone Consumer Healthcare Global Business Unit (GBU), and (ii) organizational changes to Sanofi’s Manufacturing & Supply global function (previously known as Industrial Affairs), with effect from January 1, 2023, our operations have been split into two operating segments: Biopharma and Consumer Healthcare.
The Consumer Healthcare operating segment comprises commercial operations relating to our Consumer Healthcare products, and research, development and production activities and global support functions dedicated to the segment.
The Biopharma operating segment comprises commercial operations and research, development and production activities relating to our Specialty Care, General Medicines and Vaccines franchises, for all geographical territories. The segment’s results include the costs of global support functions that are outside of the managerial responsibility of the Consumer Health Care GBU.
Our internal reporting, our segment information, and the layout of this section have been adapted so as to reflect the new organizational structure of our operations in a coherent manner.
A. History and development of the Company
The current Sanofi corporation was incorporated under the laws of France in 1994 as a société anonyme, a form of limited liability company, for a term of 99 years. Since May 2011, we have operated under the commercial name “Sanofi” (formerly known as Sanofi-Aventis). Our registered office is located at 46, avenue de la Grande Armée – 75017 Paris – France, our main telephone number is +33 1 53 77 40 00, and our website (which contains information about the company and information filed with and provided to the SEC) is www.sanofi.com. Our principal US subsidiary’s office is located at 55 Corporate Drive, Bridgewater, NJ 08807; telephone: +1 (908) 981 5000.
The SEC maintains an Internet site at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Main events over the last three years
On April 8, 2021, Sanofi acquired the entire share capital of Kymab, a clinical-stage biopharmaceutical company developing fully human monoclonal antibodies with a focus on immune-mediated diseases and immuno-oncology therapeutics.
On September 14, 2021, Sanofi finalized the acquisition of Translate Bio, a clinical-stage mRNA therapeutics company.
On November 9, 2021, Sanofi completed the acquisition of Kadmon Holdings, Inc. a biopharmaceutical company that discovers, develops, and markets transformative therapies for disease areas of significant unmet medical need.
On February 8, 2022, Sanofi acquired the entire share capital of the immuno-oncology company Amunix Pharmaceuticals, Inc. (Amunix), thereby gaining access to Amunix’s innovative PRO-XTEN technology and a promising pipeline of immunotherapies.
On May 3, 2022, Sanofi’s General Meeting of Shareholders approved the decision to distribute approximately 58% of the share capital of EUROAPI, a European leader in the development, manufacture, marketing and distribution of Active Pharmaceutical Ingredients (APIs), in the form of an exceptional dividend in kind to Sanofi shareholders. On the dividend payment date of May 10, 2022 (further to the admission of EUROAPI shares to listing on the regulated market of Euronext Paris on May 6, 2022), Sanofi divested control over EUROAPI and its subsidiaries, resulting in their deconsolidation from the Sanofi consolidated financial statements as of that date. On June 17, 2022 (the date of delivery of the EUROAPI shares to the French State via the French Tech Souveraineté fund), EPIC Bpifrance acquired a 12% equity interest in EUROAPI. Following completion of those transactions, Sanofi retains an equity interest of 30.1% in EUROAPI, which has been accounted for using the equity method since the date of loss of control (see note D.6. to the consolidated financial statements).
On March 13, 2023, Sanofi and Provention Bio, Inc. (“Provention”), a US-based publicly-traded biopharmaceutical company developing therapies to prevent and intercept immune-mediated diseases including type 1 diabetes, entered into an agreement under which Sanofi acquired the outstanding shares of Provention common stock for $25.00 per share in an all-cash transaction valued at approximately $2.8 billion. On April 27, 2023, Sanofi announced the completion of its acquisition of Provention. The acquisition adds TZIELD (teplizumab-mzwv), a therapy for type 1 diabetes, to Sanofi’s core General Medicines asset portfolio.
On July 28, 2023, Sanofi announced that it had entered into a definitive agreement to acquire ownership of QUNOL, a market-leading US-based health & wellness brand. This transaction was intended to strengthen Sanofi CHC in the Vitamin, Mineral and Supplements (VMS) category, one of the largest and fastest-growing consumer health categories in the US, focused on the
SANOFI FORM 20-F 2023 | 15 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
dynamic healthy aging segment. Sanofi’s acquisition of QRIB Intermediate Holdings, LLC was completed on September 29, 2023, at a purchase price of $1,419 million.
More detailed information about these changes is provided in Note D.1. to our consolidated financial statements, included at Item 18. of this annual report.
B. Business overview
Sanofi’s activities are organized around the following categories: DUPIXENT, Neurology & Immunology, Rare Diseases, Oncology, Rare Blood Disorders, General Medicines Core Assets and Non-Core Assets, Vaccines, and CHC. Except for CHC, which is a standalone operating segment within the meaning of IFRS 8, all of those activities fall within the Biopharma operating segment.
B.1. Strategy
The market context for Sanofi
Several fundamental trends continue to point to a positive outlook for the pharmaceutical industry. The global population is growing, and aging, and unmet medical needs remain high. Health needs have further increased, strengthening the key roles of innovation in R&D activities and cutting-edge manufacturing. The industry has taken steps to increase R&D productivity, with the objective of launching a higher number of innovative medicines and vaccines. Patients around the world – including a rising middle class in emerging markets – are demanding better healthcare, empowered by access to more and more information. It is a particularly exciting time scientifically and technologically: the promise of genomics is being realized, immuno-oncology is transforming cancer treatments, and big data is generating new insights into how to diagnose and treat diseases. Digital technologies and advanced data analytics are having a transformative effect across sales and marketing activities, R&D and manufacturing, and are acting as enablers for new businesses.
At the same time, increased geopolitical uncertainties, inflation, supply shortages, and issues around budget tightening will continue to put pressure on healthcare costs, and on the entire healthcare value chain. Although we believe that pharmaceuticals and vaccines will remain a fundamentally attractive business within that value chain, the bar for innovation will most likely continue to rise. Payers will continue to put scrutiny on prices and reimbursement criteria, and demand demonstration of real-life outcomes to confirm the efficacy of medicines and vaccines. This will be coupled with more innovative pricing and contracting practices, and more transparent policies. In view of growing concerns over increasing healthcare costs across global markets, the pharmaceutical industry will be increasingly judged by its contribution to improved access for patients and to the development of innovative, highly cost-effective medicines.
Strategic framework
The Sanofi “Play to Win” strategy is organized around four key priorities: 1) focus on growth; 2) lead with innovation; 3) accelerate efficiency; and 4) reinvent how we work to drive innovation and growth.
1) Focus on growth
•DUPIXENT (dupilumab)(1) – By leveraging the product’s unique mechanism of action targeting the type 2 inflammation pathway and its favorable safety profile, we have raised our DUPIXENT sales peak ambition.
•Vaccines – Our Vaccines business is expected to deliver mid-to-high single digit net sales growth(2) through differentiated products, market expansions and launches. Contributors to growth are expected to be pediatric combinations; boosters; influenza vaccines; meningitis; and BEYFORTUS, which addresses Respiratory Syncytial Virus (RSV)(3). Sanofi has progressed in the field of mRNA technology with our Center of Excellence. At least five First in class / best in class vaccine candidates expected in phase 3 by 2025 across diverse preventative and therapeutic areas.
•Pipeline – We are focusing our investments on priority projects, including six potentially transformative therapies in immunology, hematology, neurology, vaccines and oncology.
2) Lead with innovation
We have been able to shift from a priority asset list to a steady flow of assets in a refocused, consistent pipeline. Our pipeline is showing potential opportunities for market-leading products.
To continue fueling our promising pipeline and enhance our position in our core therapeutic areas, we have:
i.entered into a strategic collaboration with Teva to co-develop and co-commercialize asset TEV’574, currently in Phase 2b clinical trials for the treatment of Ulcerative Colitis and Crohn’s Disease, two types of inflammatory bowel disease;
ii.established a strategic collaboration with Recludix Pharma to advance a novel oral STAT6 inhibitor in multiple immunological and inflammatory indications;
iii.expanded an existing collaboration with Scribe Therapeutics to access CRISPR X-Editing (XE) genome editing technologies to advance in vivo genetic medicines for sickle cell and other genomic diseases;
iv.acquired Provention Bio, adding to our portfolio TZIELD, the first disease-modifying treatment for the delay of Stage 3 type 1 diabetes;
(1) In partnership with Regeneron.
(2) Cumulative Annual Growth Rate (CAGR), 2018-2025.
(3) In partnership with AstraZeneca.
16 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
v.established a strategic collaboration with BioMap to co-develop Artificial Intelligence (AI) modules to accelerate drug discovery for biotherapeutics;
vi.entered into an exclusivity agreement with Adocia for M1Pram, an innovative combination of insulin and pramlintide developed by Adocia to become the reference rapid-acting insulin for people with diabetes and obesity;
vii.entered into an agreement with Janssen Pharmaceuticals to develop and commercialize the vaccine candidate for extraintestinal pathogenic E. coli (9-valent) developed by Janssen, currently in Phase 3;
viii.modified an existing collaboration agreement with AstraZeneca, giving Sanofi full commercial control of BEYFORTUS (nirsevimab) and enhanced agility in the US;
ix.out-licensed the Capeserod Phase 2 program to FirstWave Biopharma so it can be repurposed in gastrointestinal disorders with significant unmet needs, enabled by AI-empowered analyses of the drug’s properties.
3) Accelerate efficiency
In October 2023, we announced new Strategic Costs Initiatives. We are striving to accelerate our efforts to improve our cost structure, launching efficiency initiatives across our Biopharma business that we expect to free operational resources to support accelerated R&D investment and unlock value-creation opportunities. This will include prioritizing our investments in R&D and modernizing our approach to commercial delivery. Given our decision to support the full realization of our pipeline’s long-term potential, our ongoing investment around new launches as well as pricing headwinds in General Medicines, we are no longer targeting 32% business operating income(4) margin for 2025, while maintaining our focus on long-term profitability.
To embrace the transformative effect offered by digital technologies and advanced data analytics, we are investing to become the leading digital healthcare platform for employees, patients and providers. This is expected to help us discover, test and deliver medicines faster, run our business more efficiently, and create engaging digital experiences. The digital transformation required to meet our ambition is under way. We are using advanced algorithms to harvest real world data to support our R&D efforts. We are also developing new go-to-market models by enhanced physician engagement through a variety of channels, building precision marketing, and providing better e-commerce capabilities. And in parallel, we are investigating the possibility of integrating drugs, devices, data and services, to bring innovative solutions to patients across many different disease areas such as diabetes and atopic dermatitis.
In 2023, we made further progress regarding the implementation of AI across the business:
•In partnership with Aily Labs, we rolled out plai, an application developed with AI, to more than 23,000 internal users. The application delivers real-time, reactive data interactions and gives a 360° view across our activities, using AI to provide users with timely insights and personalized “what if” scenarios to support informed decision making.
•In Research, we have built multiple AI programs to reduce research times through improved predictive modelling and automate time-sink activities. As a result, AI assists R&D teams to scale and accelerate research processes from a matter of weeks to hours and improve potential target identification in therapeutic areas like immunology, oncology or neurology.
•In Manufacturing & Supply, we have developed an in-house AI-enabled yield optimization solution which learns from past and current batch performance in an effort to enable consistently higher yield levels. This optimizes usage of raw materials, contributes to our environmental objectives and supports improved cost efficiency.
4) Reinvent how we work
Transformation and simplification have started, with the aim of increasing empowerment and accountability. To drive implementation of our new culture built on stronger focus, diversity and teamwork, we have streamlined our executive leadership team from 15 to 12 members. Four new members were appointed to the executive leadership team: Madeleine Roach as Head of Business Operations; Houman Ashrafian as Head of Research & Development (succeeding Dr John Reed), Emmanuel Frenehard as Chief Digital Officer (succeeding Arnaud Robert), and Brian Foard as Head of Specialty Care (succeeding Bill Sibold). The complete Sanofi Executive Committee now includes the four managers who head up our global business units (Specialty Care, Vaccines, General Medicines, and CHC) as well as the heads of each of the following support functions: Research and Development, Manufacturing & Supply (previously named Industrial Affairs), Finance, People & Culture, Digital, Legal, Ethics, Business Integrity & Global Security and Business Operations.
In 2023, we progressed further in building and simplifying our stand-alone CHC organization. We have further reduced our portfolio, mainly through divestments, to 117 brands (47% fewer than in 2019). In September 2023 we announced the acquisition of QUNOL, a leading US health and wellness brand that strengthens our CHC operations in a key market, and within the promising Vitamins, Minerals and Supplements (VMS) segment.
By the end of 2023, all the CHC legal entities required to establish a standalone organization had been established, except in India, and staffed with dedicated teams. Sanofi has announced its intention to separate the CHC Business as it increases its focus on innovative medicines and vaccines. The intended separation will seek to create two entities, each better equipped to pursue its own business strategy, resourcing and capital allocation and enabling each to focus on long-term growth in its respective markets. Sanofi believes that the separation will unlock further opportunities for CHC to leverage its portfolio of leading brands and continue to drive growth and shareholder value. Sanofi is reviewing potential separation scenarios, but believes that the most likely path would be through a capital markets transaction, by creating a listed entity headquartered in France. The timing is driven by the desire to maximize value creation and reward Sanofi shareholders. Subject to market conditions, the separation could be achieved at the earliest in the fourth quarter of 2024, following consultation with social partners.
(4) Non-IFRS financial measure: see definition in “Item 5 — A.1.5. Segment Information — 3. Business Net Income” .
SANOFI FORM 20-F 2023 | 17 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
In line with our high sustainable development ambitions, Sanofi CHC North America achieved B-corp accreditation in July 2023.
Sanofi’s Corporate Social Responsibility (CSR) strategy aims to build a healthier, more resilient world by ensuring access to healthcare for the world’s poorest people and bringing focus to addressing broader unmet needs. Sanofi’s commitment to society also aims to accelerate our goal of reducing the environmental impact of our products and of our worldwide operations. Key to tackling the global challenges that face our company are our people, who each have a role to play in building a diverse and inclusive workplace.
Sanofi’s CSR Strategy focuses on four building blocks integrated into with our Play to Win core business strategy:
•affordable access – to ensure affordable global access to health, while helping healthcare systems to remain sustainable;
•R&D for unmet needs – to be at the cutting edge of R&D innovation, to help people live fully and drive growth;
•Planet Care – to minimize the environmental impact of our business through environmental sustainability; and
•in & beyond the workplace – to give all Sanofi colleagues the chance to become a leader of change, unlocking the potential of our diverse teams.
Capital allocation policy
We will continue to pursue our focused and disciplined capital allocation policy. Our priorities in deploying the cash generated from our three core GBUs and the standalone CHC business are, in the following order: (i) organic investment; (ii) business development and merger & acquisition activities, focusing on bolt-on, value-enhancing opportunities to drive scientific and commercial leadership in core therapeutic areas; (iii) growing the annual dividend; and (iv) anti-dilutive share buybacks. We also have the potential to raise capital through asset disposals, including streamlining “tail” brands in our Established Products and Consumer Healthcare business.
B.2. Main biopharma products
The sections below provide additional information on our main products. Our intellectual property rights over our pharmaceutical products are material to our operations and are described at “B.6. Patents, Intellectual Property and Other Rights” below. As disclosed in “Item 8. Financial Information — A. Consolidated Financial Statements and Other Financial Information — Patents” of this annual report, we are involved in significant litigation concerning the patent protection of a number of these products. For more information on sales performance, see “Item 5. Operating and Financial Review and Prospects — A. Operating Results.”
Specialty Care
DUPIXENT
DUPIXENT (dupilumab) is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways and is not an immunosuppressant. Dupilumab is jointly developed by Sanofi and Regeneron under a global collaboration agreement. To date, dupilumab has been studied across more than 60 clinical trials involving more than 10,000 patients with various chronic diseases driven in part by type 2 inflammation. The dupilumab development program has shown significant clinical benefit and a decrease in type 2 inflammation in Phase 3 trials, establishing that IL-4 and IL-13 are key and central drivers of the type 2 inflammation that plays a major role in multiple inflammatory diseases, such as atopic dermatitis (AD), asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis and prurigo nodularis. DUPIXENT comes in either a pre-filled syringe for use in a clinic or at home by self-administration as a subcutaneous injection or in a pre-filled pen for at-home administration, providing patients with a more convenient option. DUPIXENT is available in all major markets including the US (since April 2017), most European Union countries (the first launch was in Germany in December 2017), Japan (since April 2018), and China (since June 2020).
Atopic Dermatitis
Moderate-to-severe AD, a form of eczema and a chronic inflammatory disease, is characterized by rashes that sometimes cover much of the body and can include intense, persistent itching and skin dryness, cracking, redness, crusting and oozing. Eighty-five to ninety percent of patients first develop symptoms before five years of age, which can often continue through adulthood.
In 2014 the FDA granted DUPIXENT Breakthrough Therapy designation and after a Priority Review evaluation: in March 2017 it granted DUPIXENT marketing authorization for the treatment of adults with moderate-to-severe AD whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable. In 2016, the FDA granted DUPIXENT Breakthrough Therapy designation for adolescent patients aged 12 to 17 years and in March 2019, the FDA extended the marketing authorization for this age group.
In 2016, the FDA granted Breakthrough Therapy designation for DUPIXENT for the treatment of severe AD in children aged six months to 11 years. On May 26, 2020, DUPIXENT was approved as the first biologic medicine for children aged six to 11 years with moderate-to-severe AD. Having accepted DUPIXENT for Priority Review in February 2022, the FDA approved DUPIXENT on June 7, 2022 for children aged six months to five years with moderate-to-severe AD and whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable, making DUPIXENT the first biologic medicine to significantly reduce signs and symptoms in children as young as six months.
18 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
The European Commission (EC) approved DUPIXENT in September 2017 for use in adults with moderate-to-severe AD who are candidates for systemic therapy, and extended the marketing authorization in August 2019 to include adolescents aged 12 to 17 years. On November 30, 2020, the EC extended the marketing authorization to children aged six to 11 years with severe AD and on June 28, 2021, the DUPIXENT Summary of Product Characteristics (SmPC) was updated with long-term data for up to three years, reinforcing the product’s well-established safety profile in adults with moderate-to-severe AD. On January 27, 2023 the CHMP adopted a positive opinion for DUPIXENT, recommending expanded approval in the EU to treat severe AD in children aged six months to five years who are candidates for systemic therapy. In March 2023, DUPIXENT was approved by the EC as the first and only targeted medicine for children as young as six months old with severe AD.
On June 19, 2020, the National Medical Products Administration (NMPA) in China approved DUPIXENT for adults for the treatment of moderate-to-severe AD after identifying dupilumab as an overseas medicine regarded as urgently needed in clinical practice, leading to an expedited review and approval process. On December 28, 2020, the National Healthcare Security Administration (NHSA) officially announced the results of the 2020 National Reimbursement Drug List (NRDL) negotiations, with DUPIXENT 300 mg included in the updated NRDL effective March 1, 2021. DUPIXENT was approved in China in September 2021 for adolescents aged 12-17 years with moderate-to-severe AD. The indication for children aged six years and over, along with the adolescent and adult AD indications, was included in the current NRDL reimbursement scope, which was reviewed during the DUPIXENT NRDL renewal in 2022 in accordance with the two-year cycle for the China access process. In May 2023, DUPIXENT was approved in China to treat moderate to severe AD in infants and children aged six months and older.
On January 22, 2018, the Ministry of Health, Labor and Welfare (MHLW) in Japan granted marketing and manufacturing authorization for DUPIXENT for the treatment of AD in adults not adequately controlled with existing therapies. More recently, on September 25, 2023 DUPIXENT was approved in Japan to treat patients with moderate-to-severe AD aged six months and older.
In April 2023, new late-breaking abstract data from a long-term efficacy open-label study presented at the Revolutionizing Atopic Dermatitis (RAD) 2023 Spring Conference in Washington, DC showed that DUPIXENT demonstrated robust and sustained efficacy with progressive improvement of AD signs and symptoms in patients with moderate-to-severe AD who completed up to five years of treatment: the longest duration of data for any biologic medicine in this disease. Additionally, the long-term safety data from a 52-week open-label extension trial in children aged six months to five years reinforced the well-established safety profile of DUPIXENT observed across all other approved age groups. This data is built on the existing evidence supporting the selective way DUPIXENT inhibits IL4/IL-13 pathways, both key and central drivers of the type 2 inflammation, thereby significantly improving itch and skin lesions and other important measures that impact a patient’s quality of life. In June 2023, the inclusion of the results from the five-year OLE Trial for Adults into the DUPIXENT label was approved in Europe, and in October 2023, by the FDA in the US.
In March 2023, positive results from the clinical trial assessing DUPIXENT in adults and adolescents with uncontrolled moderate-to-severe atopic hand and foot dermatitis were presented in a late-breaking session, one of more than 20 DUPIXENT scientific presentations, at the American Academy of Dermatology (AAD) 2023 Annual Meeting. The trial evaluating a biologic for this difficult-to-treat population met its primary and key secondary endpoints. In August 2023, the clinical section of the DUPIXENT label in Europe was updated to include the hand and foot dermatitis population. In January 2024, the DUPIXENT US label was updated with data further supporting use in atopic dermatitis with moderate-to-severe hand and foot involvement.
These Phase 3 data are from the first and only trial evaluating a biologic specifically for this difficult-to-treat population and have also been added to the DUPIXENT label in the European Union, with regulatory submissions underway in additional countries.
Asthma
DUPIXENT was granted marketing authorization by the FDA in October 2018 as an add-on maintenance therapy in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma. In May 2019, the EC approved DUPIXENT for use as an add-on maintenance treatment in severe asthma patients aged 12 years and older with type 2 inflammation whose symptoms are inadequately reduced by other treatments.
In September 2020, new long-term data from a Phase 3 open-label extension trial showed sustained improvement in lung function and reduction in severe exacerbations in adults and adolescents with moderate-to-severe asthma. On May 17, 2021, detailed results from a Phase 3 trial showed DUPIXENT significantly reduced severe asthma attacks, and within two weeks rapidly improved lung function in children aged six to 11 years with uncontrolled moderate-to-severe asthma with evidence of type 2 inflammation. Moreover, DUPIXENT significantly improved overall asthma symptom control and reduced an airway biomarker of type 2 inflammation, called fractional exhaled nitric oxide (FeNO), that plays a major role in asthma.
In October 2021, the FDA approved DUPIXENT as an add-on maintenance treatment for patients aged six to 11 years with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid-dependent asthma, thereby bringing a new treatment for children who may be suffering from life-threatening asthma attacks and poor lung function affecting their ability to breathe, which could potentially continue into adulthood. On April 7, 2022, the EC approved DUPIXENT for use in children aged six to 11 years as an add-on maintenance treatment for severe asthma with type 2 inflammation characterized by raised blood eosinophils and/or raised FeNO, whose symptoms are inadequately reduced with medium to high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
In March 2019, DUPIXENT was approved in Japan for treating patients aged 12 years and over with severe or refractory asthma and whose symptoms are inadequately controlled with existing therapies. In November 2023, DUPIXENT received approval in China for treatment of moderate to severe asthma patients aged 12 years and over with T2 inflammation.
SANOFI FORM 20-F 2023 | 19 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Chronic rhinosinusitis with nasal polyposis (CRSwNP)
CRSwNP is a chronic disease of the upper airway that obstructs the sinuses and nasal passages. It can lead to breathing difficulties, nasal congestion and discharge, reduced or loss of sense of smell and taste, and facial pressure.
In June 2019, the FDA approved DUPIXENT for use with other medicines to treat CRSwNP in adults whose disease is not controlled. In October 2019, the EC approved DUPIXENT for use as an add-on therapy with intranasal corticosteroids in adults with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control. In March 2020, the Japanese Pharmaceuticals and Medical Devices Agency approved DUPIXENT as add-on maintenance treatment for adults with inadequately controlled CRSwNP.
Eosinophilic esophagitis (EoE)
EoE is a chronic and progressive inflammatory disease that damages the esophagus and prevents it from working properly; swallowing even small amounts of food can be a painful and worrisome choking experience. In severe cases, a feeding tube may be the only option to ensure proper calorific intake and adequate nutrition. As the disease progresses, patients may continue to experience symptoms despite multiple treatments.
On September 14, 2020, the FDA granted Breakthrough Therapy designation to DUPIXENT for the treatment of patients aged 12 years and older with EoE, and subsequently accepted the file for Priority Review on April 4, 2022. On May 20, 2022, the FDA approved DUPIXENT to treat patients with EoE aged 12 years and older. With this approval, DUPIXENT became the first and only medicine specifically indicated to treat EoE in the US.
On December 16, 2022, the EMA CHMP adopted a positive opinion, recommending the approval of dupilumab in the EU to treat adults and adolescents with EoE. On January 30, 2023, the EC expanded the marketing authorization for DUPIXENT in the EU to include the treatment of EoE in adults and adolescents aged 12 years and older.
There are currently no approved treatments specifically indicated for children under 12 years of age with EoE. On July 14, 2022, a DUPIXENT Phase 3 trial showed positive results in children aged 1 to 11 years with EoE, making this the fifth pediatric pivotal trial across three type 2 inflammatory diseases to reinforce the well-established efficacy and safety profile of DUPIXENT. In January 2024, DUPIXENT was approved for the treatment of adult and pediatric patients aged one year or older, weighting at least 15 kg, with oesinophilic esophagitis (EoE). The EoE pediatric indication was submitted in the EU in the fourth quarter of 2023.
Prurigo Nodularis (PN)
PN is a chronic, debilitating skin disease with underlying type 2 inflammation and has one of the highest impacts on a patient’s quality of life among inflammatory skin diseases due to the extreme itch it causes. Those with prurigo nodularis experience intense, persistent itch, with thick skin lesions (called nodules) that can cover most of the body. The disease is often painful – with burning, stinging and tingling of the skin – and can negatively affect mental health, daily living activities and social interactions. High-potency topical steroids are commonly prescribed but are associated with safety risks if used long-term.
The FDA evaluated the DUPIXENT application for prurigo nodularis under Priority Review on May 31, 2022. On September 29, 2022, the FDA approved DUPIXENT for the treatment of adult patients with prurigo nodularis. With this approval, DUPIXENT became the first and only medicine specifically indicated to treat prurigo nodularis in the US. The FDA approval was based on data from two Phase 3 trials evaluating the efficacy and safety of DUPIXENT in adults with prurigo nodularis. Efficacy in these trials assessed the proportion of subjects with clinically meaningful reduction in itch, clearing of skin, or both. On December 15, 2022, the EC expanded the marketing authorization for DUPIXENT in the EU to treat adults with moderate-to-severe prurigo nodularis who are candidates for systemic therapy, after the previous positive recommendation on November 11, 2022.
The DUPIXENT prurigo nodularis indication was approved in Japan on June 26, 2023, and in China on September 22, 2023.
Chronic Spontaneous Urticaria (CSU)
CSU is a chronic inflammatory skin disease characterized by the sudden onset of hives on the skin and/or swelling deep under the skin. Despite standard-of-care treatment, people with CSU often experience symptoms including a persistent itch or burning sensation, which can be debilitating and significantly impact quality of life. Swelling often occurs on the face, hands and feet, but can also affect the throat and upper airways. On July 29, 2021 a pivotal Phase 3 trial evaluating DUPIXENT in patients with moderate-to-severe CSU met its primary endpoints and all key secondary endpoints at 24 weeks. Adding DUPIXENT to standard-of-care antihistamines significantly reduced itch and hives for biologic-naive patients, compared to those treated with antihistamines alone (placebo) in Study A (the first of two trials) of the LIBERTY CUPID clinical program.
Study B of the clinical trial evaluated DUPIXENT in adults and adolescents who remain symptomatic despite standard-of-care treatment and are intolerant or incomplete responders to an anti-IgE therapeutic (omalizumab). Although positive numerical trends in reducing itch and hives were observed, the study was stopped due to futility based on a pre-specified interim analysis. The safety data were generally consistent with the known safety profile of DUPIXENT in its approved indications. In December 2022, CSU was submitted to the FDA. In October 2023 the FDA issued a Complete Response Letter (CRL) stating that additional efficacy data are required to support approval; it did not identify any issues with safety or manufacturing. An ongoing clinical trial (Study C) continues to enroll patients; the results, due in late 2024, are expected to provide the additional efficacy data.
Japan's Ministry of Health, Labour and Welfare granted marketing and manufacturing authorization for DUPIXENT for the treatment of chronic spontaneous urticaria in February 2024.
20 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Life cycle management
DUPIXENT is currently being evaluated in clinical development programs for diseases that are driven by type 2 inflammation. These include chronic obstructive pulmonary disease (COPD), bullous pemphigoid (BP), chronic pruritis of unknown origin (CPUO), eosinophilic gastroenteritis (EoG) and ulcerative colitis (UC). See “— B.4. Global Research & Development.”
DUPIXENT is developed and commercialized in collaboration with Regeneron. For additional information on the collaboration, see “Item 5. Operating and Financial Review and Prospects — A.1.7. Financial Presentation of Alliances — Alliance Arrangements with Regeneron.”
Neurology & Immunology
Multiple Sclerosis (MS)
MS is an autoimmune neurological disease in which a person’s immune system attacks the central nervous system, damaging myelin, the protective sheath that covers nerve fibers. This causes a break in communication between the brain and the rest of the body, ultimately destroying the nerves themselves, and causing irreversible damage. More than 2.5 million people suffer from MS worldwide.
Our MS franchise consists of AUBAGIO (teriflunomide), a once-daily, oral immunomodulator, and LEMTRADA (alemtuzumab), a monoclonal antibody. Both products treat patients with relapsing forms of MS.
AUBAGIO
AUBAGIO (teriflunomide), a small molecule immunomodulatory agent with anti-inflammatory properties, is a once-daily oral therapy.
AUBAGIO is approved in more than 80 countries around the world including the US (since September 2012) for the treatment of patients with relapsing forms of MS; the EU (since August 2013) for the treatment of adult patients with relapsing remitting MS; and China (since July 2018). In June 2021, the EC approved AUBAGIO for the treatment of pediatric patients aged 10 to 17 years with relapsing-remitting multiple sclerosis (RRMS).
In 2017, Sanofi reached settlement with all 20 generic AUBAGIO ANDA first filers, granting each a royalty-free license to enter the US market on March 12, 2023. In the European Union, the first generic competitors to AUBAGIO became available in the third quarter of 2023.
LEMTRADA
LEMTRADA (alemtuzumab) is a humanized monoclonal antibody targeting the CD52 antigen. LEMTRADA is administered by intravenous infusion as two short courses 12 months apart; for the majority of patients no further treatment is necessary, making LEMTRADA the only disease-modifying therapy (DMT) that can provide long term durable efficacy in the absence of continuous dosing.
LEMTRADA is approved in more than 70 countries including the EU (since September 2013) and the US (since November 2014). Because of its safety profile, the FDA approved the use of LEMTRADA in patients with relapsing forms of MS who have had an inadequate response to two or more drugs indicated for the treatment of MS, and included a black-box warning on potential side effects. In the US, LEMTRADA is only available through a restricted distribution program called the LEMTRADA Risk Evaluation and Mitigation Strategy (REMS) Program. In January 2020, the EMA updated the indication for LEMTRADA to include treatment of relapsing-remitting multiple sclerosis if the disease is highly active despite treatment with at least one disease-modifying therapy, or if the disease is worsening rapidly. The EMA also added new contra-indications for patients with certain heart, circulation or bleeding disorders, and those who have autoimmune disorders other than MS.
Bayer Healthcare received contingent payments based on alemtuzumab global sales revenue through September 2023. For additional information, see Note D.18. to our consolidated financial statements, included at Item 18. of this annual report.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease causing inflammation, pain, and eventually joint damage and disability.
KEVZARA
KEVZARA (sarilumab) is a human monoclonal antibody that binds to the interleukin-6 receptor (IL-6R) and has been shown to inhibit IL-6R mediated signaling. IL-6 is a cytokine in the body that, in excess and over time, can contribute to the inflammation associated with rheumatoid arthritis. KEVZARA is available in 20 countries, including the US.
In May 2017, the FDA approved KEVZARA for the treatment of adult patients with moderately to severely active RA who have had an inadequate response or intolerance to one or more disease modifying anti-rheumatic drugs (DMARDs), such as methotrexate. In June 2017, the European Commission granted marketing authorization for KEVZARA in combination with methotrexate for the treatment of moderately to severely active RA in adult patients who have responded inadequately to – or who are intolerant to – one or more DMARDs, such as methotrexate. In September 2017, KEVZARA obtained manufacturing and marketing approval in Japan as a treatment for rheumatoid arthritis not responding well to conventional treatments. In February 2023, the FDA approved KEVZARA for the treatment of adult patients with polymyalgia rheumatica (PMR) who have had an inadequate response to corticosteroids or who cannot tolerate a corticosteroid taper. In October 2023, the FDA accepted the filing for polyarticular Juvenile Idiopathic Arthritis.
KEVZARA is developed and commercialized in collaboration with Regeneron. For additional information, see “Item 5. Operating and Financial Review and Prospects — A.1.7. Financial Presentation of Alliances — Alliance Arrangements with Regeneron.”
SANOFI FORM 20-F 2023 | 21 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Rare diseases
Our Rare Diseases business is focused on products for the treatment of rare genetic diseases and other rare chronic debilitating diseases of high unmet medical need, including lysosomal storage disorders (LSDs), a group of metabolic disorders caused by enzyme deficiencies.
CEREZYME
CEREZYME (imiglucerase) is an enzyme replacement therapy used to treat Gaucher disease, a chronic, inherited, progressive and potentially life-threatening LSD. Gaucher disease is caused by deficiency of the enzyme glucocerebrosidase; this causes a fatty substance called glucosylceramide (also called GL-1) to build up in certain areas of the body including the spleen, liver, and bone. Gaucher disease exhibits diverse manifestations, a broad range of age of onset of symptoms, and a wide clinical spectrum of disease severity. It is estimated that Gaucher disease occurs in approximately one in 120,000 newborns in the general population and one in 850 in the Ashkenazi Jewish population worldwide, but incidence and patient severity vary among regions. CEREZYME has been marketed in the US since 1994, in the EU since 1997, in Japan since 1998 and in China since 2008, and is approved to treat Type 1 Gaucher disease in more than 85 countries. It has also been approved to treat the systemic symptoms of Type 3 Gaucher disease in most non-US markets, including the EU and Japan.
CEREZYME is typically given by intravenous infusions for 1-2 hours every two weeks at an infusion center, a doctor’s office, or at home as medically appropriate.
CERDELGA
CERDELGA (eliglustat) is the first and only first-line oral therapy for Gaucher disease Type 1 adult patients. A potent, highly specific ceramide analog inhibitor of GL-1 synthesis with broad tissue distribution, CERDELGA has demonstrated efficacy in the treatment of naive Gaucher disease patients and in patients who switch from enzyme replacement therapy. CERDELGA has been approved to treat type 1 Gaucher disease in the US (2014), and in the EU and Japan (2015). It is also in development for the treatment of type 1 Gaucher disease in pediatric patients. See “— B.4. Global Research & Development.”
MYOZYME and LUMIZYME
MYOZYME (alglucosidase alfa) is an enzyme replacement therapy used to treat both Infantile Onset and Late Onset Pompe disease (IOPD and LOPD). Pompe disease is an inherited, progressive and often fatal neuromuscular disease, caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA) that results in the build-up of glycogen in the muscles’ cells. For infantile-onset Pompe disease, symptoms begin within a few months of birth and there is impact to the heart in addition to skeletal muscle weakness. Other symptoms include difficulties breathing, frequent chest infections, problems feeding that result in failure to gain weight as expected, and failure to meet certain developmental milestones. Patients with late-onset Pompe disease typically present symptoms any time after the first year of life to late adulthood and rarely manifest cardiac problems. The hallmark symptom of late-onset Pompe disease is skeletal muscle weakness, which often leads to walking disability and reduced respiratory function. Patients often require wheelchairs to assist with mobility and may require mechanical ventilation to help with breathing. Pompe disease occurs in approximately one in 40,000 newborns worldwide, but incidence and patient severity vary among regions.
MYOZYME was first approved in 2006 in the EU and has since been approved in more than 70 countries. In the US, alglucosidase alfa has been marketed as LUMIZYME since 2010.
NEXVIAZYME
NEXVIAZYME / NEXVIADYME (avalglucosidase alfa-ngpt) is a novel mannose-6-phosphate (M6P) enriched enzyme replacement therapy (ERT) treatment designed as a monotherapy for the entire spectrum of infantile-onset and late-onset Pompe disease (IOPD, LOPD), for both switch and naive patients. NEXVIAZYME/NEXVIADYME is scientifically designed to specifically target the M6P receptor, the key pathway for ERT, to effectively clear glycogen build-up in muscle cells. It helps replace the GAA enzyme for people whose bodies do not produce enough. Investment in the clinical development of NEXVIAZYME is continuing, with an ongoing Phase 3 trial in treatment-naive IOPD patients aged less than 12 months. NEXVIAZYME/NEXVIADYME is administered as a monotherapy enzyme replacement therapy every two weeks.
NEXVIAZYME was first approved in the US by the FDA on August 6, 2021 for LOPD patients aged 1 and over. On June 24, 2022, the EC granted marketing authorization for NEXVIADYME as a potential new standard of care for the long-term treatment of both LOPD and IOPD. NEXVIAZYME/NEXVIADYME has successfully launched in 25 countries including the US, Japan and Australia. In all launched markets, the vast majority of eligible patients have switched or will be switching to NEXVIAZYME/NEXVIADYME.
FABRAZYME
FABRAZYME (agalsidase beta) is an enzyme replacement therapy used to treat Fabry disease. Fabry disease (FD) is a multisystemic, progressive, X-linked inherited disorder of glycosphingolipid metabolism due to deficient or absent lysosomal α-galactosidase A activity resulting in progressive globotriaosylceramide (GL-3) accumulation in the lysosomes of various tissues. Fabry disease affects both genders. With age, progressive organ damage develops, leading to potentially life-threatening renal, cardiac and/or cerebrovascular complications. Fabry disease is characterized by different symptom severities and rates of progression, ranging from classic disease with early symptom onset to late onset disease with cardiac and/or renal complications later in life. Fabry disease is seen in all racial and ethnic groups and is an under-diagnosed condition. Prevalence estimates vary across regions. Classic Fabry disease mutations are estimated to be approximately 1:40,000 in males with more wide-ranging estimates for atypical presentations of Fabry in both males and females. FABRAZYME has been marketed in the EU since 2001 and in the US since 2003 and is approved in more than 70 countries.
22 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
ALDURAZYME
ALDURAZYME (laronidase) is the only approved enzyme replacement therapy for mucopolysaccharidosis type 1 (MPS I), an inherited lysosomal storage disorder caused by a deficiency of alpha-L-iduronidase, a lysosomal enzyme normally required for the breakdown of certain complex carbohydrates known as glycosaminoglycans (GAGs). MPS I is multi-systemic, and children with MPS I are described as having either a severe or attenuated form of the disorder based on age of onset, severity of symptoms, rate of disease progression and whether there is early and direct involvement of the brain. MPS I occurs in approximately one per 100,000 live births worldwide, but incidence and patient severity vary among regions. ALDURAZYME has been marketed in the EU and the US since 2003 and is approved in more than 75 countries.
XENPOZYME
XENPOZYME (olipudase alfa) is an enzyme replacement therapy (ERT) designed to replace deficient or defective acid sphingomyelinase (ASMD), an enzyme that allows for the breakdown of the lipid sphingomyelin. In individuals with ASMD, an insufficiency of the ASM enzyme means sphingomyelin is poorly metabolized, potentially leading to lifelong accumulation in and damage to multiple organs.
The significance of the unmet need that XENPOZYME addresses has been recognized by Japan’s PMDA with Sakigake designation, by the EU with PRIME designation, and by the FDA with Breakthrough designation.
XENPOZYME was approved first in Japan on March 28, 2022, followed by Europe on June 24, 2022, a few months before the FDA approval on August 31, 2022
XENPOZYME is the first and only ERT for the treatment of non central nervous system manifestations of ASMD, with demonstrated improvements in hepatosplenomegaly, pulmonary, liver and hematologic function, dyslipidemia, and growth (children only) in clinical trials of adults and children with ASMD. XENPOZYME is given as an intravenous infusion once every two weeks, and the dose is based on body weight.
XENPOZYME has to date been launched in eight countries. In 2024, It is anticipated that XENPOZYME will be launched in many additional markets worldwide.
Oncology
SARCLISA
SARCLISA (isatuximab) is a monoclonal antibody that binds a specific epitope on the human CD38 receptor and has antitumor activity via multiple mechanisms of action. It was approved in March 2020 in the US in combination with pomalidomide and dexamethasone for the treatment of adults with relapsed refractory multiple myeloma (RRMM) who have received at least two prior therapies including lenalidomide and a proteasome inhibitor, and by the EC in May 2020 in combination with pomalidomide and dexamethasone, for the treatment of adult patients with relapsed and refractory multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on the last therapy. SARCLISA is now approved for this indication in more than 50 countries. In addition the first biologic license application (BLA) for SARCLISA, in combination with pomalidomide and dexamethasone, was accepted by the Chinese health authorities in late 2023.
SARCLISA was approved for a label extension in combination with carfilzomib and dexamethasone in March 2021 in the US for the treatment of adults with relapsed or refractory multiple myeloma (RRMM) who have received one to three prior lines of therapy, and by the EC in April 2021 for the treatment of adult patients with multiple myeloma (MM) who have received at least one prior therapy. The Japanese Ministry of Health, Labor and Welfare (MHLW) granted approval for SARCLISA in combination with carfilzomib and dexamethasone, in combination with dexamethasone, and as monotherapy for RRMM patients in November 2021. SARCLISA is under investigation in the Phase 3 IMROZ trial as a first line treatment for patients with newly diagnosed multiple myeloma who are transplant ineligible. In December 2023, the IMROZ Trial was reported to have met its primary endpoint; the results will form the basis of a future regulatory submission. In addition, the Phase 3 IRAKLIA trial investigating the development of a new subcutaneous formulation with an on-body device system was initiated in the second half of 2022 and is enrolling in over 20 countries.
SARCLISA is also being investigated with several innovative agents in multiple myeloma in an umbrella Phase 1/2 trial.
JEVTANA
JEVTANA (cabazitaxel), a chemotherapy drug and cytotoxic agent, is a semi-synthetic second-generation taxane that prevents many cancer cells from dividing, which ultimately results in destroying many such cells. It is approved in combination with prednisone for the treatment of patients with metastatic castration resistant prostate cancer previously treated with a docetaxel-containing treatment regimen. JEVTANA was granted marketing authorization by the FDA in June 2010, by the EC in March 2011, and in Japan in July 2014. The product is marketed in over 75 countries. In Europe, generic competition started for JEVTANA from the end of March 2021. In the US, the JEVTANA composition of matter patent expired in September 2021. Sanofi has filed patent infringement suits under the US Hatch-Waxman Act against generic manufacturers for cabazitaxel in the US District Court for the District of Delaware asserting three Orange Book listed US patents for JEVTANA. Sanofi entered settlement agreements with most of the defendants and went to trial against the remaining defendant, Sandoz on one of the patents (the ‘777 patent) in January 2023; see Note D.22.b. to the consolidated financial statements, included at Item 18. of this annual report. The district court issued a final judgment in favor of Sanofi in connection with the JEVTANA patent litigation against Sandoz and on August 2, 2023, Sandoz appealed to the Court of Appeals for the Federal Circuit. On October 5, 2023, Sanofi and Sandoz filed a joint stipulation voluntarily dismissing Sandoz’s Appeal, bringing this matter to conclusion.
SANOFI FORM 20-F 2023 | 23 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
FASTURTEC/ELITEK
FASTURTEC/ELITEK is used for the management of plasma uric levels in patients with leukemia, lymphoma, and solid tumor malignancies receiving anticancer therapies.
Rare blood disorders
The Rare Blood Disorders franchise was created in 2018 following Sanofi’s acquisition of Bioverativ and Ablynx.
ALTUVIIIO
ALTUVIIIO (Antihemophilic Factor (Recombinant), Fc-VWF-XTEN Fusion Protein) is a first-in-class high-sustained factor VIII therapy that is designed to extend protection from bleeds with once-weekly prophylactic dosing for adults and children with hemophilia A. Hemophilia A is a rare, x-linked genetic bleeding disorder characterized by a deficiency of functional coagulation factor VIII, resulting in a prolonged patient plasma-clotting time. As a consequence, people with hemophilia A bleed for a longer time than normal.
ALTUVIIIO temporarily replaces the missing coagulation factor VIII by intravenous injection. In adults and adolescents, it is the first factor VIII therapy that has been shown to break through the von Willebrand factor ceiling, which imposes a half-life limitation on earlier generation factor VIII therapies. ALTUVIIIO builds on innovative Fc fusion technology by adding a region of von Willebrand factor and XTEN polypeptides to extend its time in circulation.
ALTUVIIIO was first approved in February 2023 by the FDA, which had previously granted Breakthrough Therapy designation in May 2022 (the first factor VIII therapy to receive this designation), Fast Track designation in February 2021, and Orphan Drug designation in 2017. ALTUVIIIO has since been approved the by regulatory authorities in Japan and Taiwan. The EC granted Orphan Drug designation in June 2019, and a marketing authorization application was filed at the European Medecines Agency in May 2023.
ELOCTATE
ELOCTATE (antihemophilic factor (recombinant), Fc fusion protein) is an extended half-life factor VIII therapy clotting-factor therapy to control and prevent bleeding episodes in adults and children with hemophilia A. In the US, it is indicated for use in adults and children with hemophilia A for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
Hemophilia A is a rare, x-linked genetic bleeding disorder characterized by a deficiency of functional coagulation Factor VIII, resulting in a prolonged patient plasma-clotting time. As a consequence, people with hemophilia A bleed for a longer time than normal. ELOCTATE temporarily replaces the missing coagulation Factor VIII by intravenous injection.
We market ELOCTATE primarily in the US (since 2014), Japan, Canada, Australia, South Korea, Taiwan and Hong Kong.
ELOCTATE is developed and commercialized in collaboration with Swedish Orphan Biovitrum AB (Sobi), whose territories include Europe, Russia, the Middle East, and some countries in North Africa.
ALPROLIX
ALPROLIX (coagulation Factor IX (recombinant), Fc fusion protein) is an extended half-life factor IX therapy clotting-factor therapy to control and prevent bleeding episodes in adults and children with hemophilia B. In the US, it is indicated for use in adults and children with hemophilia B for on-demand treatment and control of bleeding episodes, perioperative management of bleeding, and routine prophylaxis to reduce the frequency of bleeding episodes.
Hemophilia B is a rare, x-linked genetic bleeding disorder characterized by a deficiency of functional coagulation Factor IX, resulting in a prolonged patient plasma-clotting time. As a consequence, people with hemophilia B bleed for a longer time than normal. ALPROLIX temporarily replaces the missing coagulation Factor IX by intravenous injection.
We market ALPROLIX primarily in the US (since 2014), Japan, Canada, Australia, New Zealand, South Korea, Taiwan and Hong Kong.
ALPROLIX is developed and commercialized in collaboration with Swedish Orphan Biovitrum AB (Sobi), whose territories include Europe, Russia, the Middle East, and some countries in North Africa.
CABLIVI
CABLIVI (caplacizumab) is a bivalent anti-von Willebrand Factor (vWF) NANOBODY® VHH for the treatment of adults experiencing an episode of acquired thrombotic thrombocytopenic purpura (aTTP). CABLIVI is the first therapeutic specifically indicated for the treatment of aTTP.
Acquired thrombotic thrombocytopenic purpura is an ultra-rare (3.5-4.5 episodes per million of population), life-threatening, autoimmune-based blood clotting disorder characterized by extensive clot formation in small blood vessels throughout the body, leading to severe thrombocytopenia (very low platelet count); microangiopathic hemolytic anemia (loss of red blood cells through destruction); ischemia (restricted blood supply to parts of the body); and widespread organ damage, especially in the brain and heart. CABLIVI has an immediate effect on platelet adhesion and the ensuing formation and accumulation of the micro-clots.
CABLIVI was granted marketing authorization by the EC in September 2018; by the FDA in February 2019; and by the Japanese PMDA in September 2022. CABLIVI is currently available in 26 countries including the US, the majority of European countries (17), Brazil, Colombia, Japan and five Greater Gulf region states. Additional commercial launches are ongoing.
CABLIVI was developed by Ablynx, a Sanofi company since mid-2018. See “— A. History and Development of the Company.”
24 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
ENJAYMO
ENJAYMO (sutimlimab; formerly known as BIVV009) is a monoclonal antibody targeting the classical complement pathway (CP) specific serine protease (C1s), thereby inhibiting CP activity which is associated with a variety of immune disorders involving the presence of autoantibodies. ENJAYMO is the first-and-only approved therapeutic option approved for hemolytic anemia in adult patients with cold agglutinin disease (CAD).
CAD is a rare, serious, and chronic autoimmune hemolytic anemia, where the body’s immune system mistakenly attacks healthy red blood cells and causes their rupture, known as hemolysis. The disease impacts the lives of an estimated 12,000 people in the US, Europe, and Japan and is associated with profound fatigue and increased risk of thromboembolic events and mortality.
ENJAYMO has previously received Breakthrough Therapy Designation (BTD) and Orphan Drug Designations (ODD) from the FDA, and orphan medicine designation by the European Medicines Agency. After priority review, the product was approved in February 2022 as the first treatment to decrease the need for red blood cell transfusion due to hemolysis in adults with CAD. ENJAYMO was approved by the Japanese Ministry of Health, Labor and Welfare in June 2022 and granted marketing authorization by the EC in November 2022.
ENJAYMO is currently available in the US, Japan, Germany, Austria and the Netherlands. Additional commercial launches are ongoing.
General Medicines
Sanofi has prioritized core assets with differentiated and/or established profiles that have significant opportunity for growth in key markets. Some of these well-established medicines are the standard-of-care for patients living with diabetes or cardiovascular disease. These core assets include TZIELD, REZUROCK, TOUJEO, SOLIQUA, PRALUENT, MULTAQ, LOVENOX, and PLAVIX.
Core assets
TZIELD
TZIELD (Teplizumab) is a CD3-directed antibody (CD3 is a cell surface antigen present on T lymphocytes). It was approved by the FDA in November 2022 to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged eight years and older with Stage 2 T1D. The product is currently marketed in the US, with plans for pursuing regulatory approval in other regions. The product is currently being developed for another indication for patients already in Stage 3 T1D.
REZUROCK
REZUROCK (belumosudil) is a selective ROCK2 (rho-associated coiled-coil–containing protein kinase-2) inhibitor. It was approved in July 2021 by the FDA for the treatment of adult and pediatric patients aged 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy. REZUROCK’s favorable market adoption, especially in the US, is a reflection of its clinical profile. The product has been approved in Australia, Canada, Great Britain, Israel, China, and United Arab Emirates with other countries granting accelerated regulatory review processes.
THYMOGLOBULIN
THYMOGLOBULIN (anti-thymocyte Globulin) is a polyclonal anti-human thymocyte antibody preparation that acts as a broad immunosuppressive and immunomodulating agent. In the US, THYMOGLOBULIN is indicated for the prophylaxis and treatment of acute rejection in patients receiving a kidney transplant, used in conjunction with concomitant immunosuppression. Outside the US, depending on the country, THYMOGLOBULIN is indicated for the treatment and/or prevention of acute rejection in organ transplantation; immunosuppressive therapy in aplastic anemia; and the treatment and/or prevention of Graft-versus-Host Disease (GvHD) after allogeneic hematopoietic stem cell transplantation. THYMOGLOBULIN is currently marketed in over 65 countries.
MOZOBIL
MOZOBIL (plerixafor injection) is a hematopoietic stem cell mobilizer. It is indicated in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM). MOZOBIL is marketed in over 65 countries.
TOUJEO
TOUJEO (insulin glargine 300 units/mL) is a long-acting analog of human insulin, indicated for the treatment of diabetes mellitus in adults. TOUJEO has been granted marketing authorization by the FDA (February 2015); the EC (April 2015); and the Ministry of Health, Labor and Welfare (J-MHLW) in Japan, where its approved brand name is LANTUS XR (June 2015). TOUJEO has now been launched in more than 60 countries, including China since the end of 2020. In January 2020, the EC approved an expansion of the indication to include the treatment of diabetes in adolescents and children (aged six years and above).
TOUJEO is available in TOUJEO SOLOSTAR, a disposable prefilled pen which contains 450 units of insulin glargine and requires one-third of the injection volume to deliver the same number of insulin units as LANTUS SOLOSTAR. In the US (since 2018) and the EU (since 2019), TOUJEO is also available in a disposable prefilled pen which contains 900 units of insulin glargine. In India, TOUJEO is also available in a dedicated 450-unit cartridge in combination with a dedicated reusable pen (TOUSTAR).
SANOFI FORM 20-F 2023 | 25 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
SOLIQUA – SULIQUA
SOLIQUA 100/33 or SULIQUA is a once-daily fixed-ratio combination of insulin glargine 100 Units/mL, a long-acting analog of human insulin, and lixisenatide, a GLP-1 receptor agonist. The FDA approved SOLIQUA 100/33 in November 2016 for the treatment of adults with type 2 diabetes inadequately controlled on basal insulin (less than 60 units daily) or lixisenatide; and in February 2019 for patients uncontrolled on oral antidiabetic medicines. In January 2017, SULIQUA (the product’s brand name in Europe) was approved for use in combination with metformin with or without SGLT-2 inhibitors for the treatment of adults with type 2 diabetes to improve glycemic control, when this has not been provided either by metformin alone or by metformin combined with another oral glucose-lowering medicinal product or with basal insulin. In Japan, SOLIQUA was approved in May 2020 for type 2 diabetes mellitus, where treatment with insulin is required. In China SOLIQUA was approved on January 2023, for the treatment of adults with insufficiently controlled type 2 diabetes mellitus to improve glycaemic control as an adjunct to diet and exercise in addition to other oral antidiabetic drugs. SOLIQUA received National Reimbursement Drug List (NRDL) status in China in December 2023. SULIQUA is available in over 40 countries. (SOLIQUA is approved in over 80 countries).
Integrated Digital Care Solutions
Sanofi, in collaboration with Biocorp and Glooko, Glooko XT, Health2Sync and Emperra, is building a connected set of digital tools and features to support people living with diabetes and taking insulin. Sanofi intends to use aggregated de-identified data to generate insights to inform patients and providers, and to evaluate additional clinical or quality-of-life outcomes. Successful launches in several countries demonstrate the value of the integration of digital tools into a fully connected ecosystem.
PRALUENT
PRALUENT (alirocumab) is a human monoclonal antibody (mAb) for self-administered injection every two weeks or once-monthly. It blocks the interaction of proprotein convertase subtilisin/kexin type 9 (PCSK9) with low-density lipoprotein (LDL) receptors, increasing the recycling of LDL receptors and reducing LDL cholesterol levels. PRALUENT is indicated as an adjunct to diet and maximally tolerated statin therapy in certain adult patients and in paediatric patients eight years of age and older with heterozygous familial hypercholesterolaemia (HeFH) with uncontrolled LDL cholesterol. PRALUENT has been approved in more than 60 countries worldwide, including the US (in 2015), Canada and Switzerland, as well as in the European Union (in 2015). In 2018, the FDA approved a PRALUENT label update for some patients currently requiring LDL apheresis therapy. In March 2019 in the EU and in April 2019 in the US, PRALUENT was approved for use in patients with established cardiovascular disease to reduce the risk of cardiovascular events. In November 2023, the EMA approved a PRALUENT label update for paediatric HeFh patients aged eight years and older. In December 2019, PRALUENT was approved in China, where it started to be commercialized in May 2020. Since April 2020, Regeneron is responsible for commercialization of PRALUENT in the US, and Sanofi is responsible for all other markets outside the US. For additional information on the commercialization of this product, see “Item 5. Operating and Financial Review and Prospects — A.1.7 Financial Presentation of Alliances — Alliance Arrangements with Regeneron.”
LOVENOX/CLEXANE
LOVENOX or CLEXANE (enoxaparin sodium) is a low molecular weight heparin (LMWH) indicated for use in the prophylaxis and treatment of venous thromboembolism and in the treatment of acute coronary syndrome. Enoxaparin generics are available in the US, and biosimilar enoxaparin products have gradually become available across various European countries and in a growing number of international markets. LOVENOX or CLEXANE is marketed in more than 100 countries.
PLAVIX/ISCOVER
PLAVIX or ISCOVER (clopidogrel bisulfate) is a platelet adenosine diphosphate (ADP) receptor antagonist. It is indicated for the prevention of atherothrombotic events in patients with a history of recent myocardial infarction (MI), recent ischemic stroke or established peripheral arterial disease (PAD), and for patients with acute coronary syndrome (ACS). PLAVIX is also indicated in combination with acetylsalicylic acid (ASA) for the prevention of atherothrombotic and thromboembolic events in atrial fibrillation, including stroke.
COPLAVIX/DUOPLAVIN, a fixed-dose combination of clopidogrel bisulfate and ASA, is indicated for the prevention of atherothrombotic events in adult patients with acute coronary syndrome who are already taking both clopidogrel and ASA.
A number of clopidogrel bisulfate generics have been launched in most markets. PLAVIX or ISCOVER are available in more than 80 countries.
Sanofi is involved in two PLAVIX product lawsuits. See Note D.22.c) to our consolidated financial statements, included at Item 18. of this annual report.
MULTAQ
MULTAQ (dronedarone) is an oral multichannel blocker with anti-arrhythmic properties for prevention of atrial fibrillation recurrences in certain patients with a history of paroxysmal or persistent atrial fibrillation. MULTAQ was approved in the US and in the EU in 2009. MULTAQ is available in about 35 countries.
26 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Non-Core Assets
LANTUS
LANTUS (insulin glargine 100 units/mL) is a long-acting analog of human insulin, indicated for once-daily administration for the treatment of diabetes mellitus in adults, adolescents and children aged two years and above. LANTUS relies on more than 15 years of clinical evidence in diabetes treatment and a well-established safety profile. Approved in the US and the EU in 2000 and in Japan in 2008, LANTUS is available in over 130 countries worldwide. Two insulin glargine biosimilars are available in the US, two in European markets, and two in Japan.
APROVEL/AVAPRO/KARVEA
APROVEL, also known as AVAPRO or KARVEA (irbesartan), is an angiotensin II receptor antagonist indicated in the treatment of hypertension and for the treatment of renal disease in patients with hypertension and type 2 diabetes. Sanofi also markets COAPROVEL/AVALIDE/KARVEZIDE, a combination of irbesartan and the diuretic hydrochlorothiazide. A combination with amlodipine (APROVASC, Aprexevo, Aproxxamlo) has been launched in several countries.
A number of irbesartan generics have been launched in most markets. APROVEL and COAPROVEL are marketed in more than 80 countries. In Japan, the product is licensed to Shionogi Co. Ltd and BMS KK. BMS KK has sublicensed the agreement to Dainippon Pharma Co. Ltd.
DEPAKINE
DEPAKINE (sodium valproate) is a broad-spectrum anti-epileptic that has been prescribed for more than 50 years and remains a reference treatment for epilepsy worldwide. DEPAKINE is also a mood stabilizer, registered in the treatment of manic episodes associated with bipolar disorder (in some countries this indication is branded differently, for example as DEPAKOTE in France). We hold no rights to DEPAKINE in the US, and sodium valproate generics are available in most markets.
Sanofi is involved in product litigation related to DEPAKINE. See Note D.22.a) to the consolidated financial statements included at Item 18. of this annual report.
Vaccine products
The Vaccines division of Sanofi is a world leader in the vaccine industry and a key supplier of life-saving vaccines all over the world and for publicly funded international stakeholders such as UNICEF, the Pan American Health Organization (PAHO) and the Global Alliance for Vaccines and Immunization (GAVI).
The Vaccines portfolio includes the following products:
RSV protection
In 2023, Sanofi launched BEYFORTUS (nirsevimab-alip), a long-acting monoclonal antibody designed to protect all infants from RSV. It is indicated for protection of all infants in their first RSV season, and for those infants born at high risk who remain particularly vulnerable in their second RSV season as well.
BEYFORTUS is licensed in several countries, primarily in North America and Europe. In 2023, the US, France and Spain implemented broad protection programs with BEYFORTUS. In 2024, further licenses and introductions are expected worldwide, including in Japan and China. BEYFORTUS is part of an alliance with AstraZeneca, where Sanofi is the commercial lead and AstraZeneca is the development and manufacturing lead.
Influenza vaccines
Sanofi is a world leader in the production and marketing of influenza vaccines, offering several distinct influenza vaccines that are sold globally to meet growing demand.
FLUZONE Quadrivalent is a quadrivalent inactivated influenza vaccine, produced in the US, containing two type A antigens and two type B antigens in order to provide increased protection against more circulating strains of influenza viruses. FLUZONE Quadrivalent/FLUQUADRI is available in seven countries (including the US) for children aged over six months, adolescents and adults. FLUZONE 0.5 ml QIV is the currently-licensed standard dose (15 µg/strain) quadrivalent influenza vaccine for ages six months and older.
FLUZONE High-Dose Quadrivalent, designed specifically to provide greater protection against influenza for people aged 65 years and older, was approved by the FDA in November 2019. FLUZONE High-Dose Quadrivalent was approved in the EU in the second quarter of 2020, under the name EFLUELDA, indicated for adults aged 60 years and above. Both FLUZONE High-Dose Quadrivalent and EFLUELDA have been available since the 2020/21 influenza season. To date, this product has been distributed to 25 countries worldwide.
FLUBLOK is a quadrivalent recombinant protein based influenza vaccine indicated for adults aged 18 years of age and older. FLUBLOK is currently licensed in the US, Hong Kong and Australia. This same recombinant protein-based influenza vaccine is also licensed under the brand name SUPEMTEK in Canada, the United Kingdom, the European Union and Switzerland.
VAXIGRIP is a trivalent influenza vaccine, containing two antigens against type A influenza viruses and one antigen against type B influenza viruses.
VAXIGRIPTETRA is the quadrivalent (QIV) version of VAXIGRIP, including two antigens against A strains of influenza viruses and two antigens against B strains. Compared to the trivalent influenza vaccine, it contains an additional influenza B strain; it was licensed in 2016 and has been launched in more than 95 countries since 2017. VAXIGRIPTETRA is not licensed in the US where FLUZONE Quadrivalent, which is produced in the US, is distributed.
SANOFI FORM 20-F 2023 | 27 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Poliomyelitis, pertussis and Hib pediatric vaccines
Sanofi is one of the key players in pediatric vaccines in both developed and emerging markets, with a broad portfolio of standalone and combination vaccines protecting against up to six diseases in a single injection. Due to the diversity of immunization schedules throughout the world, vaccines vary in composition according to regional specificities.
TETRAXIM, a pediatric combination vaccine protecting against diphtheria, tetanus, pertussis and poliomyelitis (polio), was first marketed in 1998. To date, the vaccine has been launched in close to 90 countries outside the US.
PENTAXIM, a pediatric combination vaccine protecting against diphtheria, tetanus, pertussis, polio and Hemophilus influenzae type b (Hib), was first marketed in 1997. To date, the vaccine has been launched in more than 100 countries outside the US. In most European, Latin American, Asian and Middle Eastern markets, PENTAXIM is being gradually replaced by HEXAXIM.
HEXAXIM/HEXYON/HEXACIMA is a fully liquid, ready-to-use 6-in-1 (hexavalent) pediatric combination vaccine that provides protection against diphtheria, tetanus, pertussis, polio, Hib and hepatitis B. HEXAXIM is the only combination vaccine including acellular pertussis (acP) and inactivated polio vaccines (IPV) currently prequalified by the WHO. First marketed in 2013, HEXAXIM is now available in more than 100 countries outside the US.
PENTACEL, a pediatric combination vaccine protecting against diphtheria, tetanus, pertussis, polio and Hib, was launched in the US in 2008.
QUADRACEL is a vaccine indicated for active immunization against diphtheria, tetanus, pertussis and poliomyelitis, used in children aged four through six years as a fifth dose in the diphtheria, tetanus, pertussis vaccination (DTaP) series, and as a fourth or fifth dose in the inactivated poliovirus vaccination (IPV) series.
ACT-HIB is a standalone vaccine protecting against Hib, and is mainly distributed in the US, Japan and China in conjunction with pertussis combination vaccines that do not contain the Hib valence.
Sanofi is a leading provider of polio vaccines and has been a partner of the Global Polio Eradication Initiative (GPEI) for over 30 years, with more than 15 billion doses of oral polio vaccines (OPV) delivered during that time.
Between 2014 and 2023, Sanofi has provided more than 400 million doses of inactivated polio vaccine to UNICEF, to support the WHO “Polio End Game” strategy for the world’s 73 poorest countries.
VAXELIS
VAXELIS is a hexavalent combination vaccine protecting against diphtheria, tetanus, pertussis, polio, Hib and hepatitis B. This vaccine (developed and distributed in partnership with Merck) was approved in 2016 by the EMA and is distributed in various EU countries. VAXELIS was approved by the FDA in December 2018, becoming the first hexavalent vaccine to be approved in the US, and launched in this country in June 2021.
Sales of VAXELIS in the US are booked to the MSP JV and credited to Merck and Sanofi as equity income and therefore are not reported separately in each parent company’s net sales. Fifty percent of the JV profits are included in our share of profit/loss of associates and joint ventures line.
Booster vaccines
ADACEL is the first trivalent booster vaccine offering protection against diphtheria, tetanus and pertussis. The vaccine can be used from four years of age following primary immunization and is the first Tdap vaccine indicated for use during pregnancy for protection against pertussis in newborns. It is available in 68 countries including the US and other countries mostly in Europe, Asia and Latin America.
REPEVAX/ADACEL-POLIO is a combination vaccine that provides protection against diphtheria, tetanus, pertussis and polio. It is the first Tdap-IPV vaccine indicated for use during pregnancy for protection against pertussis in newborns. It is currently marketed in 25 countries outside the US, with a strong focus on European markets (such as France and Germany).
Meningitis and Travel & endemic vaccines
MENACTRA, the first quadrivalent conjugate vaccine against meningococcal meningitis (serogroups: A, C, Y, and W-135), one of the deadliest forms of meningitis, is indicated for people aged nine months through 55 years. Since launch, it has become a strong leader in the meningitis quadrivalent market. It is commercialized in a large number of countries (excluding Europe). MENACTRA was the first fully liquid (no reconstitution needed) meningitis quadrivalent conjugated vaccine, and more than 100 million doses of this vaccine have been distributed since launch.
MENQUADFI is a novel fully-liquid meningococcal quadrivalent conjugated vaccine expected to have a broad age indication from infants (six weeks) to the elderly, with flexible dosing schedules. MENQUADFI is the first and only quadrivalent ACWY vaccine to demonstrate superior immune response against serogroup C in toddlers compared to a monovalent serogroup C vaccine (standard-of-care in multiple markets in Europe and internationally). MENQUADFI will progressively fully replace MENACTRA. It is already available in the US (for people over two years of age), and in Australia, Canada, Europe, Japan, Argentina, Brazil, and Chile for people aged 12 months and above. Marketing authorization is also pending in numerous other countries. Extension of the age indication down to six weeks of age will follow submission of additional Phase 3 data.
Sanofi provides a wide range of travel and endemic vaccines including hepatitis A, typhoid, cholera, yellow fever and rabies vaccines. These products are used in endemic settings in the developing world and are the foundation for important partnerships with governments and organizations such as UNICEF. They are also used by travelers and military personnel in industrialized countries and in endemic areas.
28 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
COVID Vaccine
COVID-19 recombinant adjuvanted vaccine: VIDPREVTYN Beta is a recombinant spike protein vaccine developed in partnership with GSK and using GSK’s AS03 adjuvant. It is indicated as an adult booster to protect against SARS-CoV-2 infections. Phase 3 results demonstrated significant vaccine efficacy against symptomatic infection with a Beta variant-containing vaccine in the face of an Omicron variant predominated pandemic period. Significant efficacy was demonstrated in naive individuals and individuals previously infected or vaccinated. These results, coupled with data from comprehensive studies to evaluate the vaccine as a heterologous booster for people initially vaccinated with Emergency Use Authorization (EUA) vaccines, supported VIDPREVTYN Beta’s full marketing authorization for the booster indication in both the EU and the UK. The first doses were supplied at the end of 2022 and Sanofi fulfilled all of its commitments in 2022 and 2023.
B.3. Consumer Healthcare
In 2023, we progressed further in building and simplifying our stand-alone CHC organization. We have further reduced our portfolio, mainly through divestments, to 117 brands (47% fewer than in 2019). In September 2023 we announced the completion of the acquisition of QUNOL, a leading US health and wellness brand that strengthens our CHC portfolio in a key market, and within the promising VMS segment.
By the end of 2023, all the CHC legal entities required to establish a standalone organization had been established, except in India, and staffed with dedicated teams. Our CHC business now publishes its results as a separate dedicated segment, including all its support function costs, within the Sanofi environment. We also announced our intention to separate our CHC operations at the earliest in the fourth quarter of 2024, most likely through a capital markets transaction, via the creation of a listed entity headquartered in France.
In line with our high sustainable development ambitions, Sanofi CHC North America achieved B-corp accreditation in July 2023.
Our CHC sales are supported by a range of products, including the following brands:
Allergy, Cough & Cold
•ALLEGRA comprises a range of fexofenadine HCl-based products. Fexofenadine is an anti-histamine for relief from allergy symptoms including sneezing, runny nose, itchy nose or throat, and itchy, watery eyes. The ALLEGRA brand family is sold in more than 80 countries across the world.
•MUCOSOLVAN is a cough brand with many different formulations. It contains the mucoactive agent ambroxol; this stimulates synthesis and release of surfactant. It is sold in various countries in Europe, Latin America, Asia and Russia.
Pain
•DOLIPRANE offers a range of paracetamol/acetaminophen-based products for pain and fever with a wide range of dosage options and pharmaceutical forms, and is sold mainly in France and various African countries.
•The BUSCOPAN range (hyoscine butylbromide) has an antispasmodic action that specifically targets the source of abdominal pain and discomfort. It is sold across the globe.
•We also have local pain brands such as EVE in Japan; DORFLEX and NOVALGINA in Brazil; and ICY HOT and ASPERCREME in the US.
Digestive
•DULCOLAX products offer a range of constipation solutions from predictable overnight relief to comfortable natural-feeling relief. The products are sold in over 80 countries. DULCOLAX tablets contain the active ingredient bisacodyl or sodium picosulfate, which works directly on the colon to produce a bowel movement.
•ENTEROGERMINA is a probiotic indicated for the maintenance and restoration of intestinal flora in the treatment of acute or chronic intestinal disorders. ENTEROGERMINA is sold primarily in Europe, Latin America and parts of Asia.
•ESSENTIALE is a natural soybean remedy to improve liver health. It is composed of essential phospholipids extracted from highly purified soya and contains a high percentage of phosphatidylcholine, a major component of the cell membrane. ESSENTIALE is used in fatty liver disease and is sold mainly in Russia, Eastern Europe, various countries in Southeast Asia, and China.
Nutritional
•Nutritionals include a range of products to maintain general health, provide immune system support, or supplement vitamin deficiencies. These products help manage energy, stress, sleep and anxiety, and include a number of brands across the globe including NATURE’S OWN in Australia to improve and maintain health; PHARMATON (mainly in Europe and Latin America); QUNOL in the US for healthy aging; MAGNE B6 in Europe; and a range of sleep brands, including NOVANUIT in Europe, UNISOM in the US, and DREWELL in Japan.
Other
•GOLD BOND offers products for daily hydration, aging skin, cracked skin, overnight nourishment, and specialty skincare needs like eczema-prone skin. GOLD BOND is only sold in the US and Canada. Sanofi is involved in GOLD BOND product litigation in the US. See Note D.22.c) to our consolidated financial statements, included at Item 18. of this annual report.
SANOFI FORM 20-F 2023 | 29 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
B.4. Global research & development
For the past three years, Sanofi has reshaped its discovery and development of therapeutics, built a robust pipeline, and sharpened its research focus, employing cutting-edge therapeutic platforms and creating a culture that responds to the urgent needs of patients. Discovering and developing new medicines is a costly, lengthy, and uncertain process and our continuous investments in R&D for future products and for the launches of newly registered medicines could result in increased costs without a proportionate increase in revenues. See “Item 3. Key Information — D. Risk Factors” for further information.
Sanofi’s new Head of Research & Development (R&D), Houman Ashrafian, was appointed in September 2023 with responsibility for bolstering the company’s strategy to develop first or best-in-class medicines. Focusing on its portfolio of 12 new molecular entities, including multiple late-stage assets, Sanofi’s strategy is to prioritize development and leverage its leadership in immunology across all other therapeutic areas.
Details about Sanofi’s R&D pipeline and three “pipeline-in-a-product” assets are presented in the sections “— B.4.1. Biopharma pipeline” below.
B.4.1. Biopharma pipeline
For 2023, the main changes related to the pharmaceuticals and vaccines pipeline were:
| Project | Potential Indication | Change | Reason | ||||||||
TZIELD – Anti-CD3 mAb | Type 1 diabetes | Added | Acquired from Provention Bio | ||||||||
SAR447189 – Anti-TL1A mAb | Ulcerative colitis; Crohn’s disease | Added | Co-developed with Teva Pharmaceuticals | ||||||||
SAR445399 – Anti-IL1R3 mAb | Inflammatory indication | Added | Entered confirmatory development | ||||||||
SAR445611 – Anti-CX3CR1 NANOBODY® VHH | Inflammatory indication | Added | Entered confirmatory development | ||||||||
SAR446422 – Anti CD28/OX40 bispecific antibody | Inflammatory indication | Added | Entered confirmatory development | ||||||||
SAR445514 – Trifunctional anti-BCMA NK-Cell engager | Relapsed, refractory multiple myeloma | Added | Entered confirmatory development | ||||||||
SAR445953 – Anti-CEACAM5-Topo1 ADC | Colorectal cancer | Added | Entered confirmatory development (a) | ||||||||
SAR444836 – PAH replacement AAV-based gene therapy | Phenylketonuria | Added | Entered confirmatory development | ||||||||
ALTUVIIIO – Factor VIII replacement therapy | Hemophilia A | Removed | Commercialized | ||||||||
SAR444419 – Anti-TNFa/IL6 NANOBODY® VHH | Inflammatory indication | Removed | Development discontinued | ||||||||
atuzabrutinib (SAR444727) – BTK inhibitor (topical) | Atopic dermatitis | Removed | Development discontinued | ||||||||
tusamitamab ravtansine (SAR408701) – Anti-CEACAM5 ADC | NSCLC and other solid tumors | Removed | Development discontinued | ||||||||
SAR441000 – Cytokine mRNA | Solid tumors | Removed | Development discontinued (b) | ||||||||
SAR442257 – CD38/CD28/CD3 T-Cell engager | Multiple myeloma/non-Hodgkin lymphoma | Removed | Development discontinued | ||||||||
SAR443216 – CD3/CD28/HER2 T-Cell engager | Solid tumors | Removed | Development discontinued | ||||||||
alomfilimab (SAR445256) – Anti-ICOS mAb | Solid tumors | Removed | Development discontinued | ||||||||
SAR445710 – Anti PD-L1/IL-15 fusion | Solid tumors | Removed | Development discontinued | ||||||||
Shan6 DTwP-HepB-Polio-Hib | Pediatric hexavalent vaccine | Removed | Strategic decision | ||||||||
| ExPEC vaccine (9-valent) | Prevention of invasive E.coli disease | Added | Partnered with Janssen | ||||||||
BEYFORTUS (nirsevimab), anti RSV mAb | Passive prevention of RSV infections in all infants | Removed | Commercialized | ||||||||
mAb: monoclonal antibody. ADC: antibody-drug conjugate. NSCLC: Non-small cell lung cancer. RSV, respiratory syncitial virus.
(a) SAR445953: co-developed with Seagen.
(b) SAR441000: termination decided jointly with BioNTech.
The portfolio of products in clinical development (from Phase 1 to Phase 3) and in registration as of December 31, 2023 is described in the section “Item 4. Information on the Company — E. R&D Appendix.”
Phase 1 studies are the first studies performed in humans, who are mainly healthy volunteers, except for studies in oncology, where Phase 1 studies are performed in patients. Their main objective is to assess the tolerability, the pharmacokinetic profile (the way the product is distributed and metabolized in the body and the manner by which it is eliminated) and where possible the pharmacodynamic profiles of the new drug (i.e. how the product may react on some receptors).
Phase 2 studies are early controlled studies in a limited number of patients under closely monitored conditions to show efficacy and short-term safety, and to determine the dose and regimen for Phase 3 studies.
Phase 3 studies have the primary objective of demonstrating or confirming the therapeutic benefit and the safety of the new drug in the intended indication and population. They are designed to provide an adequate basis for registration.
30 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
B.4.1.1. Products in development
Products in clinical development include three ‘pipeline-in-a-product’ assets – amlitelimab, frexalimab and the oral TNFR1 signaling inhibitor SAR441566 – intended to address unmet patient needs in markets with low penetration of advanced therapies. In addition, our clinical pipeline features nine innovative medicines and vaccines: tolebrutinib, rilzabrutinib, itepekimab, lunsekimig, the IRAK4 degrader SAR444656, the anti-TL1A monoclonal antibody SAR447189, the ExPEC vaccine, and two mRNA vaccines respectively against respiratory syncytial virus and acne. The pipeline of potential market leading opportunities is summarized below:
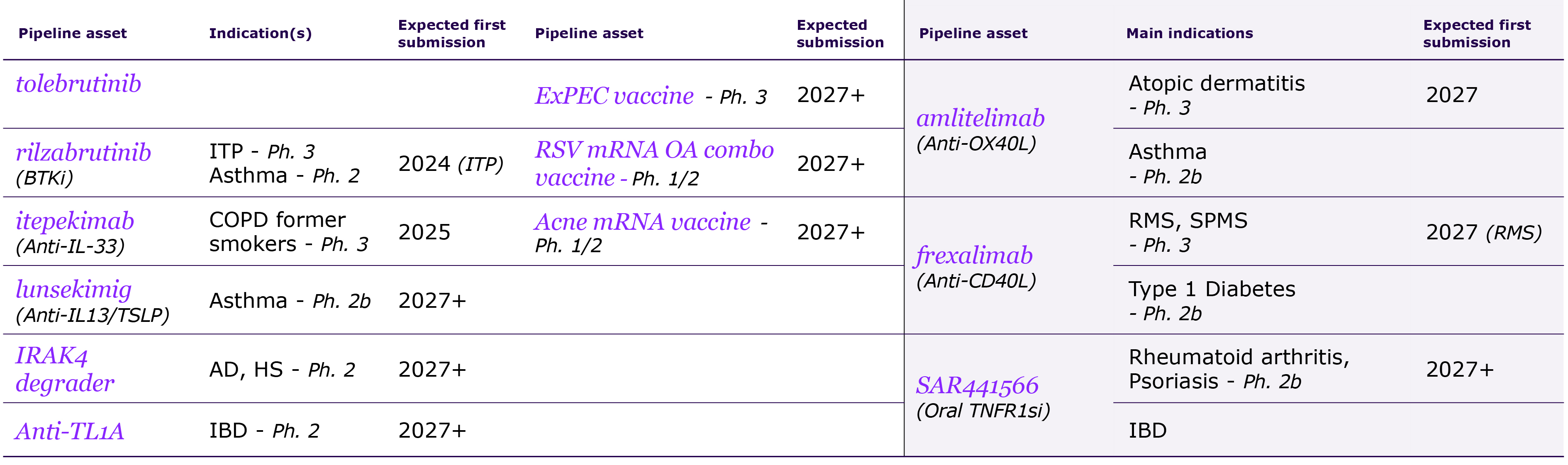
Phase 1/2 study of acne mRNA vaccine to be initiated in the first half of 2024.
Further details on these products, and on other products in clinical development, are given below.
a) Immunology & Inflammation
amlitelimab (SAR445229), a human anti-OX40L monoclonal antibody, is currently being assessed in clinical programs for the treatment of atopic dermatitis (AD), asthma and hidradenitis suppurativa (HS).
The Phase 2b study (STREAM-AD) in adults with moderate-to-severe AD whose disease cannot be adequately controlled with topical medications or for whom topical medications are not a recommended treatment approach, met its primary endpoint of percentage change in Eczema Area and Severity Index (EASI) score from baseline at 16 weeks. Four subcutaneous doses were studied, and continued improvement was seen through 24 weeks. The Phase 3 program started in 2023 with the first study (Coast 1) initiated for treatment of participants diagnosed with moderate to severe AD, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The Phase 2b study (TIDE-asthma) is assessing add-on therapy with amlitelimab in adult participants with moderate-to-severe asthma; results are expected in 2024.
In November 2023, a Phase 2 proof-of-concept study assessing amlitelimab in adult participants with moderate to severe HS was initiated.
The Phase 2b study (TIDE-asthma) is assessing add-on therapy with amlitelimab in adult participants with moderate-to-severe asthma; results are expected in 2024.
In November 2023, a Phase 2 proof-of-concept study assessing amlitelimab in adult participants with moderate to severe HS was initiated.
frexalimab (SAR441344), a second generation anti-CD40L monoclonal antibody that blocks the costimulatory CD40/CD40L pathway which is important for activation and function of adaptive (T and B cells) and innate (macrophages/microglia and dendritic cells) immunity. Sanofi is developing frexalimab under an exclusive license from ImmuNext Inc.
Frexalimab is being evaluated in multiple sclerosis (see details in section “— c) Neurology”). The compound is also being evaluated in Phase 2 trials for the treatment of Sjogren’s syndrome and systemic lupus erythematosus, respectively. In 2023, a Phase 2b study assessing frexalimab in adults and adolescents with newly diagnosed type 1 diabetes was initiated.
Frexalimab is being evaluated in multiple sclerosis (see details in section “— c) Neurology”). The compound is also being evaluated in Phase 2 trials for the treatment of Sjogren’s syndrome and systemic lupus erythematosus, respectively. In 2023, a Phase 2b study assessing frexalimab in adults and adolescents with newly diagnosed type 1 diabetes was initiated.
SAR441566, the first small molecule TNFR1 signaling inhibitor, is intended to provide patients with an oral alternative to anti-TNFa monoclonal antibodies in the range of inflammatory indications where these have been approved. SAR441566 is currently being evaluated in two Phase 2b clinical studies that were initiated in 2023, respectively for the treatment of patients with psoriasis and with rheumatoid arthritis.
itepekimab (SAR440340) is a human anti-IL33 monoclonal antibody co-developed with Regeneron that is currently being evaluated in a Phase 3 clinical program (AERIFY-1 and AERIFY-2 studies) for the treatment of COPD in former smokers; itepekimab is also being assessed in a cohort of current smokers in AERIFY-2. In addition, an exploratory Phase 2a study (AERIFY-3) is evaluating the mechanism of action of itepekimab and its impact on airway inflammation in former and current smokers with COPD. Itepekimab has FDA Fast Track Designation for the treatment of COPD.
rilzabrutinib (SAR444671) is a covalent and reversible inhibitor of Bruton’s tyrosine kinase under development for the treatment of autoimmune/inflammatory diseases.
The Phase 2 study evaluating rilzabrutinib in adults with moderate-to-severe chronic spontaneous urticaria (CSU) met its primary endpoint consisting of improvement in weekly itch severity score (primary endpoint in the US) or in weekly urticaria activity score (itch and hives; primary endpoint outside the USA). Improvements in secondary endpoints were also observed. The Phase 3 clinical program in CSU will be initiated in 2024. Further Phase 2 studies have been pursued, assessing the product for the treatment of patients with moderate-to-severe asthma and IgG4-related disease, respectively.
SANOFI FORM 20-F 2023 | 31 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
In 2023, the development of rilzabrutinib for the treatment of atopic dermatitis was discontinued, based on the results of a Phase 2 study in which the primary endpoint was not met.
In addition, rilzabrutinib is being evaluated for the treatment of immune thrombocytopenia and warm autoimmune hemolytic anemia (see details in section “— e) Rare Blood Disorders”).
lunsekimig (SAR443765) is a bispecific NANOBODY® VHH which blocks both TSLP and IL-13, key upstream and downstream mediators (respectively) of asthma. After Proof-of-Mechanism findings were obtained earlier this year, a Phase 2b study (AIRCULES) enrolled the first participants in 2023.
SAR444656 is a selective, orally administered small molecule targeting Interleukin-1 Receptor Associated Kinase 4 (IRAK4), which is necessary for proinflammatory signaling and cytokine production. SAR444656 is developed in partnership with Kymera Therapeutics. Based on the encouraging results of a Phase 1 clinical trial, Sanofi has initiated two Phase 2 studies, respectively in atopic dermatitis and in HS.
SAR447189 (also known as TEV-48574), an anti-TL1A monoclonal antibody, entered our Immunology & Inflammation portfolio in 2023 and is co-developed with Teva Pharmaceuticals. SAR447189 is currently being evaluated in a Phase 2 clinical program for the treatment of adults with ulcerative colitis and Crohn’s disease (inflammatory bowel disease).
eclitasertib (SAR443122) is a small molecule targeting the receptor-interacting serine/threonine-protein kinase 1 (RIPK1), which is being co-developed with Denali. In 2023, it was decided to discontinue eclitasertib in cutaneous lupus erythematosus, based on the efficacy results of the proof-of-concept Phase 2 study. The compound was found to be generally well-tolerated and a Phase 2 study evaluating eclitasertib in patients with ulcerative colitis is ongoing.
riliprubart (SAR445088) is a complement C1s inhibitor under clinical development in various indications (see details in section “— e) Rare Blood Disorders” and section “— c) Neurology”). A Phase 2 study is currently evaluating the compound for the treatment of patients at risk of antibody-mediated rejection (AMR) or diagnosed with AMR.
SAR442970, a bispecific NANOBODY® VHH that combines the blockade of TNFa and the immune co-stimulatory regulator OX40L progressed to Phase 2 in 2023 for the treatment of Hidradenitis Suppurativa.
SAR444336, a non-beta IL-2 SYNTHORIN molecule designed to selectively engage CD4+ regulatory T cells (and not on effector T or NK cells), is currently being evaluated in Phase 1 for the treatment of inflammatory indications.
SAR444559, an anti-CD38 monoclonal antibody with engineered format, is in clinical development for the treatment of inflammatory indications.
In 2023, new products originating from Sanofi’s research entered Phase 1 for the treatment of inflammatory indications:
•SAR445399, an anti-IL1R3 mAb monoclonal antibody;
•SAR445611, an anti-CX3CR1 NANOBODY® VHH; and
•SAR446422, a bispecific antibody targeting CD28 and OX40.
b) Oncology
pegenzileukin (SAR444245) is a non-alpha IL-2 SYNTHORIN molecule currently being evaluated in a Phase 1/2 program initiated in 2023, to optimize the dose schedule for the treatment of solid tumors.
SAR444881, a monoclonal antibody targeting the Ig-like transcript 2 (ILT2) receptor co-developed with Biond Biologics for the treatment of solid tumors, is currently being evaluated in Phase 1.
SAR445419 is an off-the-shelf NK cell therapy currently being evaluated in Phase 1 for the treatment of relapsed/refractory acute myeloid leukemia.
SAR443579 is a trifunctional anti-CD123 NK cell engager developed in partnership with Innate Pharma. SAR443579 is being investigated in a Sanofi-sponsored Phase 1/2 clinical trial in patients with relapsed or refractory acute myeloid leukemia, B-cell acute lymphoblastic leukemia or high risk-myelodysplasia. SAR443579 has FDA Fast Track Designation for the treatment of acute myeloid leukemia.
SAR446309, an HER2-based T cell engager, is currently being evaluated as a single agent and in combination with pembrolizumab, in a Phase 1 clinical trial for the treatment of locally advanced or metastatic HER2-expressing cancers.
SAR444200 is a GPC3-based T cell engager designed with a NANOBODY® VHH format that is currently being evaluated in Phase 1 in patients with advanced solid tumors.
SAR445877 is an anti PD1/IL-15 fusion protein under assessment in a Phase 1 trial in patients with solid tumors.
SAR445514 is a trifunctional anti-BCMA NK cell engager developed in partnership with Innate Pharma for the treatment of relapsed or refractory multiple myeloma; a Phase 1/2 study was initiated in 2023.
SAR445953, an antibody drug conjugate (ADC) that binds to human CEACAM-5, is under Phase 1 clinical evaluation for the treatment of patients with colorectal cancer or other solid tumors. SAR445953 is developed in collaboration with Seagen Inc.
32 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
c) Neurology
tolebrutinib (SAR442168) is an oral investigational brain-penetrant and bioactive Bruton’s tyrosine kinase (BTK) inhibitor that achieves cerebrospinal fluid concentrations predicted to modulate B lymphocytes and microglial cells. Tolebrutinib is being evaluated in Phase 3 clinical trials for the treatment of relapsing forms of multiple sclerosis (RMS), non-relapsing secondary progressive MS (nrSPMS), and primary progressive MS (PPMS).
frexalimab (SAR441344) is a monoclonal antibody against CD40L (see section “— b) Immunology & Inflammation”), a key immune costimulatory component involved in MS. Through its unique upstream mechanism of action, frexalimab has the potential to address both acute and chronic neuroinflammation in MS. Data from the placebo-controlled part of the Phase 2 study data in adults diagnosed with RMS demonstrated significant reductions in new gadolinium-enhancing lesions at week 12 (primary objective), plus sustained reduction of disease activity over week 24 in both high-dose and low-dose frexalimab groups. The treatment continuation open-label part of the study is ongoing. Two Phase 3 studies were initiated late 2023 to evaluate the efficacy and safety of frexalimab for the treatment of adults with RMS and nrSPMS, respectively.
SAR443820 is an oral brain-penetrant RIPK1 inhibitor that targets inflammatory cell signaling and activation of cell death pathways in MS pathophysiology. Its multitargeted mechanism of action and flexibility to be used as a monotherapy or in combination gives the compound the potential to address neurodegeneration and disability accumulation. SAR443820 is in-licensed from Denali and has been evaluated in Phase 2 clinical trials for amyotrophic lateral sclerosis (ALS) and MS. Results of the Phase 2 trial in ALS (HIMALAYA) obtained in February 2024 indicated that the primary endpoint was not met; development of SAR443820 in this indication will not be pursued. Sanofi will continue the Phase 2 study (K2) of SAR443820 in participants with MS.
riliprubart (SAR445088), a complement C1s inhibitor (see details in section “— e) Rare Blood Disorders”), is being assessed in a Phase 2 trial in patients with chronic inflammatory demyelinating polyneuropathy (CIDP).
SAR446159 is a bispecific antibody targeting both alpha-synuclein and insulin-like growth factor 1 receptor (IGF1R) developed in collaboration with ABL Bio for the treatment of Parkinson’s disease. A Phase 1 study assessing the safety and tolerability of intravenous SAR446159 is ongoing.
d) Rare Diseases
venglustat (GZ402671) is an orally administered brain penetrant glucosylceramide synthase (GCS) inhibitor that blocks the conversion of ceramide to glucosylceramide (GL-1). Venglustat is currently being evaluated in Phase 3 trials for the treatment of three lysosomal storage diseases: late-onset GM2 gangliosidosis (Tay-Sachs disease and Sandhoff disease), Fabry disease, and Gaucher disease type 3. The data of the Phase 3 study in late-onset GM2 gangliosidosis and subsequent submission are expected in 2024.
SAR442501 is an antibody (Fab format) that directly targets fibroblast growth factor receptor 3 (FGFR3), which has a gain-of-function genetic mutation leading to premature closure of bone growth plates and the achondroplasia condition, the most common cause of dwarfism in the world. In 2023, a Phase 2 clinical trial was initiated to evaluate SAR442501 for the treatment of achondroplasia in children from birth up to 12 years of age.
SAR443809, a humanized monoclonal antibody that selectively inhibits the activated fragment of factor B (termed Bb) in the alternative pathway of the complement system, is being evaluated in a Phase 1 trial for the treatment of rare renal diseases.
SAR439459 is a monoclonal antibody targeting transforming growth factor beta (TGFβ) currently evaluated in a Phase 1 study in adult participants with Osteogenesis imperfecta, also called brittle bone disease, a rare disease in which bones fracture easily. Orphan Drug Designation was granted by the FDA for this indication.
SAR444836 is a phenylalanine hydroxylase replacement gene therapy based on adeno-associated virus vector technology that is developed in collaboration with MediciNova, Inc. In 2023, a Phase 1 study was initiated to treat patients with phenylketonuria. Orphan Drug Designation has been granted by the FDA for this indication.
e) Rare Blood Disorders
rilzabrutinib (SAR444671) is being investigated in a Phase 3 trial for the treatment of adults and adolescents with persistent or chronic immune thrombocytopenia (ITP), for which the FDA has granted Fast Track Designation. Rilzabrutinib is also being evaluated in a Phase 2b study for the treatment of adults with warm autoimmune hemolytic anemia (wAIHA).
fitusiran (SAR439774), a first-in-class, subcutaneously administered antithrombin siRNA therapy, is currently in Phase 3 clinical development for the treatment of hemophilia A or B, with or without inhibitors, which includes lower doses and less frequent dosing while maintaining an antithrombin target range of 15-35% (antithrombin-based dosing regimen, AT-DR) in all ongoing studies. The Phase 3 open-label extension study (ATLAS-OLE) demonstrated during an interim analysis a substantially improved safety profile versus AT-DR, and the risk of thrombosis was reduced with rates comparable to those reported in the general hemophilia population. Pre-specified efficacy analyses confirmed that fitusiran provides consistent protection with as few as six injections per year. Sanofi is currently in discussions with the US FDA regarding filing in 2024. Planned submissions in the European Union and Japan will require data from the ongoing Phase 3 ATLAS-NEO study.
riliprubart (SAR445088) is a humanized IgG4 monoclonal antibody that binds to and inhibits C1s, thereby inhibiting classical pathway (CP) of complement activity. Activation of the CP of complement is associated with a variety of immune disorders involving the presence of autoantibodies. Inhibition of autoantibody-mediated CP activation on the surface of erythrocytes via C1s binding prevents complement opsonin deposition on red blood cells and protects them from phagocytosis and extravascular hemolysis in autoimmune hemolytic anemia such as cold agglutinin disease (CAD). SAR445088 is currently being evaluated in a Phase 2 study for the treatment of patients with CAD.
SANOFI FORM 20-F 2023 | 33 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
f) Vaccines
The Vaccines R&D portfolio includes projects enhancing existing vaccines, and projects addressing novel targets. As shown below, several projects are in Phase 2 and Phase 3.
mRNA Quadrivalent influenza vaccine (SP0273) is a quadrivalent influenza vaccine based on mRNA technology, which is currently in Phase 1. Following results of Phase 1, the mRNA influenza vaccine program is being adapted in order to enter Phase 3 with an optimal next generation influenza vaccine.
Rabies Vaccine (SP0087): a next-generation purified human rabies vaccine (VRVg) is under development, aimed at replacing both of Sanofi’s currently commercialized rabies vaccines (IMOVAX Rabies and VERORAB). It will be cultured on Vero cells and will be free of animal or human material. VRVg is currently in Phase 3 trials in order to support pre and post exposure indications.
Vero Yellow Fever (vYF) vaccine candidate (SP0218) is a next generation freeze-dried live-attenuated yellow fever vaccine produced on a Vero cell line, for subcutaneous and intra-muscular administration in people aged nine months and older. This vaccine aims at replacing STAMARIL and YF-VAX with a single product. Following positive Phase 2 results in 2023, Phase 3 will be initiated in 2024.
ExPEC vaccine (SP0282): ExPEC is a vaccine for the prevention of invasive E.Coli disease, including sepsis, which is expected to complement our older adults vaccines portfolio . This 9-valent vaccine is partnered with Janssen Pharmaceuticals Inc., a Johnson and Johnson company; it is currently in Phase 3, which was initiated in 2021 and continues to enroll patients.
RSV toddler vaccine (SP0125): All infants can be protected against RSV during their first season with BEYFORTUS (nirsevimab), now launched in the US and several other countries. The goal of RSV toddler vaccine is to expand RSV protection by providing protection to all toddlers against RSV, from the second season onwards. Positive data from a Phase 1/2 study performed in the US evaluating the safety and effectiveness of two doses of an intranasal delivery device in infants were obtained at the beginning of 2023, which will enable a move to Phase 3 in 2024.
RSV older adult vaccine (SP0256): Sanofi has initiated Phase 2 with an mRNA vaccine against RSV for the older adults population. This vaccine aims at providing protection against RSV, and is key for a respiratory combination providing additional benefits against other unmet medical needs (hMPV, PIV). Consistent with this, we started Phase 1/2 in November 2023 with an RSV/hMPV combination vaccine.
Meningococcal Group B (Men B) and MenPenta (Men ACWYB) (SP0230): Sanofi’s MenB vaccine candidate is intended to provide active immunization against invasive meningococcal disease caused by Neisseria meningitidis serogroup B (Men B) for all age groups. Following positive results of a Phase 1/2 study initiated in March 2021, and positive pre-clinical data obtained on MenPenta, a MenPenta Phase 1/2 was initiated in October 2023.
Pneumococcal Conjugate Vaccine (PCV) (SP0202): Sanofi is collaborating with SK Biosciences (South Korea) to develop a 21-valent pneumococcal conjugate vaccine that will provide expanded protection against pneumococcal disease. Data from Phase 2 studies assembled between mid-2022 and mid-2023 will allow us to move to Phase 3 in the pediatric population in 2024.
B.4.1.2. Line extensions
For more information on DUPIXENT, KEVZARA, TZIELD, SARCLISA, REZUROCK, NEXVIAZYME, FLUZONE and MENQUADFI see also “ — B.2. Main biopharma products”.
DUPIXENT is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, jointly developed with Regeneron. DUPIXENT has received regulatory approvals in several countries for use in patients with AD, asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis (EoE) or prurigo nodularis (PN) in different age populations; details about clinical and regulatory activities pursued in 2023 are provided below.
DUPIXENT is under assessment for the treatment of dermatology, respiratory and gastrointestinal diseases:
a.in October 2023, the FDA issued a Complete Response Letter (CRL) for the sBLA for DUPIXENT in CSU, an inflammatory skin condition, which causes sudden and debilitating hives and swelling on the skin. The CRL states that additional efficacy data are required to support an approval; it did not identify any issues with safety or manufacturing. An ongoing Phase 3 clinical trial (Study C) continues to enroll patients, with results expected in late 2024 that are anticipated to provide the additional efficacy data. Sanofi and Regeneron remain committed to working with the FDA to advance the study of DUPIXENT for patients living with CSU who are inadequately controlled by antihistamines;
b.the primary and all key secondary endpoints were met in the BOREAS Phase 3 trial evaluating DUPIXENT compared to placebo in adults currently on maximal standard-of-care inhaled therapy (triple therapy) with uncontrolled COPD and evidence of type 2 inflammation. Clinically meaningful and highly significant reduction (30%) in moderate or severe acute exacerbations of COPD (rapid and acute worsening of respiratory symptoms) was obtained, while the trial also demonstrated significant improvements in lung function, quality of life and COPD respiratory symptoms. The second, replicate Phase 3 trial (NOTUS) met its primary endpoint (significant reduction of exacerbations of COPD with DUPIXENT compared to placebo), confirming the results from the BOREAS pivotal trial. Supplemental BLA in the US was completed in December 2023, following Marketing Authorization Application submission in Europe; the respective approvals are expected in 2024;
c.a Phase 3 clinical study (LIBERTY-BP) is ongoing to evaluate DUPIXENT in adult patients with bullous pemphigoid; results are expected in 2024;
d.the product is being assessed in a Phase 3 study (LIBERTY-CPUO-CHIC) for the treatment of chronic pruritis of unknown origin in adults;
34 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
e.a Phase 2/3 trial evaluating DUPIXENT in adult and adolescent patients with eosinophilic gastritis with or without eosinophilic duodenitis was initiated in 2023;
f.a Phase 2 clinical study was initiated in 2023 for the treatment of patients with ulcerative colitis.
The Phase 3 study (LIBERTY-CINDU) evaluating DUPIXENT in chronic inducible cold urticaria did not meet the required efficacy endpoints to continue this program.
Discontinuation of DUPIXENT programs in allergic fungal rhinosinusitis and chronic rhinosinusitis without nasal polyps was decided due to portfolio prioritization; the respective studies will be completed.
KEVZARA, a monoclonal antibody against the IL-6 receptor (developed with Regeneron) is already marketed for the treatment of moderate to severe rheumatoid arthritis.
The product is currently being developed for the treatment of polyarticular juvenile idiopathic arthritis. Based on the 52-week data of the pivotal Phase 2 study assessing the pharmacokinetic profile, safety, and efficacy of sarilumab in children aged two to 17 years old with polyarticular juvenile idiopathic arthritis, KEVZARA was submitted for approval to the FDA and to the European Medical Agency (EMA), respectively in August and November 2023. The extension of indication is further supported by extrapolation of efficacy data in adults with rheumatoid arthritis to the pediatric population with polyarticular juvenile idiopathic arthritis.
A repeat dose-finding Phase 2 study evaluating KEVZARA in children and adolescents with systemic juvenile idiopathic arthritis (SKYPS) is ongoing in Europe and in the rest of the world, as part of the pediatric investigation plan.
TZIELD is a CD3-directed monoclonal antibody acquired by Sanofi in 2023. TZIELD is the first and only disease modifying therapy in type 1 diabetes (T1D), a chronic autoimmune condition where the body’s ability to regulate blood sugar levels is impacted due to the gradual destruction of insulin producing beta cells by one’s own immune system. The product was approved by the FDA in November 2022 to delay the onset of Stage 3 T1D in adults and children eight years and older diagnosed with Stage 2 T1D.
The potential of TZIELD to slow the progression of Stage 3 T1D in newly diagnosed children and adolescents is currently being evaluated in a Phase 3 clinical program.
SARCLISA is a monoclonal antibody designed to selectively bind to CD38, a cell surface antigen expressed in multiple myeloma (MM) cancer cells and other hematological malignancies. SARCLISA is approved in several countries in combination settings for the treatment of adults with relapsed refractory multiple myeloma (RRMM).
SARCLISA is under assessment in combination with current standard and novel treatments across the MM treatment continuum:
a.the Phase 3 study (IMROZ) evaluating SARCLISA in combination with standard-of-care bortezomib, lenalidomide and dexamethasone (VRd) met its primary endpoint at a planned interim analysis for efficacy, demonstrating statistically significant improvement in progression-free survival compared with VRd alone in transplant-ineligible patients with newly diagnosed MM. These results will form the basis of a regulatory submission planned in 2024;
b.a Phase 3 study (IRAKLIA) investigating the development of a new subcutaneous formulation with an on-body device system in RRMM patients who have received at least one prior line of therapy is ongoing; this program is managed in collaboration with Blackstone Life Sciences;
c.a Phase 3 study (GMMG HDF) is evaluating SARCLISA in combination with lenalidomide, bortezomib and dexamethasone for induction and with lenalidomide for maintenance in patients with newly diagnosed MM. This clinical trial is being conducted in collaboration with the German-speaking Myeloma Multicenter Group (GMMG);
d.a Phase 3 trial (ITHACA) is assessing SARCLISA in combination with lenalidomide and dexamethasone versus lenalidomide and dexamethasone in patients with high-risk smoldering MM;
e.SARCLISA is under evaluation in new combinations with emerging novel mechanisms of action in a Phase 2 study for the treatment of patients with RRMM or newly diagnosed MM patients.
In 2023, the Phase 2 study evaluating SARCLISA in combination with chemotherapy in pediatric patients with relapsed/refractory acute lymphoblastic leukemia or acute myeloid leukemia (ISAKIDS) was terminated.
Finally, the Phase 2 study evaluating the safety, pharmacokinetics and efficacy of subcutaneous SARCLISA in adults with Warm Autoimmune Hemolytic Anemia (wAIHA), a rare blood disorder, was discontinued based on the preliminary results and portfolio prioritization.
REZUROCK is a selective ROCK2 (rho-associated coiled-coil–containing protein kinase-2) inhibitor that is approved by the FDA for the treatment of adult and pediatric patients aged 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
Clinical development of the drug has been pursued in 2023 in two additional indications:
a.A Phase 3 study is evaluating REZUROCK in combination with corticosteroids for the treatment of newly diagnosed moderate or severe chronic GVHD;
b.Another Phase 3 study is assessing REZUROCK on top of azithromycin and standard-of-care regimen of immunosuppression in adult participants who have evidence of progressive chronic lung allograft dysfunction (CLAD) despite azithromycin therapy.
NEXVIAZYME is a long-term enzyme replacement therapy targeting the mannose-6-phosphate receptor to effectively clear glycogen build-up in muscle cells. This enzyme replacement therapy is approved for the treatment of patients with Pompe disease, a rare disease caused by a deficiency of the enzyme acid alpha-glucosidase (GAA). In Europe, the treatment is marketed under the brand name NEXVIADYME.
SANOFI FORM 20-F 2023 | 35 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
A Phase 3 program is currently ongoing for the treatment of patients aged six months or younger who are affected by infantile onset Pompe disease.
FLUZONE: QIV HD is a higher dose quadrivalent influenza vaccine licensed in the US and in Europe for the elderly population, who do not respond as well to standard-dose influenza vaccines due to aging of the immune system (immuno-senescence). Safety and efficacy in the pediatric population will be assessed in a Phase 3 trial.
MENQUADFI: Sanofi’s Men ACYW-TT vaccine is our latest advance in meningococcal quadrivalent conjugate vaccination, designed to help protect an expanded patient group including infants and adolescents through older adults. MENQUADFI is already licensed in the US (for people aged two years and over), and in Europe and several other countries (for people aged 12 months and over). MENQUADFI has also received WHO pre-qualification for people aged 12 months and above. Phase 3 trials are ongoing to evaluate immunogenicity and safety in infants aged 6 weeks and above, and allow for extension of the age indication down to six weeks of age.
B.4.2. R&D Expenditures for late stage development
Expenditures on research and development amounted to €6,728 million in 2023 (€6,706 million in 2022), comprising €6,509 million in the Biopharma segment and €219 million in the Consumer Healthcare segment. Research and development expenditures represented approximately 15.6% of our net sales in 2023, stable compared to 2022.
Within the Biopharma segment, investments in Pharmaceuticals and Vaccines R&D represented respectively €3,816 million and €885 million in 2023 (€3,978 million and €724 million in 2022), mainly reflecting additional spend in Immunology and reduced spend in investment in oncology (Pharmaceuticals), while Vaccines made further investments in the mRNA vaccines platform. Medical Affairs investment and R&D Support functions came to €2,027 million in 2023 (€2,004 million in 2022), while cost control efforts continued overall.
B.5. Markets
A breakdown of revenues by business segment and by geographical region for 2023, 2022, and 2021 can be found at Note D.35. to our consolidated financial statements, included at Item 18. of this annual report.
The following market shares and ranking information are based on consolidated national pharmaceutical sales data (excluding vaccines), in constant euros, on a September 2023 MAT (Moving Annual Total) basis. The data are mainly from IQVIA MIDAS local sales audit supplemented by various other country-specific sources including Knobloch (Mexico), GERS (France) and HMR (Portugal).
B.5.1. Marketing and distribution
We have business operations in approximately 70 countries and our products are available in more than 180 countries. A breakdown of our aggregate net sales by geographical region is presented in “Item 5. Operating and Financial Review and Prospects — Results of Operations — Year Ended December 31, 2023 Compared with Year Ended December 31, 2022.” Sanofi is the seventh largest pharmaceutical company globally by sales. Our main markets in terms of net sales are respectively:
•United States: we rank ninth with a market share of 3.9%;
•Europe: we are the fourth largest pharmaceutical company in France where our market share is 5.5%, and we rank third in Germany with a 4.2% market share; and
•other countries: we are ranked sixteenth in Japan with a market share of 1.9%, and ninth in China with a market share of 1.4%.
Although specific distribution patterns vary by country, we sell prescription drugs primarily to wholesale drug distributors, independent and chain retail drug outlets, hospitals, clinics, managed-care organizations and government institutions. Some products in Rare Diseases and Oncology may also be sold directly to physicians. With the exception of Consumer Healthcare products, our drugs are ordinarily dispensed to patients by pharmacies upon presentation of a doctor’s prescription. Our Consumer Healthcare products are also sold and distributed through e-commerce, which is a growing trend in consumer behavior. Our vaccines are sold and distributed through multiple channels including physicians, pharmacies, hospitals, private companies and distributors in the private sector, and governmental entities and non-governmental organizations in the public and international donor markets.
We use a range of channels from in-person to digital to disseminate information about and promote our products among healthcare professionals, ensuring that the channels not only cover our latest therapeutic advances but also our established prescription products, which satisfy patient needs in some therapy areas. We regularly exhibit at major medical congresses. In some countries, products are also marketed directly to patients by way of television, radio, newspapers and magazines, and digital channels (such as the Internet). National education and prevention campaigns can be used to improve patients’ knowledge of their conditions.
Our sales representatives, who work closely with healthcare professionals, use their expertise to promote and provide information on our drugs. They represent our values on a day-to-day basis and are required to adhere to a code of conduct and to internal policies on which they receive training.
Although we market most of our products through our own sales forces, we have entered into and continue to form partnerships to co-promote/co-market certain products in specific geographical areas. Our major alliances are detailed at “Item 5. Operating and Financial Review and Prospects — Financial Presentation of Alliances.” See also “Item 3. Key Information — D. Risk Factors — We rely on third parties for the discovery, manufacture and marketing of some of our products.”
36 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
B.5.2. Competition
The pharmaceutical industry continues to experience significant changes in its competitive environment.
There are four primary types of competition in the prescription pharmaceutical market:
•competition among pharmaceutical companies to research and develop new patented products or address unmet medical needs;
•competition among different patented pharmaceutical products for the same therapeutic indication;
•competition among original and generic products or original biological products and biosimilars, at the end of regulatory exclusivity or patent protection; and
•competition among generic or biosimilar products.
Generics manufacturers who have received all necessary regulatory approvals for a product may decide to launch a generic version before the patent expiry date, even in cases where the owner of the original product has already commenced patent infringement litigation against the generics manufacturer. Such launches are said to be “at risk” for the promoter of the generic product because it may be required to pay damages to the owner of the original product in the context of patent infringement litigation; however, such launches may also significantly impair the profitability of the pharmaceutical company whose product is challenged.
Drug manufacturers may also face intra-product competition through parallel trading, or parallel importation or reimportation, where permitted. This may take place when drugs sold in a foreign market under the same brand name as in a domestic market are permitted to be imported into that domestic market by parallel traders or importers, who may repackage or resize the original product or sell it through alternative channels such as mail order or the Internet. This situation is of particular relevance to the EU with its common market, where such practices have been encouraged by the current regulatory framework. Parallel traders or importers take advantage of the price differentials between markets arising from factors including sales costs, market conditions (such as intermediate trading stages), tax rates, or national regulation of prices.
Finally, pharmaceutical companies face illegal competition from substandard and falsified drugs. The WHO estimates that falsified products account for 10% in low- and middle-income countries. All therapeutic areas are affected, including vaccines.
Worldwide, falsified products are an issue in part due to the exponential increase in Internet connectivity of those engaged in the manufacture, distribution and supply of substandard and falsified medical products.
Similar types of competition apply in CHC.
In Vaccines, there are two primary types of competition:
•competition among vaccine companies to research and develop new patented products, including new vaccines originated from disruptive technologies (such as the emergence of mRNA vaccines to fight the COVID virus in 2020), or address unmet medical needs; and
•competition among different patented (or non-patented) vaccine products marketed for the same therapeutic indication.
Generics and biosimilars are not an issue in vaccines at present, since vaccines are still mostly produced from proprietary viral or bacterial strains. As with pharmaceutical drugs, vaccine manufacturers can face competition through parallel trading. However, the extent of such practices is limited by the need for cold chain distribution of vaccines, and by the fact that vaccines are sold and administered through pharmacies or dispensing physicians.
B.5.3. Regulatory framework
The pharmaceutical and health-related biotechnology sectors are highly regulated. National and supranational health authorities administer a vast array of legal and regulatory requirements that dictate pre-approval testing (including testing in human subjects) and quality standards to maximize the safety and efficacy of a new medical product. These authorities also regulate product labeling, manufacturing, importation/exportation, safety reporting and marketing, as well as mandatory post-approval requirements and commitments.
The submission of an application to a regulatory authority does not guarantee that a license or approval to market will be granted. Furthermore, each regulatory authority may impose its own requirements during product development or during the application review. It may refuse to grant approval or require additional data before granting approval, even in circumstances in which the same product has already been approved in other countries. Regulatory authorities also have the authority to request product recalls and product withdrawals, to impose penalties for violations of regulations, and ultimately the ability to revoke product licensure or approval.
Product review and approval can vary from six months or less to several years from the date of application submission depending upon the country and regulatory jurisdiction. Factors such as the quality of data and evidence, the review procedures, the nature of the product and the condition to be treated, play a major role in the length of time a product is under review, and whether or not the product is ultimately licensed or approved.
In the EU, there are three main procedures for applying for marketing authorization:
•the centralized procedure is mandatory for drugs derived from biotechnologies; new active substances designed for human use to treat HIV, viral diseases, cancer, neurodegenerative diseases, diabetes and auto-immune diseases; orphan drugs; and innovative products for veterinary use. When an application for human use is submitted to the EMA, the scientific evaluation of the application is carried out by the EMA’s CHMP and a scientific opinion is prepared. This opinion is sent to the EC, which adopts the final decision and grants an EU marketing authorization. Such a marketing authorization is valid throughout the EU, and the drug may be marketed within all EU Member States;
SANOFI FORM 20-F 2023 | 37 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
•if a company is seeking a national marketing authorization in more than one Member State, two procedures are available to facilitate the granting of harmonized national authorizations across Member States: the mutual recognition procedure or the decentralized procedure. Both procedures are based on the recognition by national competent authorities of a first assessment performed by the regulatory authority of one Member State; and
•national authorizations are still possible, but are only for products intended for commercialization in a single EU Member State or for line extensions to existing national product licenses.
In the EU, vaccines are treated as pharmaceutical products, and therefore have to obtain marketing authorization under the centralized procedures described above.
Generic products are subject to the same marketing authorization procedures. A generic product must contain the same active medicinal substance as a reference product approved in the EU. Generic applications are abridged: generic manufacturers only need to submit quality data and demonstrate that the generic drug is “bioequivalent” to the originator product (i.e., performs in the same manner in the patient’s body), but do not need to submit safety or efficacy data since regulatory authorities can refer to the reference product’s dossier.
Another relevant aspect in the EU regulatory framework is the “sunset clause” under which any marketing authorization ceases to be valid if it is not followed by marketing within three years, or if marketing is interrupted for a period of three consecutive years.
In the US, the FDA has broad regulatory jurisdiction over all pharmaceutical and biological products that are intended for sale and marketing in the US. To commercialize a new drug or biologic in the US, an applicant must submit to the FDA a New Drug Application (NDA) under the Food, Drug and Cosmetic (FD&C) Act or a Biologics License Application (BLA) under the Public Health Service (PHS) Act, respectively, for filing and pre-market review. Specifically, the FDA must decide whether the product is safe and effective for its proposed use; if the benefits of the product outweigh its risks; whether the product labeling is adequate; and if the manufacturing of the product and the controls used for maintaining quality are adequate to preserve the product’s identity, strength, quality and purity. Based upon this review, the FDA can stipulate post-approval commitments and requirements. Changes to an approved product, including but not limited to a new indication, require submission of a supplemental NDA (sNDA) for a drug or a supplemental BLA (sBLA) for a biological product.
The FD&C Act provides another option for NDA product approval via the 505(b)(2) pathway. This 505(b)(2) application contains full reports of investigations of safety and effectiveness but at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. For example, under the 505(b)(2) pathway an applicant may seek to rely on literature or earlier FDA findings of safety and effectiveness for approved drugs.
Sponsors wishing to market a generic drug or biosimilar product can file an Abbreviated NDA (ANDA) under 505(j) of the FD&C Act or abbreviated BLA (aBLA) under 351(k) of the PHS Act, respectively.
•ANDA applications are “abbreviated” because they are generally not required to include data to establish safety and efficacy but need to demonstrate that their product is bioequivalent (i.e., performs in humans in the same manner as the originator’s product) to a reference product. Consequently, the length of time and cost required for development of generics can be considerably less than for the innovator’s drug. The ANDA pathway in the US can only be used for generics of drugs that can be referenced as having been approved under the FD&C Act.
•aBLA applications contain evidence that the potential product is biosimilar to a reference product already approved by the FDA. A biosimilar is highly similar to and has no clinically meaningful differences in terms of safety, purity, and potency (i.e., safety and effectiveness) from an FDA-approved reference product. The abbreviated approval pathway for biosimilars was created to help reduce the time and cost of development of biologics without compromising safety and effectiveness. Consequently, the length of time and cost required for development of biosimilars may be less than for the innovator’s reference product.
In Japan, the entire process of approval review from review-related inspections and clinical trial consultation to review for the drugs approved by the Ministry of Health, Labour and Welfare (MHLW) is undertaken by the Pharmaceuticals and Medical Devices Agency (PMDA). The PMDA conducts first scientific review of the NDA submitted, assessing particularly the safety, efficacy and quality of the product or medical device proposed. Results of this primary evaluation are then submitted to the PMDA’s external experts. After a second evaluation based on the external experts’ feedback, a report is provided; the Pharmaceutical Affairs and Foods Sanitation Council (PAFSC) – one of the councils organized under the MHLW as advisory commission – is consulted, and advises the MHLW on final approvability.
For Japanese registrations, clinical data for Japanese patients are necessary. The regulatory authorities can require local clinical studies, though they also accept multi-regional studies including Japan. In some cases, bridging studies have been conducted to verify extrapolability of foreign clinical data to Japanese patients and to obtain data to determine the appropriateness of the dosages for Japanese patients.
The MHLW may require additional post-approval studies (Phase 4) for some specific cases, to further evaluate safety and/or to gather information on the use of the product under specified conditions. In approval of new drugs, new indications, new dosages or new administrations, the re-examination period is determined by the MHLW. Post-marketing information on a drug for the predetermined period after approval is collected to reconfirm its efficacy, safety and quality at the end of the period. This collection process involves both post-marketing surveillance (PMS), which is a non-interventional study, and post-marketing clinical trials.
For generic products, the data necessary for filing are similar to EU and US requirements. Companies only need to submit quality data, and data demonstrating bioequivalence to the originator product, unless the drug is biopharmaceutical. Common Technical Document (CTD) submission for generics has been mandatory since March 2017.
38 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) was created in 1990 and reformed in 2015.
The ICH currently includes 21 Members and 37 Observers. Harmonization is achieved through the development of ICH Guidelines via a process of scientific consensus with regulatory and industry experts working side-by-side.
In addition to the joint efforts, Free Trade Agreements (FTAs) have proven to be one of the best ways to open up foreign markets to exporters and to allow for discussions on harmonization topics for regulatory authorities. Some agreements, such as the Agreement on Trade Related Aspects of Intellectual Property Rights (TRIPS), are international in nature, while others are between specific countries. The requirements of many countries (including Japan and several EU Member States) to negotiate selling prices or reimbursement rates for pharmaceutical products with government regulators significantly extend the time to market entry beyond the initial marketing approval. While marketing authorizations for new pharmaceutical products in the EU have been largely centralized within the EC in collaboration with the EMA, pricing and reimbursement remain a matter of national competence.
For a description of risks relating to the regulatory environment in which we operate, refer to “— Item 3.D. Risk Factors — Product liability claims could adversely affect our business, results of operations and financial condition.”
B.5.4. Pricing & reimbursement
We are operating in a highly volatile and competitive market access and launch environment globally. The pharmaceuticals and health biotech industries are highly regulated (see “— Item 3. Key information — D. Risk Factors — Our activities (including our products and manufacturing activities) are subject to significant government regulations and regulatory approvals, which are often costly and could result in adverse consequences to our business if we fail to anticipate the regulations, comply with them, maintain the required approvals, and/or adapt to changes in applicable regulations”).
Faced with mounting budget pressure, governments and payers are using several drug price control policies such as price referencing for imported drugs, increased patient co-payments, restrictive formularies, prescribing guidelines, tendering procedures, generic and biosimilar substitution, and medico-economic evaluations of healthcare products.
In addition, companies are required to demonstrate the value and cost-effectiveness of their products throughout their life cycle (e.g. comparative efficacy studies, real-world patient data, budget modelling) to meet diverse and stringent payer evidence requirements, raising the bar for market entry in many countries.
Despite numerous pricing and reimbursement challenges, payers and regulators remain committed to providing access to new innovative therapies, with greater emphasis on real-world evidence (RWE).
These trends are likely to continue in the coming year amid macroeconomic and geopolitical headwinds.
United States
Overview of the US health insurance system:
Commercial insurance is offered widely as part of employee benefit packages and is the main source of employee access to subsidized healthcare. Some individuals purchase private health plans directly or through marketplaces established under the Affordable Care Act, while publicly subsidized programs provide coverage for retirees, the indigent, the disabled, uninsured children, and serving or retired military personnel. Double coverage can occur.
Commercial insurance includes:
•Managed Care Organizations (MCOs), which combine the functions of health insurance, delivery of care, and administration. MCOs use specific provider networks and specific services and products. There are four primary types of managed care plans: Health Maintenance Organizations (HMOs), Preferred Provider Organizations (PPOs), Exclusive Provider Organizations (EPOs), and Point of Service (POS) plans; and
•Pharmacy Benefit Managers (PBMs), which serve as intermediaries between insurance companies, pharmacies and manufacturers to negotiate rebates and discounts on formulary placement for commercial health plans, self-insured employer plans, Medicare Part D plans, and federal and state government employee plans.
Government insurance includes:
•Medicare, which provides health insurance for people aged 65 and older and for people with permanent disabilities. The basic Medicare scheme (Part A) provides hospital insurance only, and the vast majority of beneficiaries purchase additional coverage through Part B, Part C and/or Part D. Part D enables Medicare beneficiaries to obtain outpatient drug coverage. Almost two-thirds of all Medicare beneficiaries have enrolled in Part D plans;
•Medicaid, which provides health insurance for low-income families, certain qualified pregnant women and children, individuals receiving supplemental security income, and other eligible persons determined on a state-by-state basis; and
•TRICARE, which provides health insurance for uniformed service members, retirees, and their families including comprehensive healthcare, prescription and dental coverage.
In August 2022, the Inflation Reduction Act (IRA) was signed into law by President Biden. This legislation contains three core provisions related to drug pricing which are to be phased between 2022 and 2026: Medicare drug price negotiation, inflation penalties on list price increases, and Medicare Part D redesign. Importantly, all of the policy changes enacted under the IRA apply to the two Medicare programs that cover separately reimbursable prescription drugs: Part B (physician-administered outpatient medicines) and Part D (self-administered medicines).
SANOFI FORM 20-F 2023 | 39 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Under the IRA, for the first time ever, the federal government will negotiate the prices of selected drugs with high budget impact on Medicare Part B and Part D, starting with 10 drugs with high Part D expenditures, whose negotiated pricing will take effect in 2026; up to 15 more high-Part D expenditure drugs, whose negotiated pricing will take effect in 2027; and up to 15 more drugs with high expenditures under Parts B and/or D drugs; whose negotiated pricing will take effect in 2028. Up to 20 more drugs with high Part B and/or Part D expenditures will be selected for negotiated pricing with effect in 2029 and each subsequent year. No Sanofi product is among the initial 10 drugs that the government has identified for negotiations in 2023 and 2024 and for which negotiated pricing will take effect in 2026.
The IRA also imposes inflation penalties applied to Medicare Parts B and D if prices rise faster than inflation (based on the consumer price index, CPI), beginning in October 2022 for Part D and January 2023 for Part B.
Other measures of the Act redesign the Medicare Part D benefit, including a monthly $35 insulin cap in 2023 and an annual $2,000 out-of-pocket (OOP) spending cap in 2025 for Medicare beneficiaries.
Altogether, the IRA’s prescription drug-related provisions are expected to reduce the federal deficit by about $237 billion through 2031 according to estimates from the Congressional Budget Office (CBO). The new legislation is also likely to have a negative impact on industry revenue growth and future innovation, though significant uncertainties remain over the process and methods of Medicare price negotiations.
In addition to the IRA, the industry is exposed to increased price pressure from the continuing vertical integration and consolidation within the US health insurance market. With the three largest PBM group purchasing organizations (GPOs) (i.e. Ascent, Zinc and Emisar) now covering over 85% of US prescription drug claims, consolidation has led to more aggressive formulary utilization management controls, resulting in the GPOs having strong bargaining power for negotiating discounted prices, thereby adversely impacting manufacturer’s revenues.
China
China continues to pursue reforms towards Healthy China 2030. Healthcare is one of the growth priorities under the country’s fourteenth Five-Year Plan (2021-2025), with policies aimed at addressing a large and increasing burden of disease (especially cancer, diabetes and cardiovascular diseases), while balancing access to innovation and costs.
China continues to improve regulatory timelines. For example, DUPIXENT received approval for the treatment of adults with moderate-to-severe atopic dermatitis in June 2020, within six months of filing through an accelerated review process.
Pricing pressure is expected to continue and further intensify as a growing number of products are subject to National Reimbursement Drug List (NRDL) price negotiations and volume-based procurement (VBP) tenders, with the lowest price prevailing to compete with local champions.
Access to innovative therapies has been accelerating in the last five years, fueled by annual NRDL updates, albeit with steep price cuts across therapy areas. According to the National Healthcare Security Administration (NHSA), 126 new drugs were added to the National Reimbursement Drug List (NRDL) in December 2023, with an average price reduction of 61.7% in line with recent years. Over the past six years, 744 drugs have been added to the NRDL, bringing the total number of covered medicines to 3,088 (1,698 Western drugs and 1,390 traditional Chinese medicines).
Further expansion of the VBP policy will drive down the prices of a growing number of products, with more than 500 drugs targeted for inclusion by 2025.
Europe
In Europe, health systems have been under mounting budget pressures. These remain high in the wake of COVID-19, with rising inflation and slow economic growth translating into an acceleration of cost-containment policies in major EU markets.
The industry is also facing high uncertainty in the context of the new Pharmaceutical Strategy for Europe, including numerous policy changes to EU pharmaceutical legislation, health technology assessment (HTA) and joint procurement, among others. These reforms will have a far-reaching impact on the pricing and market access landscape in Europe and will likely pose additional challenges for industry.
On April 26, 2023, the EC adopted a proposal for a new Directive and a new Regulation, which represent the largest pharmaceutical reform in over 20 years. The most concerning proposals under review relate to the potential reduction of market exclusivity for orphan medicines; greater transparency of R&D costs; faster availability of generics and biosimilars; and more stringent obligations for the supply of medicines. If passed in its current form, the legislation could have a detrimental impact on access, innovation and competitiveness in Europe.
The Commission also adopted the new EU Health Technology Assessment (HTA) regulation in December 2021, after years of joint work between Member States. The regulation will introduce EU-level joint scientific consultations (JSCs) and joint clinical assessments (JCAs) that will serve as the basis for national value assessments and price negotiations. The joint HTA process will be implemented in a phased approach starting in January 2025 with oncology medicines and advanced therapy medicinal products (ATMPs), before expanding to include orphan drugs in 2028 and all EMA centrally authorized medicinal products by 2030.
Additional uncertainty is caused by the evolving joint procurement approaches of vaccines and medicines at EU level, building on the lessons learned during the COVID-19 pandemic. The Commission may promote the use of EU joint procurement as a means to improve affordability and security of supply, which is already occurring to some extent with existing cross-country collaboration initiatives such as the BeNeLuxA alliance, the FINOSE collaboration and the Nordic Pharmaceutical Forum.
40 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Pricing and reimbursement pressures are being felt in other regions and countries around the globe.
To overcome these challenges, we are developing tailored market access strategies early in the drug development process, engaging in dialogue with payers and multiple stakeholders throughout the lifecycle, based on a thorough understanding of evolving market dynamics.
B.6. Patents, intellectual property and other rights
B.6.1. Patents
Patent protection
We own a broad portfolio of patents, patent applications and patent licenses worldwide. These patents are of various types and may cover: active ingredients; pharmaceutical formulations; product manufacturing processes; intermediate chemical compounds; therapeutic indications/methods of use; technology platforms; delivery systems; digital applications; and enabling technologies, such as assays.
Patent protection for individual products typically extends for 20 years from the patent filing date in countries where we seek patent protection. A substantial part of the 20-year life span of a patent on a new molecule (small molecule or biologic) has generally already passed by the time the related product obtains marketing authorization. As a result, the effective period of patent protection for an approved product’s active ingredient is significantly shorter than 20 years. In some cases, the period of effective protection may be extended by procedures established to compensate regulatory delay in Europe (via Supplementary Protection Certificate or SPC), in the US (via Patent Term Extension or PTE), and in Japan (PTE).
The protection a patent provides to the related product depends upon the type of patent and its scope of coverage, and may also vary from country to country.
In Europe, applications for new patents may be submitted to the European Patent Office (EPO). A European Patent (EP) may cover 39 European Patent Convention Member States, including all Member States of the EU. The granted EP establishes corresponding national patents with uniform patent claims among the Member States. Since June 1, 2023, it has been possible to obtain a Unitary Patent, currently covering 17 states, which are all Member States of the EU and of the European Patent Convention.
We monitor our competitors and vigorously seek to challenge patent infringers when such infringement would negatively impact our business objectives. See “Item 8. — A. Consolidated Financial Statements and Other Financial Information — Information on Legal or Arbitration Proceedings — Patents” of this annual report.
The expiration or loss of a patent covering a new molecule, typically referred to as a compound patent, may result in significant competition from generic or biosimilar products and can result in a dramatic reduction in sales of the original branded product (see “Item 3. Key Information — D. Risk Factors”). In some cases, it is possible to continue to benefit from a commercial advantage through product manufacturing trade secrets or other types of patents. Certain categories of products, such as traditional vaccines and insulin, were historically relatively less reliant on patent protection and may in many cases have no patent coverage. It is nowadays increasingly frequent for novel vaccines also to be patent protected.
Regulatory exclusivity
In some markets, including the EU and the US, many of our pharmaceutical products may also benefit from multi-year regulatory exclusivity periods, during which a generic or biosimilar competitor may not rely on our clinical trial and safety data in its drug application. This exclusivity operates independently of patent protection and may protect the product from generic or biosimilar competition even if there is no patent covering the product.
In the US, the FDA may not grant final marketing authorization to a generic competitor for a New Chemical Entity (NCE) until the expiration of the regulatory exclusivity period (five years) that commences upon the first marketing authorization of the reference listed drug. Significant new uses of existing NCEs, including new indications, may qualify for an additional three years of regulatory exclusivity if certain conditions are met. In the US, a different regulatory exclusivity paradigm applies to biological drugs. The BPCIA (Biologics Price Competition and Innovation Act) provides that the FDA may not approve a biosimilar application until 12 years after the date on which the reference product was first licensed.
In the EU, regulatory exclusivity is available in two forms: data exclusivity and marketing exclusivity. Generic or biosimilar drug applications will not be accepted for review until eight years after the first marketing authorization (data exclusivity). This eight-year period is followed by a two-year period during which generics or biosimilars cannot be marketed (marketing exclusivity). The marketing exclusivity period can be extended to three years if, during the first eight-year period, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which are deemed to provide a significant clinical benefit over existing therapies. This is known as the “8+2+1” rule.
In Japan, the regulatory exclusivity period varies. In general, the regulatory exclusivity period is of four to six years for drugs for a specific use, and for medicinal products with new indications or with new dosages; eight years for drugs containing a new chemical entity; ten years for orphan drugs, and for new drugs requiring pharmaco-epidemiological study; six to eight years for Innovative drugs (“SAKIGAKE” products), and for orphan drugs with a new ethical combination or new mode of administration; and six years for other medicinal products, such as new prescription combination drugs or drugs requiring a new mode of administration.
SANOFI FORM 20-F 2023 | 41 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Pediatric extension
In the US and the EU, under certain conditions, it is possible to extend a product’s regulatory exclusivity for an additional period of time by providing data on pediatric studies.
In the US, under certain conditions of the Hatch-Waxman Act, it may result in the FDA extending regulatory exclusivity and patent life by six months, to the extent these protections have not already expired (the so-called “pediatric exclusivity”).
In Europe, a regulation on pediatric medicines provides for pediatric research obligations with potential associated rewards including extension of supplementary patent protection and six-month regulatory exclusivity for pediatric marketing authorization (for off-patent medicinal products).
In Japan, there is no pediatric research extension of patent protection for patented medicinal products. However, regulatory exclusivity may be extended from eight to ten years.
Orphan drug exclusivity
Under certain conditions, orphan drug exclusivity may be granted in the US to drugs intended to treat rare diseases or conditions. Orphan drug exclusivities also exist in the EU and Japan.
Emerging markets
One of the main limitations on our operations in emerging market countries is the lack of effective intellectual property protection or enforcement for our products, which frequently do not provide non-patent exclusivity for innovative products. While the situation has gradually improved, the lack of protection for intellectual property rights or the lack of robust enforcement poses difficulties in certain countries. Additionally, in recent years and especially during the pandemic, a number of countries have waived or threatened to waive intellectual property protection for specific products, for example through compulsory licensing of generics. See “Item 3. Key Information — D. Risk Factors — Risks Relating to Sanofi’s Structure and Strategy — The globalization of our business exposes us to increased risks in specific areas”.
Product overview
We summarize below the intellectual property coverage (in some cases through licenses) of our most significant marketed products in terms of sales, in our major markets. In the discussion of patents below, we focus on active ingredient patents (compound patents) and, in the case of NCEs, on any later filed patents listed as applicable in the FDA’s list of Approved Drug Products with Therapeutic Equivalence Evaluations (the “Orange Book”) or in its foreign equivalents. For biologics, the Orange Book listing does not apply.
These patents or their foreign equivalents tend to be the most relevant in the event of an application by a competitor to produce a generic or a biosimilar version of one of our products (see “— Challenges to Patented Products” below). In some cases, products may also benefit from pending patent applications or from patents not eligible for Orange Book listing (in the case of NCEs for example, patents claiming industrial processes). In each case below, we specify whether the active ingredient is claimed by an unexpired patent. Where patent terms have been extended to compensate for US Patent and Trademark Office (USPTO) delays in patent prosecution (Patent Term Adjustment – PTA) or for other regulatory delays, the extended dates are indicated below. The US patent expirations presented below reflect USPTO dates, and also reflect six-month pediatric extensions when applicable. Where patent terms have expired we indicate such information and mention whether generics or biosimilars are on the market.
We do not provide later filed patent information relating to formulations already available as an unlicensed generic. References below to patent protection in Europe indicate the existence of relevant patents in most major markets in the EU. Specific situations may vary by country.
We additionally set out any regulatory exclusivity from which these products continue to benefit in the US, EU or Japan. Regulatory exclusivities presented below incorporate any pediatric extensions obtained. While EU regulatory exclusivity is intended to be applied throughout the EU, in some cases Member States have taken positions prejudicial to our exclusivity rights.
42 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
| United States | European Union | Japan | |||||||||
AUBAGIO (teriflunomide) | Compound: expired | Compound: expired | Compound: expired | ||||||||
Later filed patent: coverage ranging through April 2027 with SPC* | Later filed patent: coverage ranging through March 2024 | ||||||||||
Generics on the market | Regulatory exclusivity: August 2024 Generics on the market | ||||||||||
ALPROLIX (eftrenonacog alfa) | Use: March 2028 with PTA* and PTE* | Compound: May 2024 (May 2029 with SPC* in most EU countries) | Compound: May 2024 (February 2026 with PTE*) | ||||||||
| Later filed patents: coverage ranging through December 2037 (pending) | Later filed patents: coverage ranging through December 2037 (pending) | Later filed patents: coverage ranging through December 2037 (pending) | |||||||||
Regulatory exclusivity: March 2026 | Regulatory exclusivity: May 2028 | ||||||||||
ALTUVIIIO (efanesoctocog alfa) | Compound: February 2037 with PTA* (PTE* pending) | Compound: January 9, 2035 | Compound: January 9, 2035 (PTE* pending) | ||||||||
Later filed patents: coverage ranging through March 2043 (pending) | Later filed patents: coverage ranging through March 2043 (pending) | Later filed patents: coverage ranging through March 2043 (pending) | |||||||||
Regulatory exclusivity: February 2035 | Regulatory exclusivity: September 2031 | ||||||||||
| BEYFORTUS (nirsevimab-alip) | Compound: January 2035 (PTE* pending) | Compound: January 2035 (SPC* in process of being granted across EU countries) | Compound: January 2035 | ||||||||
Later filed patent: coverage ranging through September 2042 (pending) | Later filed patent: coverage ranging through September 2042 (pending) | Later filed patent: coverage ranging through September 2042 (pending) | |||||||||
Regulatory exclusivity: July 2035 | Regulatory exclusivity: November 2032 | ||||||||||
| CEREZYME (imiglucerase) | Patent: expired | Patent: expired | Patent: expired | ||||||||
DUPIXENT (dupilumab) | Compound: March 2031 with PTE* | Compound: September 2032 with SPC* (March 2033 with pediatric extension of SPC* in process of being granted across EU countries) | Compound: May 2034 with PTE* | ||||||||
Later filed patents: coverage ranging through December 2042 (pending) | Later filed patents: coverage ranging through January 2042 (pending) | Later filed patents: coverage ranging through October 2041 (pending) | |||||||||
| Regulatory exclusivity: March 2029 | Regulatory exclusivity: September 2028 | Regulatory exclusivity: January 2026 | |||||||||
ELOCTATE (efmoroctocog alfa) | Compound: June 2028 with PTA* and PTE* | Use: May 2024 (November 2029 with SPC* in most EU countries) | Compound: May 2024 (August 2026 with PTE*) | ||||||||
| Later filed patents: coverage ranging through December 2037 (pending) | Later filed patents: coverage ranging through December 2037 (pending) | Later filed patents: coverage ranging through December 2037 (pending) | |||||||||
Regulatory exclusivity: June 2026 | Regulatory exclusivity: November 2025 | ||||||||||
ENJAYMO (sutimlimab) | Compound: November 2033 (PTE* pending) | Compound: November 2033 (SPC* in process of being granted across EU countries) | Compound: November 2033 (PTE* pending) | ||||||||
Later filed patents: Coverage ranging through March 2042 (pending) | Later filed patents: Coverage ranging through March 2042 (pending) | Later filed patents: Coverage ranging through March 2042 (pending) | |||||||||
Regulatory exclusivity: February 2034 | Regulatory exclusivity: November 2032 | Regulatory exclusivity: June 2032 | |||||||||
FABRAZYME (agalsidase beta) | Patent: expired | Patent: expired | Patent: expired | ||||||||
| Regulatory exclusivity: March 2028 pediatric indication (ages 2-8 with confirmed Fabry disease) | Generics/biosimilars on the market | ||||||||||
| JEVTANA (cabazitaxel) | Compound: Expired Later filed patents: coverage ranging through October 2030 | Compound: expired Later filed patents: coverage ranging through October 2030 Generics on the market | Compound: expired Later filed patents: coverage ranging through November 2030 | ||||||||
LANTUS (insulin glargine) | Compound: expired | Compound: expired | Compound: expired | ||||||||
Generics/biosimilars on the market | Generics/biosimilars on the market | Generics/biosimilars on the market | |||||||||
LOVENOX (enoxaparin sodium) | Compound: expired | Compound: expired | Compound: expired | ||||||||
Generics on the market | Biosimilars on the market | ||||||||||
LUMIZYME/MYOZYME (alglucosidase alfa) | Compound: expired | Compound: expired | Compound: expired | ||||||||
NEXVIAZYME / NEXVIADYME (avalglucosidase alfa) | Compound: March 2030 with PTA* (PTE* pending) | Compound: January 2028 (SPC* granted in BG, ES, FR, GR, HU, NL, SI and SE; pending in many other EU countries) | Compound: December 2032 with PTE* | ||||||||
Later filed patents: coverage ranging through May 2032 | Later filed patents: coverage ranging through May 2032 | Later filed patents: coverage ranging through May 2032 | |||||||||
Regulatory exclusivity: pending | Regulatory exclusivity: no (a) | Regulatory exclusivity: September 2031 | |||||||||
PLAVIX (clopidogrel bisulfate) | Compound: expired | Compound: expired | Compound: expired | ||||||||
| Generics on the market | Generics on the market | Generics on the market | |||||||||
SANOFI FORM 20-F 2023 | 43 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
| United States | European Union | Japan | |||||||||
TOUJEO (insulin glargine) | Compound: expired | Compound: expired | Compound: expired | ||||||||
Later filed patents: coverage ranging through May 2031 | Later filed patents: coverage ranging through May 2031 | Later filed patents: coverage ranging through July 2033 with PTE* | |||||||||
XENPOZYME (olipudase alfa) | Use: March 2031 with PTA* (PTE* pending) | Use: August 2030 (SPC* in process of being granted across EU countries) | Use: August 2030 (PTE* pending) | ||||||||
Later filed patents: coverage ranging through 2043 (pending) | Later filed patents: coverage ranging through 2043 (pending) | Later filed patents: coverage ranging through 2043 (pending) | |||||||||
| Regulatory exclusivity: August 2034 | Regulatory exclusivity: June 2032 | Regulatory exclusivity: March 2030 | |||||||||
* PTE: Patent Term Extension. – SPC: Supplementary Protection Certificate. – PTA: Patent Term Adjustment.
(a) Subject to legal challenge before EU General Court.
Patents held or licensed by Sanofi do not in all cases provide effective protection against a competitor’s generic or biosimilarversion of our products. For example, notwithstanding the presence of unexpired patents, competitors launched generic versions of ALLEGRA in the US (prior to the product being switched to over-the-counter status) and MULTAQ in the EU.
We caution the reader that there can be no assurance that we will prevail when we assert a patent in litigation and that there may be instances in which Sanofi determines that it does not have a sufficient basis to assert one or more of the patents mentioned in this report, for example in cases where a competitor proposes a formulation not appearing to fall within the claims of our formulation patent; a salt or crystalline form not claimed by our composition of matter patent; or an indication not covered by our method of use patent. See “Item 3. Key Information — D. Risk Factors — Risks Relating to Legal and Regulatory Matters — We rely on our patents and other proprietary rights to provide exclusive rights to market certain of our products, and if such patents and other rights were limited, invalidated or circumvented, our financial results could be materially and adversely affected.”
As disclosed in Item 8. of this annual report, we are involved in significant litigation concerning the patent protection of a number of our products.
Challenges to patented products
— Abbreviated New Drug Applications (ANDAs)
In the US, generic companies have filed Abbreviated New Drug Applications (ANDAs) containing challenges to patents related to a number of our small molecule products. An ANDA is an application by a drug manufacturer to receive authority to market a generic version of another company’s approved product, by demonstrating that the purportedly generic version has the same properties (safety and other technical data) as the original approved product. As a result of regulatory protection of our safety and other technical data, ANDA applications are generally four years after FDA approval, and include a challenge to a patent listed in the FDA’s Orange Book. If the patent holder or licensee brings suit in response to the patent challenge within the statutory window, the FDA is barred from granting final approval to an ANDA during the 30 months following the expiry of the 5-year regulatory exclusivity (this bar is referred to in our industry as a “30-month stay”) unless, before the end of the 30 months, the parties reach settlement or a court decision has determined either that the ANDA does not infringe the listed patent or that the listed patent is invalid and/or unenforceable.
FDA approval of an ANDA after this 30-month period does not resolve outstanding patent disputes, but it does remove the regulatory impediments to a product launch by a generic manufacturer willing to take the risk of later being ordered to pay damages to the patent holder.
Accelerated ANDA-type procedures are potentially applicable to many, but not all, of the products we manufacture. See “— B.5.3. Regulatory Framework” and “- Regulation” above. We seek to defend our patent rights vigorously in these cases. Success or failure in the assertion of a given patent against a competing product is not necessarily predictive of the future success or failure in the assertion of the same patent. See “Item 3. Key Information — D. Risk Factors — Risks Relating to Legal and Regulatory Matters — We rely on our patents and other proprietary rights to provide exclusive rights to market certain of our products, and if such patents and other rights were limited, invalidated or circumvented, our financial results could be materially and adversely affected.”
— Section 505(b)(2) New Drug Applications in the US
Our products and patents are also subject to challenge by competitors via another abbreviated approval pathway, under section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act. This pathway allows for approval for a wide range of products, especially for those products that represent only a limited change from an existing approved drug. The 505(b)(2) pathway is distinct from the ANDA pathway, which allows for approval of a generic product based on a showing that it is equivalent to a previously approved product.
Similarly, entities wishing to market a generic biologic can utilize an abbreviated approval pathway established in the PHS Act. This §351(k) pathway enables an applicant to rely on a reference product sponsor’s data when seeking approval of a biological product shown to be biosimilar (highly similar with no clinically meaningful differences) or interchangeable with an FDA-licensed reference BLA product.
44 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
— Europe
In the EU, a generic drug manufacturer may only reference the data of the regulatory file for the original approved product after data exclusivity has expired. However, there is no patent listing system in Europe comparable to the Orange Book, which would allow the patent holder to prevent the competent authorities from granting marketing authorization by bringing patent infringement litigation prior to approval. As a result, generic products may be approved for marketing following the expiration of marketing exclusivity without regard to the patent holder’s rights. Nevertheless, in most of these jurisdictions once the competing product is launched, and in some jurisdictions even prior to launch (once launch is imminent), the patent holder may seek an injunction against such marketing if it believes its patents are infringed. See Item 8. of this annual report.
B.6.2. Trademarks – Domain names – Copyright
Our products are sold around the world under trademarks that we consider to be of material importance in the aggregate. Our trademarks help to identify our products and to protect the sustainability of our growth. We generate new assets (trademarks, domain names, service marks) when creating global brands for new, innovative products. We support the development of the product, from the branding of biotech platforms to the protection of service marks for patient support programs.
Trademarks are particularly important to the commercial success of our products and services in a competitive marketplace, providing a strong visibility and assuring patients of the origin of the products.
Domain names are essential to inform a range of communities about what we do. We also pay close attention to ensuring that no damage is done to our reputation online.
We aim to ensure that the product trademarks we submit to healthcare authorities to obtain marketing authorizations are available, and are protected. In certain cases, we may enter into a coexistence agreement with a third party that owns potentially conflicting rights in order to avoid any risk of confusion and to secure our rights.
Ongoing digitization emphasizes the importance of securing copyright protection for software and web layouts.
We monitor and defend our trademarks based on a specific policy designed to prevent counterfeiting, trademark infringement and/or unfair competition.
B.7. Production and raw materials
We have opted to manufacture the majority of our products in-house. There are three principal stages in our production process: the manufacture of active ingredients, the transformation of those ingredients into drug products or vaccines, and the final packaging.
Our general policy is to produce our key active ingredients and main drug products at our own plants in order to reduce our dependence on external suppliers. We also rely on third parties for the manufacture and supply of specific active ingredients, drug products and medical devices. Active ingredients are manufactured using raw materials sourced from suppliers who have been subject to rigorous selection and approval procedures, in accordance with international standards and our own internal directives. We have outsourced some of our production under supply contracts associated with acquisitions of products or businesses or with Sanofi plant divestitures, or to establish a local presence to capitalize on growth in emerging markets. Our pharmaceutical subcontractors follow our general quality and logistics policies, as well as meeting other criteria.
Our manufacturing activities require significant amounts of energy, the costs of which increased in 2022 and 2023 as a result of inflationary pressures and supply constraints due to the war in Ukraine. The Group uses supply contracts and hedging to mitigate those risks and costs. See “Item 3. Key Information — D. Risk Factors — Risks Relating to Our Business.”
We also obtain active ingredients from third parties under collaboration agreements. This applies in particular to the monoclonal antibodies developed with Regeneron.
Our production sites are divided into three categories:
•global sites, which serve all markets: located mainly in Europe, these facilities are dedicated to the manufacture of our active ingredients, injectable products, and a number of our main solid-form products;
•regional sites, which serve markets at regional level, giving us a strong industrial presence in emerging markets; and
•local sites, which serve their domestic market only.
Vaccines produces vaccines at various sites, with the main locations situated in France, the United States, Canada, India, Mexico and China. The pharmaceutical site at Le Trait (France) also contributes to Vaccines’ industrial operations by making its sterile filling facilities available for vaccine manufacturing.
All of our production facilities are good manufacturing practice (GMP) compliant, in line with international regulations.
Our main sites are approved by the FDA:
•the Specialty Care facilities in the United States (Framingham MA and Northborough MA), France (Lyon Gerland, Vitry-sur-Seine, Le Trait), Germany (Frankfurt), Ireland (Waterford) and Belgium (Geel);
•the General Medicines facilities in Germany (Frankfurt), France (Aramon, Sisteron, Ploermel, Ambarès and Tours), Italy (Anagni and Scoppito), Singapore (Jurong) and the United States (Ridgefield NJ);
•the CHC facilities in France (Compiègne) and the United States (Chattanooga TN); and
•the Vaccines facilities in France (Marcy l’Étoile, Le Trait, Val-de-Reuil and Neuville-sur-Saône), the United States (Swiftwater PA) and Canada (Toronto).
SANOFI FORM 20-F 2023 | 45 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Wherever possible, we seek to have multiple plants approved for the production of key active ingredients and our strategic finished products (this is the case with LOVENOX and DUPIXENT, for example).
More details about our manufacturing sites are given below at section “— D. Property, Plant and Equipment”.
B.8. Insurance and risk coverage
We are protected by five main insurance programs, relying not only on the traditional corporate insurance and reinsurance market but also on our direct insurance company, Carraig Insurance DAC (Carraig).
These five key programs cover Property & Business Interruption; General & Product Liability; Stock & Transit; loss and liability arising from cyber and digital risks; and Directors & Officers Liability.
Carraig participates in our coverage for various lines of insurance including Property, Stock & Transit, Cyber/Digital, and General & Product Liability. Carraig is run under the supervision of the Irish and European regulatory authorities, is wholly owned by Sanofi, and has sufficient resources to meet those portions of our risks that it has agreed to cover.
Carraig sets premiums for our entities at market rates. Claims are assessed using the traditional models applied by insurance and reinsurance companies, and the company’s reserves are regularly verified and confirmed by independent actuaries.
Our Property & Business Interruption program covers all our entities worldwide, in all territories where it is possible to use a centralized program operated by Carraig. By sharing risk between our entities, this approach enables us to set deductibles and cover appropriate to the needs of local entities before the market attachment point. It also incorporates a prevention program, including a comprehensive site visit schedule covering our production, storage, research and distribution facilities and standardized repair and maintenance procedures across all sites.
The Stock & Transit program protects all goods owned by Sanofi while they are in transit nationally or internationally whatever the means of transport, and all our inventories wherever they are located. Sharing risk between our entities through Carraig means that we can set deductibles at appropriate levels, for instance differentiating between goods that require temperature controlled distribution and those that do not. We have developed a prevention program with assistance from experts, implementing best practices in this area at our distribution sites.
Our Cyber/Digital insurance program protects our operations against loss originating from various sources, and against liability in respect of data security. Centralized through Carraig, the program enables us to set deductibles and cover appropriate to the needs of local entities before the market attachment point.
Our General & Product Liability program was renewed in 2023 for all our subsidiaries worldwide in all territories where it was possible to do so. For several years, insurers have been reducing product liability cover because of the difficulty of transferring risk for some products that have been subject to numerous claims.
The principal risk exposure for our pharmaceutical products is covered with low deductibles at country level, with a greater proportion of risk being retained. The level of risk self-insured by Sanofi (including via Carraig) before the market attachment point enables us to retain control over the management and prevention of risk. Our negotiations with third-party insurers and reinsurers are tailored to our specific risks. In particular, they allow for differential treatment of products in the development phase; for discrepancies in risk exposure between European countries and the United States; and for specific issues arising in certain jurisdictions. Coverage is adjusted every year to take account of the relative weight of new product liability risks such as those arising out of biotechnologies and new technology platforms.
Our cover for risks that are not specific to the pharma-biotech industry (general liability) is designed to address the potential impacts of our operations.
For all the insurance programs handled by Carraig, outstanding claims are covered by provisions for the estimated cost of settling all claims incurred but not paid at the balance sheet date, whether reported or not, together with all related claims handling expenses. Where there is sufficient data history from Sanofi or from the market for claims made and settled, management – with assistance from independent actuaries – prepares an actuarial estimate of our exposure to unreported claims for the risks covered. The actuaries perform an actuarial valuation of the company’s IBNR (Incurred But Not Reported) and ALAE (Allocated Loss Adjustment Expense) liabilities at year end. Two ultimate loss projections (based upon reported losses and paid losses, respectively) are computed each year using various actuarial methods including the Bornhuetter-Ferguson method; those projections form the basis for the provisions set.
The Directors & Officers Liability program protects all legal entities under our control, and their directors and officers. Carraig is not involved in this program.
We also operate other insurance programs, but these are of much lesser importance than those described above.
All our insurance programs are backed by highly-rated insurers and reinsurers and are intented to be designed in such a way that we can integrate most newly acquired businesses without interruption of cover. Our insurance cover has been designed to reflect our risk profile and the capacity available in the insurance market. By centralizing our major programs, we are able to provide what we believe to be excellent, cost effective protection.
46 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
B.9. Health, Safety and Environment
Our manufacturing and research operations are subject to increasingly stringent health, safety and environmental (HSE) laws and regulations. These laws and regulations are complex and rapidly changing, and Sanofi invests the necessary sums in order to comply with them. This investment, which aims to respect health, safety and the environment, varies from year to year.
Applicable environmental laws and regulations may require us to eliminate or reduce the effects of chemical substance discharge at our various sites. The sites in question may belong to Sanofi, and may be currently operational, or may have been owned or operational in the past. In this regard, Sanofi may be held liable for the costs of removal or remediation of hazardous substances on, under or in the sites concerned, or on sites where waste from activities has been stored, without regard to whether the owner or operator knew of or under certain circumstances caused the presence of the contaminants, or at the time site operations occurred the discharge of those substances was authorized.
As is the case for a number of companies in the pharmaceutical, chemical and intense agrochemical industries, soil and groundwater contamination has occurred at some of our sites in the past, and may still occur or be discovered at others. In Sanofi’s case, such sites are mainly located in the United States, Germany, UK and France. As part of a program of environmental surveys conducted over the last few years, detailed assessments of the risk of soil and groundwater contamination have been carried out at current and former Sanofi sites. In cooperation with national and local authorities, Sanofi regularly assesses the rehabilitation work required and carries out such work when appropriate. Remediation works have just been completed at Dagenham in the United Kingdom and Neuville in France. Long-term rehabilitation work is in progress or planned in Mount Pleasant, Portland in the United States; Frankfurt in Germany; Valernes and Limay in France; and on a number of sites divested to third parties and covered by contractual environmental guarantees granted by Sanofi.
We may also have potential liability for investigation and cleanup at several other sites. We have established provisions for the sites already identified and to cover contractual guarantees for environmental liabilities for sites that have been divested. In France specifically, we have provided the financial guarantees to the authorities as required under French regulations for environmental protection in connection with the operation of activities on French sites.
Potential environmental contingencies arising from certain business divestitures are described in Note D.22.d. to the consolidated financial statements. In 2023, Sanofi spent €33 million on rehabilitating sites previously contaminated by soil or groundwater pollution.
Due to changes in environmental regulations governing site remediation, our provisions for remediation obligations may not be adequate due to the multiple factors involved, such as the complexity of operational or previously operational sites, the nature of claims received, the rehabilitation techniques involved, the planned timetable for rehabilitation, and the outcome of discussions with national regulatory authorities or other potentially responsible parties, as in the case of multiparty sites. Given the long industrial history of some of our sites and the legacy obligations arising from the past involvement of Aventis in the chemical and agrochemical industries, it is impossible to quantify the future impact of these laws and regulations with precision. See “Item 3.D. Risk Factors — Environmental and safety risks of Our Industrial Activities.”
We have established, in accordance with our current knowledge and projections, provisions for cases already identified and to cover contractual guarantees for environmental liabilities relating to sites that have been divested. In accordance with Sanofi standards, a comprehensive review is carried out once a year on the legacy of environmental pollution. In light of data collected during this review, we adjusted our provisions to €493 million as of December 31, 2023 versus €526 million as of December 31, 2022. The terms of certain business divestitures, and the environmental obligations and retained environmental liabilities relating thereto, are described in Note D.22. to our consolidated financial statements.
To our knowledge, Sanofi did not incur any liability in 2023 for non-compliance with current HSE laws and regulations that could be expected to significantly jeopardize its activities, financial situation or operating income. We also believe that we are in substantial compliance with current HSE laws and regulations and that all the environmental permits required to operate our facilities have been obtained.
Regular HSE audits are carried out by Sanofi in order to assess compliance with standards (which implies compliance with regulations) and to initiate corrective measures (36 internal audits performed in 2023). Moreover, more than 100 specific visits were performed jointly with experts representing our insurers.
Sanofi has implemented a worldwide master policy on health, safety and environment to promote the health and well-being of the employees and contractors working on its sites and respect for the environment. We consider this master policy to be an integral part of our commitment to social responsibility. In order to implement this master policy, Sanofi key requirements have been drawn up in the key fields of HSE management, HSE leadership, safety in the workplace, process safety, occupational hygiene, health in the workplace and protection of the environment. However, despite these efforts, Sanofi may be unsuccessful in the implementation of its policy to reduce and mitigate the harmful effects of its activities on the health and safety of its employees, customers or the general public and on the environment more generally. See “Item 3.B. Risk Factors” for further information.
SANOFI FORM 20-F 2023 | 47 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Health
From the development of compounds to the commercial launch of new drugs, Sanofi research scientists continuously assess the effect of products on human health. This expertise is made available to employees through two committees responsible for chemical and biological risk assessment. Sanofi’s COVALIS (Comité des Valeurs Limites Internes Sanofi) Committee is responsible for the hazard determination and classification of all active pharmaceutical ingredients and synthesis intermediates handled at Sanofi facilities. This covers all active ingredients handled in production at company sites or in processes sub-contracted for manufacture. Any important issues involving raw materials or other substances that lack established occupational exposure limits may also be reviewed. The COVALIS Committee determines the occupational exposure limits required within Sanofi. Our TRIBIO Committee is responsible for classifying all biological agents according to their degree of pathogenicity, and applies rules for their containment and the preventive measures to be respected throughout Sanofi. See “Item 3. Key Information — D. Risk Factors — Environmental and safety risks of Our Industrial Activities — Risks from manufacturing activities and the handling of hazardous materials could adversely affect our results of operations and reputation.”
Appropriate occupational hygiene practices and programs are defined and implemented in each site. These practices consist essentially of containment measures for collective and individual protection against chemical and biological exposure in all workplaces where chemical substances or biological agents are handled. All personnel are monitored with an appropriate medical surveillance program, based on the results of professional risk evaluations linked to their duties.
In addition, dedicated resources have been created to implement the European Regulation on Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and the European Regulation on Classification, Labeling and Packaging of chemicals (CLP). To fully comply with REACH, Sanofi has registered the relevant hazardous chemical substances with the European Chemicals Agency (ECHA).
Safety
Sanofi has rigorous policies to identify and evaluate safety risks and to develop preventive safety measures, and methods for checking their efficacy. Additionally, Sanofi invests in training that is designed to instill in all employees a sense of concern for safety, regardless of their duties. These policies are implemented on a worldwide scale to ensure the safety of all employees and to protect their health. Each project, whether in research, development or manufacturing, is subject to evaluation procedures, incorporating the chemical substance and process data communicated by the COVALIS and TRIBIO Committees described above. The preventive measures are designed primarily to reduce the number and seriousness of work accidents and to minimize exposures involving permanent and temporary Sanofi employees as well as our sub-contractors.
The French chemical manufacturing sites in Aramon and Sisteron are listed Seveso III (from the name of the European directive that deals with potentially dangerous establishments where dangerous substances may be present in quantities exceeding certain thresholds to prevent major accidents and limit their consequences). In accordance with French law on technological risk prevention, the French sites are also subject to heightened security inspections due to the toxic or flammable materials stored on the sites and used in the operating processes.
Risk assessments of processes and installations are drawn up according to standards and internal guidelines incorporating the best state of the art benchmarks for the industry. These assessments are used to fulfill regulatory requirements and are regularly updated. Particular attention is paid to any risk-generating changes such as process or installation changes, as well as changes in production scale and transfers between industrial or research units.
We are using specialized process safety-testing laboratories that are fully integrated into our chemical development activities, apply methods to obtain the physico-chemical parameters of manufactured chemical substances (intermediate chemical compounds and active ingredients) and apply models to measure the effect of potentially leachable substances in the event of a major accident. In these laboratories the parameters for qualifying hazardous reactions are also determined, in order to define scale-up process conditions while transferring from development stage to industrial scale. We use these data to enhance the relevance of our risk assessments.
We believe that the safety management systems implemented at each site, the hazard studies carried out and the risk management methods implemented, as well as our third-party property insurance policies covering any third-party physical damage, are consistent with legal requirements and the best practices in the industry, although no guarantee can be given that they will prevent accidents of various kinds.
Environment
Beyond healthcare, we have developed an ambitious policy aimed at minimizing the environmental impacts of our products and activities while strengthening our resilience in the face of environmental changes. We have identified six major environmental challenges relating to our businesses: greenhouse gas emissions and climate disruption; eco-design; water; pharmaceuticals in the environment; waste; and biodiversity.
The initiatives already implemented since 2010 are continuing, and we have been keen to give them fresh impetus through the Planet Care program. Reflecting our environment strategy out to 2030 and 2045, the program sets more ambitious targets for reducing environmental impacts across the entire value chain. Planet Care is a global project that involves all of the Company’s resources in defining objectives and engaging with external partners.
Compared with 2019 figures, we are undertaking to reduce our carbon emissions by 55% (scope 1 and 2) by the end of 2030, building the path to carbon-neutral status by 2030 on our scope 1, 2 & 3 (direct and indirect emissions for all activities), and net zero by 2045. We have also set ourselves the target of achieving sustainable water resource management, especially at sites which are under hydric stress. On this new scope, by the end of 2023 we had reduced CO2 emissions by 38% (scope 1 and 2) and water withdrawals by 18%.
48 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
Overall waste valorization at sites is already above 88% and is expected to be more than 90% by the end of 2025. The landfill rate had dropped to 2.3% at the end of 2023 and we have committed to move towards no more than 1% by 2025. Biodiversity management at our sites is also a priority, with the aim of making all employees aware of this challenge and implementing risk assessment and management plans at priority sites by 2025.
Finally, we are pursuing the policy we began in 2010 of managing pharmaceutical products in the environment throughout their life cycles. At the end of 2023, all priority production sites had initiated specific risk-based programs to monitor, manage and reduce emissions of pharmaceuticals in waste water (where needed).
In line with this approach, we are engaged in the Industry Roadmap for Progress on Combating Antimicrobial Resistance. Together with the other AMR roadmap signatories we have co-developed reference guides and methodologies for producing antibiotics responsibly. The initiative includes a specific commitment with respect to antibiotic production sites that are operated by signatories or their suppliers, involving the deployment of a shared framework and safe-discharge targets for managing potential emissions of antibiotics. See “Cautionary statement regarding forward-looking statements” and “Item 3.D. Risk Factors.”
C. Organizational Structure
C.1. Significant Subsidiaries
Sanofi is the holding company of a consolidated group consisting of almost 260 companies. The table below sets forth our significant subsidiaries as of December 31, 2023. For a fuller list of the principal companies in our consolidated group, see Note F. to our consolidated financial statements, included in Item 18 of this annual report.
| Significant subsidiary | Date of incorporation | Country of incorporation | Principal activity | Financial and voting interest | ||||||||||
| Aventis Inc. | July 1, 1968 | United States | Pharmaceuticals | 100 | % | |||||||||
| Genzyme Corporation | November 21, 1991 | United States | Pharmaceuticals | 100 | % | |||||||||
| Genzyme Europe B.V. | October 24, 1991 | Netherlands | Pharmaceuticals | 100 | % | |||||||||
| Hoechst GmbH | July 8, 1974 | Germany | Pharmaceuticals | 100 | % | |||||||||
| Sanofi-Aventis Deutschland GmbH | June 30, 1997 | Germany | Pharmaceuticals | 100 | % | |||||||||
| Sanofi-Aventis Participations SAS | February 25, 2002 | France | Pharmaceuticals | 100 | % | |||||||||
| Sanofi-Aventis Singapore Pte Ltd | May 14, 1997 | Singapore | Pharmaceuticals | 100 | % | |||||||||
| Sanofi Biotechnology | December 23, 2013 | France | Pharmaceuticals | 100 | % | |||||||||
| Sanofi Foreign Participations B.V. | April 29, 1998 | Netherlands | Pharmaceuticals | 100 | % | |||||||||
| Sanofi Winthrop Industrie | December 11, 1972 | France | Pharmaceuticals | 100 | % | |||||||||
| Sanofi Pasteur Inc. | January 18, 1977 | United States | Pharmaceuticals | 100 | % | |||||||||
Since 2009, we have transformed Sanofi through numerous acquisitions and divestments (see main recent events in “A. History and Development of the Company” above), in particular the acquisitions of Genzyme in April 2011, Boehringer Ingelheim (BI) Consumer Healthcare in January 2017, Bioverativ in March 2018, Ablynx in June 2018, Synthorx in January 2020, Principia in September 2020, Kymab in April 2021, Translate Bio in September 2021, and Amunix Pharmaceuticals, Inc in February 2022; the deconsolidation of EUROAPI in May 2022; and the acquisitions of Provention Bio, Inc. and QRIB Intermediate Holdings, LLC. in 2023 The financial effects of the Genzyme acquisition are presented in Note D.1.3. to our consolidated financial statements for the year ended December 31, 2013, included in our annual report on Form 20-F for that year. At the end of December 2016, Sanofi Pasteur and MSD (known as Merck in the United States and Canada) ended their Sanofi Pasteur MSD joint venture. The financial effects of the resulting divestment/acquisition are presented in Note D.1.2. to our consolidated financial statements for the year ended December 31, 2016, included in our annual report on Form 20-F for that year. On January 1, 2017, Sanofi and Boehringer Ingelheim (BI) finalized the strategic transaction agreed in June 2016, involving the exchange of Sanofi’s Animal Health business (Merial) for BI’s Consumer Healthcare business. The financial effects of this transaction are presented in Note D.1. to our consolidated financial statements for the year ended December 31, 2017, included in our annual report on Form 20-F for that year. The financial effects of the Bioverativ and Ablynx acquisitions are presented in Note D.1.1. to our consolidated financial statements for the year ended December 31, 2018, included in our annual report on Form 20-F for that year. The financial effects of the Synthorx and Principia acquisitions are presented in Note D.1. to our consolidated financial statements for the year ended December 31, 2020, included in our annual report on Form 20-F for that year. The financial effects in 2021 of the Kymab, Kiadis, Tidal, Translate Bio, Kadmon and Origimm acquisitions, and in 2022 of the acquisition of Amunix Pharmaceuticals, Inc. and the deconsolidation of EUROAPI, are presented in Note D.2. to our consolidated financial statements for the year ended December 31, 2033, included in our annual report on Form 20-F for that year. The financial effects of the acquisition of Provention Bio, Inc and QRIB Intermediate Holdings, LLC in 2023 are presented in Note D.1. to our consolidated financial statements for the year ended December 31, 2023, included at Item 18. of this annual report on Form 20-F.
In certain countries, we carry on some of our business operations through joint ventures with local partners. In addition, we have entered into worldwide collaboration agreements in particular those with Regeneron on DUPIXENT and KEVZARA and with AztraZeneca on BEYFORTUS. For further information, refer to Note C. “Principal Alliances” to our consolidated financial statements, included at Item 18. of this annual report on Form 20F.
SANOFI FORM 20-F 2023 | 49 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
C.2. Internal organization of activities
Sanofi and its subsidiaries collectively form a group organized around two activities: Biopharma (General Medicines, Specialty Care and Vaccines) and Consumer Healthcare.
Within Sanofi, responsibility for R&D rests with Sanofi, Genzyme Corporation, Sanofi Pasteur and Sanofi Pasteur, Inc. (in vaccines) for the Biopharma segment. However, within our integrated R&D organization, strategic priorities are set and R&D efforts coordinated on a worldwide scale. In fulfilling their role in R&D, the aforementioned companies subcontract R&D to those of their subsidiaries that have the necessary resources. They also license patents, manufacturing know-how and trademarks to certain of their French and foreign subsidiaries. Those licensee subsidiaries manufacture, commercialize and distribute the majority of our products, either directly or via local distribution entities.
Our industrial property rights, patents and trademarks are mainly held by the following companies:
•Biopharma: Sanofi, Sanofi Mature IP, Sanofi Biotechnology SAS (France), Sanofi-Aventis Deutschland GmbH (Germany), Ablynx (Belgium), Genzyme Corporation, Bioverativ Inc., Kadmon Corporation LLC, Amunix Pharmaceuticals, Inc., Kymab Ltd, Principia Biopharma Inc., Sanofi Pasteur (France), Sanofi Pasteur, Inc. (US), Sanofi Pasteur Vaxdesign Corp., Translate Bio (US), Synthorx, Inc., Aventis Pharma SA and Provention Bio, Inc.;
•consumer healthcare: A. Nattermann Cie & GmbH (Germany), Chattem Inc. (US), Opella Healthcare and SSP Co. Ltd (Japan).
For a description of our principal items of property, plant and equipment, see “— D. Property, Plant and Equipment” below. Our property, plant and equipment is held mainly by the following companies:
•in France: Sanofi Pasteur SA, Sanofi Chimie, Sanofi Winthrop Industrie, Opella Healthcare International SAS and Sanofi-Aventis Recherche & Développement;
•in the United States: Sanofi Pasteur, Inc., Genzyme Therapeutics Products LP, Genzyme Corporation and Translate Bio;
•in Germany: Sanofi-Aventis Deutschland GmbH;
•in Canada: Sanofi Pasteur Limited;
•in Belgium: Genzyme Flanders BVBA; and
•in Ireland: Genzyme Ireland Limited.
C.3. Financing and financial relationships between group companies
The Sanofi parent company raises the bulk of the Company’s external financing and uses the funds raised to meet, directly or indirectly, the financing needs of its subsidiaries. The parent company operates a cash pooling arrangement under which any surplus cash held by subsidiaries is managed centrally. There is also a centralized foreign exchange risk management system in place, whereby the parent company contracts hedges to meet the needs of its principal subsidiaries.
Consequently, at December 31, 2023, the Sanofi parent company held 91% of our external financing and 75% of our surplus cash.
Sanofi European Treasury Center SA (SETC), a 100%-owned Sanofi subsidiary incorporated in 2012 under the laws of Belgium, is dedicated to providing financing and various financial services to our subsidiaries.
D. Property, plant and equipment
D.1. Overview
Our headquarters are located in Paris, France. See “— D.4. Office Space” below.
We operate our business through office premises and research, production and logistics facilities in approximately 70 countries around the world. Our office premises house all of our support functions, plus operational representatives from our subsidiaries and the Company.
A breakdown of our sites by use and by ownership status (owned versus leasehold) is provided below. This breakdown is based on surface area. All surface area figures are unaudited.
| Breakdown of sites by use | Breakdown of sites by ownership status | |||||||||||||
| Industrial | 59 | % | Leasehold | 26 | % | |||||||||
| Research | 16 | % | Owned | 74 | % | |||||||||
| Offices | 12 | % | ||||||||||||
| Logistics | 10 | % | ||||||||||||
| Other | 3 | % | ||||||||||||
50 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
D.2. Description of our sites
Sanofi industrial sites
As part of the process of transforming Sanofi and creating Global Business Units, we are continuing to adapt the organization of the Manufacturing & Supply department in support of our new business model.
The Manufacturing & Supply department focuses on customer needs and service quality; the sharing of “Sanofi Manufacturing System” good manufacturing practices; and the development of a common culture committed to quality.
The organizational structure of Manufacturing & Supply is aligned on our corporate structure and our four Global Business Units: Specialty Care, General Medicines, Vaccines and CHC.
EUROAPI is a standalone business created by Sanofi and dedicated to the production, development and marketing of active pharmaceutical ingredients (API) in Europe. EUROAPI houses the activities of six manufacturing sites (located in Italy, Germany, the UK, France and Hungary), and was first listed on Euronext Paris on May 6, 2022. Following that initial listing, Sanofi no longer controls EUROAPI, and now accounts for its residual interest (29.8 % at December 31, 2023) using the equity method.
The Manufacturing & Supply department is also responsible for Sanofi Global HSE and Global Supply Chain.
At the end of 2023, we were carrying out industrial production at 54 sites in 24 countries:
•8 sites for our Specialty Care operations;
•22 sites for our General Medicines operations;
•10 sites for the industrial operations of Vaccines; and
•13 sites for our CHC operations.
The quantity of units sold in 2023, including in-house and outsourced production, was 4.3 billion. This comprised:
•Biopharma: 2.3 billion units; and
•CHC: 2.0 billion units.
We believe that our production facilities are in compliance with all material regulatory requirements, are properly maintained and are generally suitable for future needs. We regularly inspect and evaluate those facilities with regard to environmental, health, safety and security matters, quality compliance and capacity utilization. For more information about our property, plant and equipment, see Note D.3. to our consolidated financial statements, included at Item 18. of this annual report, and section “B.7. Production and Raw Materials” above.
Our main production sites by volume are:
•Le Trait (France), Frankfurt (Germany), Waterford (Ireland), Geel (Belgium) and Framingham (United States) for Specialty Care;
•Aramon, Sisteron and Ambarès (France), Frankfurt (Germany), Csanyikvölgy (Hungary), Lüleburgaz (Turkey), Campinas (Brazil), Jurong (Singapore) and Hangzhou (China) for General Medicines products;
•Compiègne and Lisieux (France), Cologne (Germany), Origgio (Italy), Chattanooga (United States) and Ocoyoacac (Mexico) for Consumer Healthcare products; and
•Marcy-l’Étoile and Val-de-Reuil (France), Toronto (Canada) and Swiftwater (United States) for vaccines.
Research & Development sites
In Pharmaceuticals, research and development activities are conducted at the following sites:
•four operational sites in France: Chilly-Mazarin/Longjumeau, Montpellier, Strasbourg and Vitry-sur-Seine/Alfortville;
•three sites in the rest of Europe (Germany, Belgium and the Netherlands), the largest of which is in Frankfurt (Germany);
•six sites in the United States: Bridgewater, Cambridge, Framingham/Waltham, Great Valley and San Francisco ; and
•three sites in China (Beijing, Shanghai and Chengdu).
Vaccines research and development sites are:
•Swiftwater, Cambridge and Orlando (United States);
•Marcy-l’Étoile/Lyon (France); and
•Toronto (Canada).
D.3. Acquisitions, capital expenditures and divestitures
The carrying amount of our property, plant and equipment at December 31, 2023 was €10,160 million. During 2023, we invested €1,693 million (see Note D.3. to our consolidated financial statements, included at Item 18. of this annual report), mainly in increasing capacity and improving productivity at our various production and R&D sites.
Our principal acquisitions, capital expenditures and divestitures in 2021, 2022 and 2023 are described in Notes D.1. & D.2. (“Changes in the scope of consolidation”), D.3. (“Property, plant and equipment”) and D.4. (“Goodwill and other intangible assets”) to our consolidated financial statements, included at Item 18. of this annual report. For associated commitments, and in particular future contingent milestone payments, refer to Notes D.18 and D.21. to our consolidated financial statements, which provide disclosures about liabilities related to business combinations and our principal research and development collaboration agreements, respectively.
SANOFI FORM 20-F 2023 | 51 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
As of December 31, 2023, our firm commitments in respect of future capital expenditures amounted to €638 million. The principal locations involved are: for the Biopharma segment, the industrial facilities at Frankfurt (Germany); Le Trait, Maisons-Alfort, Compiègne, and Ambares (France); Cambridge (United States); Geel (Belgium); Origgio, Anagni, Brindisi, and Scoppito (Italy); and for the Vaccines segment, the facilities at Swiftwater (United States), Toronto (Canada), Marcy-l’Étoile, Neuville-sur-Saône and Val-de-Reuil (France), and Singapore.
In the medium term and assuming no changes in the scope of consolidation, we expect to invest on average approximately €1.5 billion a year in property, plant and equipment. We believe that our own cash resources and the undrawn portion of our existing credit facilities will be sufficient to fund these expenditures.
Our principal ongoing capital expenditures are described below.
Specialty Care
Our Specialty Care industrial operations are organized around two end-to-end clusters. We have four dedicated biotechnology hubs: Paris/Lyon (France), Frankfurt (Germany), Geel (Belgium) and Boston Area (United States). Exploiting the innovative techniques on which biotech relies, including cell and microbiological culture and the development of viral vectors, our Specialty Care operations call for highly specific knowledge and expertise backed by dedicated production platforms to support global product launches.
The Waterford and Le Trait sites manufacture pre-filled DUPIXENT syringes.
General Medicines
Our General Medicines industrial operations are organized through end-to-end clusters, with chemistry, pharmaceutical and injectable sites organized through a network of 22 regional and local industrial sites in 14 countries, supporting growth in those markets.
This new organization encompasses a dedicated Launch Sites cluster from API manufacturing to finished goods packaging (Sisteron, Aramon, Ambarès, Scoppito).
The Frankfurt facility is our principal site for the manufacture of diabetes treatments.
Vaccines
The industrial operations of our Vaccines business are in a major investment phase, preparing for the upcoming growth of our influenza and Polio/Pertussis/Hib franchises, plus the mid-term growth linked to our mRNA roadmap and New Vaccines pipeline. Major investments were announced in 2020 and 2021 with a new Evolutive Facility in France (Neuville-Sur-Saone) and a new facility in Singapore for our New Vaccines pipeline. Other major investments are under way in France (including construction of a new influenza vaccine building at Val-de-Reuil), Canada (a new pertussis vaccine building), the US and Mexico.
Consumer Healthcare
The pharmaceutical industrial operations of our Consumer Healthcare (CHC) business are spread across a dedicated network. Global markets are supplied from our facilities at Compiègne (France), Cologne (Germany) and Origgio (Italy). We have recently invested in new production capacities in Narita (Japan), Origgio (Italy) and Lisieux (France).
Innovation and culture of industrial excellence
The ambition of our Manufacturing & Supply department is to continue to raise safety, quality and operating standards in Sanofi’s production activities, and to remain a world leader and a benchmark in the global pharmaceutical industry. To achieve this goal, all our activities share a common culture of industrial excellence, enshrined in the Sanofi Manufacturing System. This sets out a series of priorities (such as customer service, constant improvement, site network optimization and transverse optimization) that constitute our industrial vision and will be crucial to our mutual success.
In terms of operational excellence, we continue to build on our Top Decile performance program, focused on core sites and fully leveraging digital opportunities and technology innovations. We are also reinforcing the Sanofi Manufacturing System to drive more improvement directly from the sites and reach our performance goals, while creating a culture of best practices shared across the industrial network.
D.4. Office space
We continue to evolve our roadmap to ensure our sites reflect the best-in-class experience and reinforce connections between people, no matter where they are based.
In addition, we have enhanced our services to provide even more productivity, wellbeing, collaboration, innovation and ultimately foster greater employee engagement.
A number of projects are currently under way to deliver the next generation of work experience and hybrid work.
We continue to actively revisit our real estate footprint to ensure we are taking the right steps in the carbon neutrality race.
52 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 4. Information on the company | ||
E. R&D Appendix
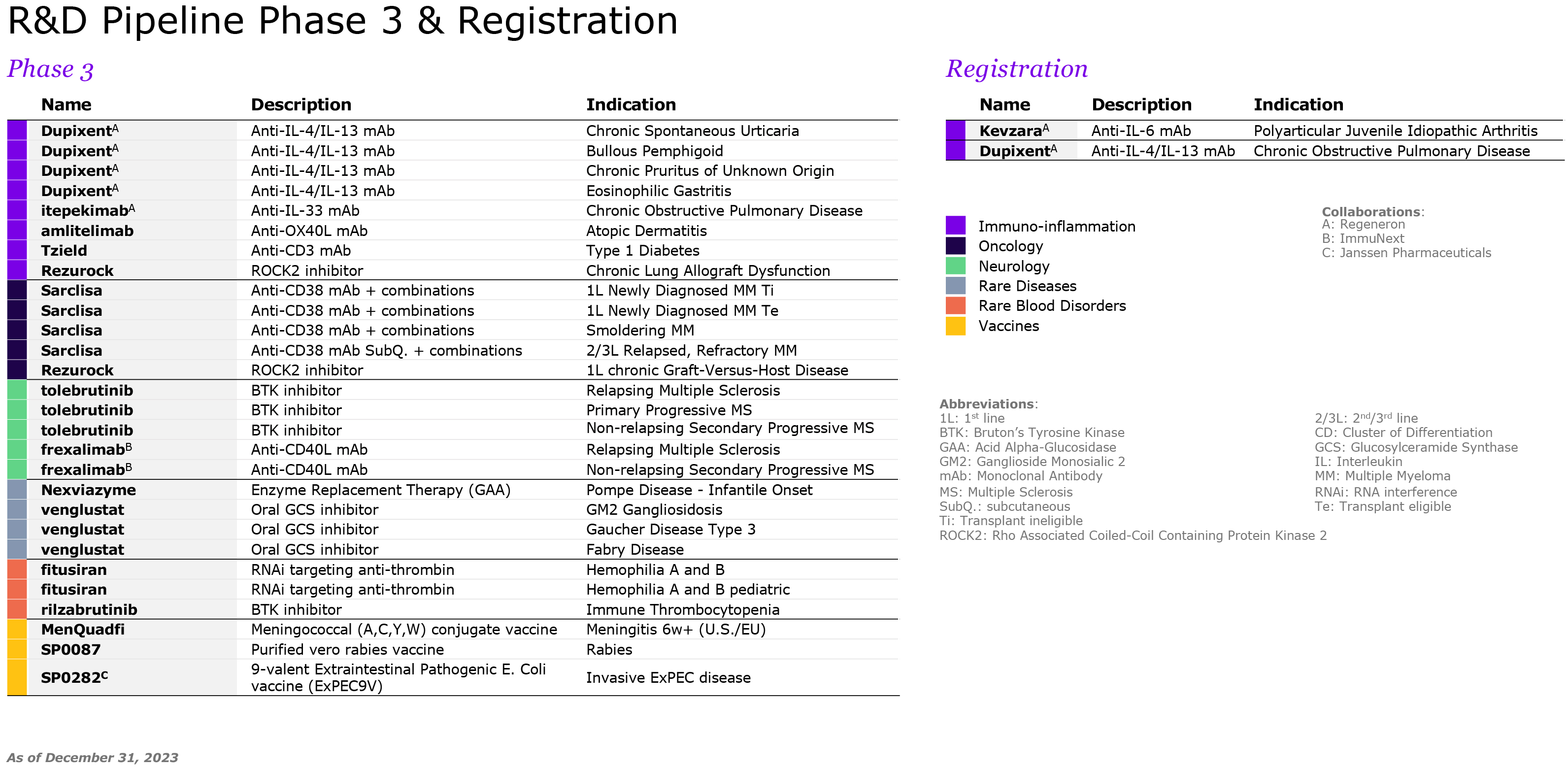
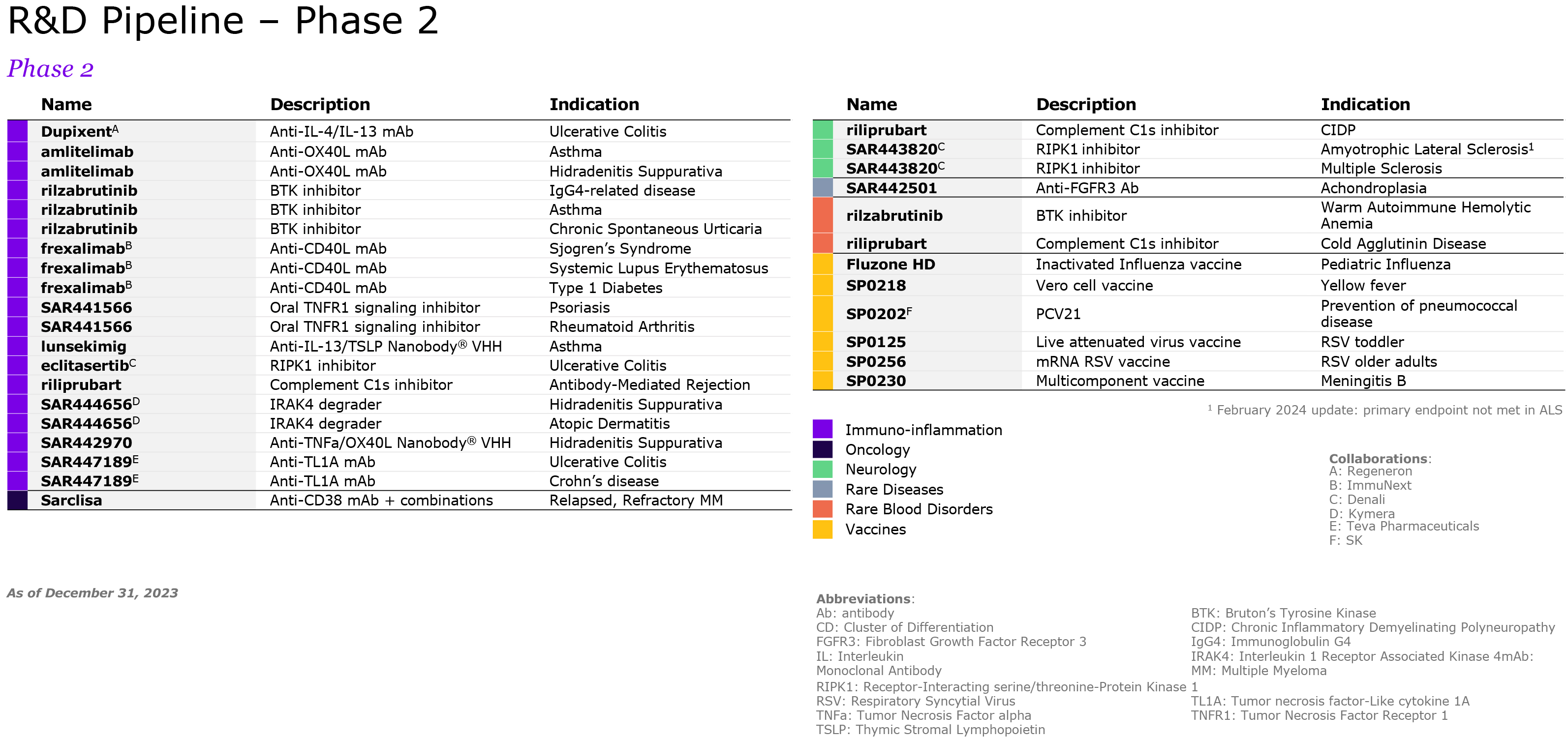
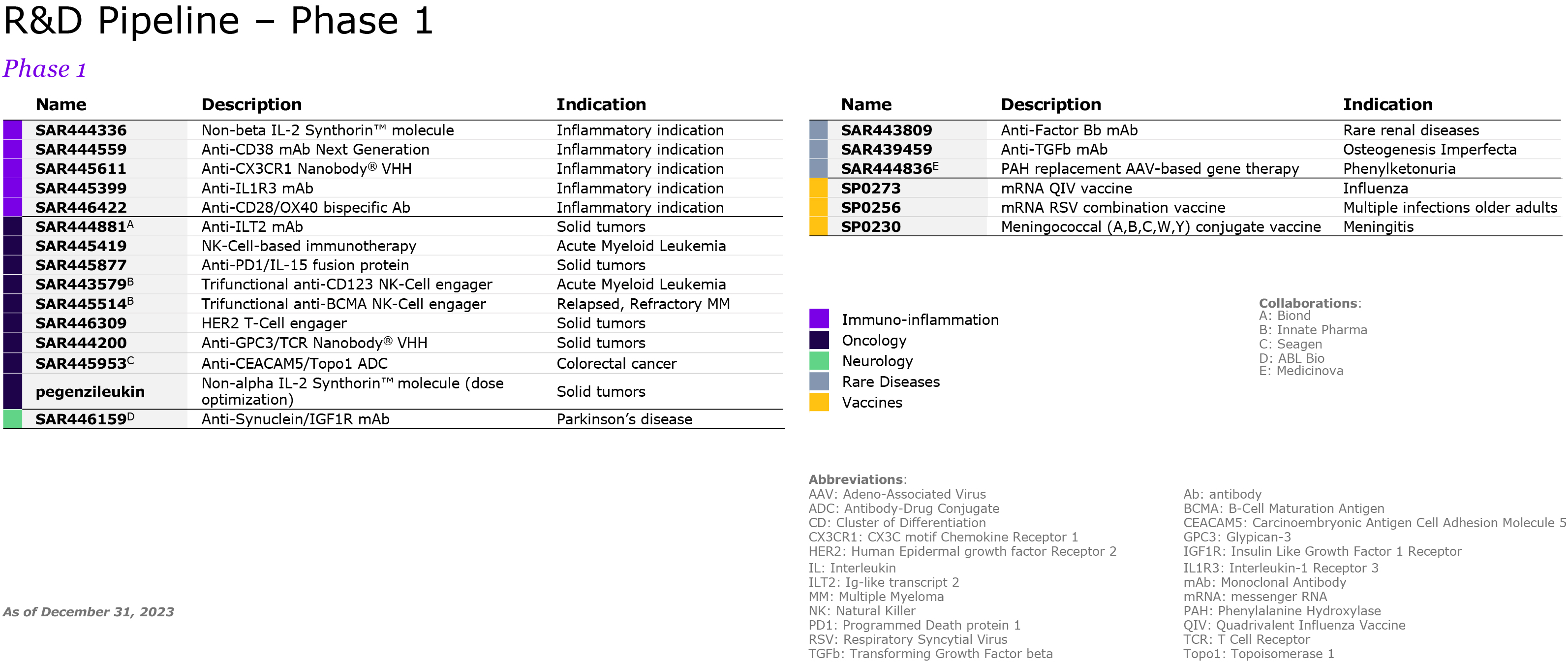
SANOFI FORM 20-F 2023 | 53 | ||||
| PART I | ||
ITEM 4A. Unresolved Staff Comments | ||
Item 4A. Unresolved Staff Comments
N/A
Item 5. Operating and Financial Review and Prospects
You should read the following discussion in conjunction with our consolidated financial statements and the notes thereto included in this annual report at Item 18.
Our consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB) and with IFRS endorsed by the European Union as of December 31, 2023.
The following discussion contains forward-looking statements that involve inherent risks and uncertainties. Actual results may differ materially from those contained in such forward-looking statements. See “Cautionary Statement Regarding Forward-Looking Statements” at the beginning of this document.
Unless otherwise stated, all financial variations in this item are given on a reported basis.
The discussion of our operating and financial review and prospects for the years ended December 31, 2022 and December 31, 2021, including a presentation of our consolidated income statements for the years ended December 31, 2022 and December 31, 2021, can be found in “Item 5. Operating and Financial Review and Prospects — A. Operating results — A.2. Results of operations — Year ended December 31, 2022 compared with year ended December 31, 2021” of our annual report filed on February 24, 2023.
A. Operating results
A.1. Significant operating information
A.1.1. 2023 Overview
During 2023, Sanofi continued to implement its “Play to Win” strategy, initiating the second phase which aims to launch major innovations, redeploy resources and develop leading innovative R&D. For further information about our strategy, refer to “Item 4. Information on the Company — B. Business Overview — B.1. Strategy.” Other significant events of the year are described below.
On January 11, 2023, Sanofi Ventures announced an additional multi-year commitment from Sanofi, which will increase the capital of the evergreen venture fund to more than $750 million. In addition to serving as a financial partner to top-tier early-to-mid-stage portfolio companies, the fund supports future efforts for business development and M&A opportunities within Sanofi. The additional capital, confirmed by our Executive Committee, will also be allocated to advance the expansion and investment capacity of the Sanofi Ventures investment team on a global scale.
On March 13, 2023, Sanofi and Provention Bio, Inc. (Provention), a US-based publicly-traded biopharmaceutical company developing therapies to prevent and intercept immune-mediated diseases including type 1 diabetes, entered into an agreement under which Sanofi acquired the outstanding shares of Provention common stock for $25.00 per share in an all-cash transaction valued at approximately $2.8 billion. On April 27, 2023, Sanofi announced the completion of its acquisition of Provention. The acquisition added TZIELD (teplizumab-mzwv), a therapy for type 1 diabetes, to Sanofi’s core General Medicines asset portfolio, and further drives our strategic shift toward products with a differentiated profile.
On April 9, 2023, Sanofi and AstraZeneca simplified their contractual agreements for the development and commercialization of BEYFORTUS (nirsevimab) in the US. Sanofi thereby obtained control of all commercial rights to BEYFORTUS (nirsevimab) in the US, and ended the sharing of commercial profits between the two partners in that territory. In line with the terms of the revised agreements and in accordance with IAS 38, Sanofi recognized an intangible asset of €1.6 billion for the fair value of the additional US rights. On the same date, AstraZeneca and Sobi ended their participation agreement, signed in 2018, which transferred the economic rights for the US territory to Sobi.
Sanofi simultaneously entered into an agreement with Sobi relating to direct royalties on US net sales of BEYFORTUS (nirsevimab). In line with the terms of that agreement, on April 9, 2023 Sanofi recognized a financial liability of €1.6 billion. That liability is classified as a financial liability at amortized cost under IFRS 9. Other than royalty payments, subsequent movements in the liability comprise (i) the effects of the unwinding of discount and (ii) changes in estimates of future cash outflows for royalty payments. Those movements are recognized in the income statement within Net financial income/(expenses) in accordance with paragraph B5.4.6 of IFRS 9. As of December 31, 2023 the liability was remeasured by an amount of €541 million, reflecting the strong success of the US launch of BEYFORTUS (nirsevimab), which led to sales forecasts being revised upward from the initial estimate.
For territories other than the US (except for China, which is now considered a “major market,” with profits/losses shared 50/50 with AstraZeneca), the existing agreement between AstraZeneca and Sanofi continues to govern the principal terms of the collaboration: Sanofi recognizes the sales and cost of sales, and shares the Alliance’s commercial profits with AstraZeneca.
54 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
On June 20, 2023, Sanofi announced that in a dispute referred to the International Chamber of Commerce arbitration panel, the panel had dismissed an indemnification claim against Sanofi from Boehringer Ingelheim and confirmed that Sanofi will not be liable for any potential losses in relation to the ongoing ZANTAC litigation in the US. This decision is final and non-appealable.
On July 28, 2023, Sanofi announced that it had entered into an agreement to acquire QRIB Intermediate Holdings, LLC (QRIB), the owner of QUNOL, a market-leading US-based health & wellness brand. The deal reflects Sanofi’s ongoing drive to pursue growth opportunities and value creation for its CHC business. Sanofi’s acquisition of QRIB was completed on September 29, 2023, at a purchase price of $1,419 million.
On October 3, 2023, Sanofi announced that it had entered into an agreement with Janssen Pharmaceuticals, Inc. (Janssen), a Johnson & Johnson company, to develop and commercialize the vaccine candidate for extra-intestinal pathogenic E. coli (9-valent) developed by Janssen, currently in Phase 3. The deal was closed on November 9, 2023. Under the terms of the agreement, both parties will co-fund current and future research and development costs. Sanofi paid $175 million upfront to Janssen, and may pay to Janssen milestone payments contingent on the attainment of certain development and commercial objectives.
On October 4, 2023, Sanofi entered into a collaboration with Teva Pharmaceuticals to co-develop and co-commercialize asset TEV’574, currently in Phase 2b clinical trials for the treatment of Ulcerative Colitis and Crohn’s Disease, two types of inflammatory bowel disease. Under the terms of the new collaboration agreement, Teva received an upfront payment of €469 million ($500 million) and may receive up to €940 million ($1 billion) in milestone payments, depending on the achievement of development and commercialization goals.
On October 23, 2023 Sanofi, along with WhiteLab Genomics (a specialist in applying AI to genomic medicine), the TaRGeT lab at the University of Nantes (INSERM UMR 1089, one of France’s leading gene therapy labs) and Imagine Institute (AP-HP, INSERM, Paris Cité University, Europe’s leading center for research, education and care in the field of genetic diseases), collectively launched the WIDGeT (Viral Vector Intelligent Design for Gene Therapy) consortium to accelerate the development of adeno-associated virus (AAV) based gene therapies for the treatment of rare-to-frequent diseases (especially kidney and eye diseases) by using AI to develop next-generation AAVs. The consortium receives financial support from the Santé de France (Healthy France) 2030 tranche of the Innovation Santé (Health Innovation) Plan, overseen by the French Healthcare Innovation Agency and operated by Bpifrance, the French public investment bank.
On October 27, 2023, Sanofi provided a comprehensive update regarding its Play to Win strategy. This strategy continues to focus on the critical goals of executing transformative medicine and vaccine launches, driving agile and efficient resource deployment and enhancing R&D productivity. To that end, Sanofi announced plans to increase its R&D investments in an effort to fully realize its pipeline potential, drive long-term growth and enhance shareholder value. Sanofi also announced its intention to separate its Consumer Healthcare (CHC) business, to enable greater management focus and resource allocation to the needs of the Biopharma business, where value-creating opportunities and longer-term operational levers have been identified to support the accelerated R&D investments. The intended separation would be completed at the earliest in the fourth quarter of 2024, most likely through a capital markets transaction, via the creation of a listed entity headquartered in France.
On December 5, 2023, Sanofi announced the signature of a major research collaboration agreement with Aqemia to use artificial intelligence to accelerate the discovery of future medicines. This new collaboration dovetails with the discovery process, from identifying initial active ingredients through to selection of preclinical candidates.
In 2023, various health authorities granted marketing approvals to several Sanofi products, paving the way for innovative new treatments.
In Europe and China, DUPIXENT (dupilumab) was approved for the treatment of severe atopic dermatitis in children aged six months to five years who are candidates for systemic therapy. DUPIXENT was also approved in the European Union for the treatment of eosinophilic esophagitis in adults and adolescents aged 12 and over.
In the US and Taiwan, the FDA and the Taiwan Food and Drug Administration approved ALTUVIIIO (efanesoctocog alfa), the first sustained-acting replacement factor VIII in its pharmacotherapeutic class, indicated for routine prophylaxis and on-demand treatment and control of bleeding episodes, as well as for perioperative management, in adults and children with hemophilia A.
Nirsevimab (marketed as BEYFORTUS) was approved in Canada and the United States during 2023. It has also been accepted for priority review in China, and submitted in Japan.
In Europe, the EMA issued a positive opinion on Fexinidazole Winthrop as the first oral treatment of the acute form of sleeping sickness (rhodesiense) found in East and Southern Africa.
On December 21, 2023, Sanofi announced that the program evaluating tusamitamab ravtansine had ended after a Phase 3 trial as a second-line treatment in non-small cell lung cancer (NSCLC) failed to meet a primary endpoint.
For further information about the biopharma products we sell, and about our research and development portfolio, refer to “Item 4. Information on the Company — B. Business Overview.”
Our net sales for 2023 amounted to €43,070 million, an increase of 0.2% from 2022. At constant exchange rates (CER)(1), net sales rose by 5.3%, mainly reflecting strong growth for DUPIXENT and increased sales for our Vaccines business, more than offsetting lower sales for our Non Core Assets franchise.
(1) Non-IFRS financial measure: see definition in “— Presentation of Net Sales” below.
SANOFI FORM 20-F 2023 | 55 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
Net income attributable to equity holders of Sanofi amounted to €5,400 million for 2023, compared with €8,371 million in 2022, a €2,971 million decrease. Earnings per share was €4.31 in 2023, compared with €6.69 in 2022. Business net income(2) was €10,155 million, down 1.8% on 2022, while business earnings per share (business EPS(2)) was 1.8% lower than in 2022 at €8.11.
At the Annual General Meeting on April 30, 2024, we will ask our shareholders to approve a dividend of €3.76 per share for the 2023 financial year, representing a payout of 46.3% of our Business net income (see “— Consolidated balance sheet and debt.”).
A.1.2. Impacts of competition from generics and biosimilars
Some of our flagship products continued to suffer sales erosion in 2023 under the impact of competition from generics and biosimilars. We do not believe it is possible to state with certainty what level of net sales would have been achieved in the absence of generic competition. A comparison of our consolidated net sales for the years ended December 31, 2023 and 2022 (see “— Results of Operations — Year Ended December 31, 2023 Compared with Year Ended December 31, 2022” below) for the main products affected by generic and biosimilar competition shows a loss of €651 million of net sales on a reported basis. However, other parameters can also contribute to the loss of sales, such as a fall in the average selling price of certain products.
The table below sets forth the change by product.
(€ million) | 2023 | 2022 | Change on a reported basis | Change on a reported basis (%) | ||||||||||
APROVEL Europe | 78 | 82 | (4) | -4.9 | % | |||||||||
LANTUS Europe | 357 | 426 | (69) | -16.2 | % | |||||||||
LOVENOX Europe | 622 | 658 | (36) | -5.5 | % | |||||||||
PLAVIX Europe | 96 | 101 | (5) | -5.0 | % | |||||||||
JEVTANA Europe | 12 | 33 | (21) | -63.6 | % | |||||||||
LANTUS United States | 281 | 757 | (476) | -62.9 | % | |||||||||
LOVENOX United States | 7 | 17 | (10) | -58.8 | % | |||||||||
APROVEL Japan | 16 | 22 | (6) | -27.3 | % | |||||||||
LANTUS Japan | 9 | 13 | (4) | -30.8 | % | |||||||||
PLAVIX Japan | 33 | 53 | (20) | -37.7 | % | |||||||||
| Total | 1,511 | 2,162 | (651) | -30.1 | % | |||||||||
We expect the erosion caused by generic competition to continue in 2024, with a negative impact on our net income. The products likely to be impacted in 2024 include those that already faced generic competition in 2023, but whose sales can reasonably be expected to be subject to further sales erosion in 2024 (see products listed in the table above). In addition, we have experienced generic competition in the United States for AUBAGIO since March 2023 and for MOZOBIL since July 2023, following expiry of exclusivity in that country. In Europe, AUBAGIO generic competition began in in the fourth quarter of 2023 and will intensify in 2024, while generic competition for MOZOBIL is expected to begin between February and August 2024.
In 2023, aggregate consolidated net sales of those products in Europe, the United States and Japan amounted to €1,511 million; this comprised €288 million in the United States (including €281 million in net sales of LANTUS); €1,165 million in Europe; and €58 million in Japan. The negative impact on our 2024 net sales is likely to represent a substantial portion of those sales, but the actual impact will depend on a number of factors, such as the impact of generics and biosimilars on our molecules, but also the market entry of generics of molecules that are in competition with our products.
In China, the authorities have implemented a range of healthcare cost containment measures, including the Volume Based Procurement (VBP) reverse auction that particularly impacts our insulin-based products, PLAVIX, APROVEL, and LOVENOX (see also “Item 4. Information on the Company — B. Business Overview — B.5.4. Pricing & Reimbursement”). A large number of molecules were selected to submit tenders under successive waves of the VBP program, with the successful bidders being awarded a high level of market share in return for offering lower prices. We participated in a number of VBP bids and were selected for only part of the volumes awarded for 2022, 2023 and 2024 in respect of insulins (TOUJEO and LANTUS), PLAVIX and APROVEL, in return for a considerable reduction in unit prices. Sanofi was not among the nine companies selected to supply enoxaparin sodium based products in the latest VBP allocation, which covers 50% of volumes for the 2024-25 period.
(2) Non-IFRS financial measure: see definition in “— Segment Information — Business Net Income” below.
56 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
A.1.3. Purchase accounting effects
Our results of operations and financial condition for the years ended December 31, 2023, and 2022 have been significantly affected by our past acquisitions (acquisition of Aventis in August 2004, acquisition of Genzyme in April 2011, exchange of our Animal Health business (Merial) for Boehringer Ingelheim’s CHC business in January 2017, acquisition of Bioverativ in 2018, and certain other transactions). See “— Critical accounting and reporting policies — Business combinations” below for an explanation of the impact of business combinations on our results of operations.
The Genzyme business combination has generated significant amortization of intangible assets (€405 million in 2023, and €513 million in 2022). The exchange of Merial for Boehringer Ingelheim’s CHC business has generated amortization of intangible assets (€184 million in 2023, and €188 million in 2022). The Bioverativ business combination has generated significant amortization of intangible assets (€633 million in 2023, and €375 million in 2022). The Kadmon acquisition has generated amortization of intangible assets (€156 million in 2023, and €160 million in 2022). The Provention acquisition has generated amortization of intangible assets (€144 million in 2023).
In order to isolate the purchase accounting effects of all acquisitions and certain other items, we use a non-IFRS financial measure that we refer to as “business net income” (see definition and discussion of reconciliation to the IFRS financial measure Operating income in “— Segment Information and Business Net Income —Business Net Income” below).
A.1.4. Sources of revenues and expenses
Revenues. Revenue arising from the sale of goods is presented in the income statement within Net sales. Net sales comprise revenue from sales of biopharma products, consumer healthcare products, and active ingredients, net of sales returns, of customer incentives and discounts, and of certain sales-based payments paid or payable to the healthcare authorities. Returns, discounts, incentives and rebates are recognized in the period in which the underlying sales are recognized, as a reduction of sales revenue. See Note B.13.1. to our consolidated financial statements included at Item 18. of this annual report. We sell biopharma products directly, through alliances, and by licensing arrangements throughout the world. When we sell products directly, we record sales revenues as part of our consolidated net sales. When we sell products through alliances, the revenues reflected in our consolidated financial statements are based on the contractual arrangements governing those alliances. For more information about our alliances, see “— Financial Presentation of Alliances” below.
The line item Other revenues is used to recognize all revenue that falls within the scope of IFRS 15 but does not relate to sales of Sanofi products. It mainly comprises (i) royalties received from licensing intellectual property rights to third parties; (ii) VaxServe sales of products sourced from third-party manufacturers; and (iii) revenue received under agreements for Sanofi to provide manufacturing services to third parties. Royalties received under licensing arrangements are recognized over the period during which the underlying sales are recognized. VaxServe is a Biopharma segment entity whose operations include the distribution within the United States of vaccines and other products manufactured by third parties.
Cost of Sales. Our cost of sales consists primarily of the cost of purchasing raw materials and active ingredients, labor and other costs relating to our manufacturing activities, packaging materials, payments made under licensing agreements and distribution costs. We have license agreements under which we manufacture, sell and distribute products that are patented by other companies. When we pay royalties, we record them in Cost of sales.
Operating Income. Our operating income reflects our revenues, our cost of sales and the remainder of our operating expenses, the most significant of which are research and development expenses and selling and general expenses. For our operating segments, we also measure our results of operations through an indicator referred to as “Business Operating Income,” which we describe below under “— Segment Information and Business Net Income — Business Operating Income.”
A.1.5. Segment information and Business net income
1/ Operating segments
In accordance with IFRS 8 (Operating Segments), the segment information reported by Sanofi is prepared on the basis of internal management data provided to our Chief Executive Officer, who is the chief operating decision maker of Sanofi. The performance of those segments is monitored individually using internal reports and common indicators. The operating segment disclosures required under IFRS 8 are provided in Note D.35. to the consolidated financial statements included at Item 18. of this annual report.
In 2022, Sanofi reported three operating segments (Pharmaceuticals, Vaccines and CHC). The costs of the global support functions (Corporate Affairs, Finance, People & Culture, Legal, Ethics, Business Integrity & Global Security, Information Solutions & Technology, Sanofi Business Services, etc.), which are mainly managed centrally at group-wide level, were presented within the “Other” category.
In 2023, Sanofi reviewed the presentation of its segment information following adjustments to its internal reporting systems in order to reflect (i) progress on the “Play to Win” strategy leading to the creation of the standalone CHC Global Business Unit (GBU) which, in addition to integrated research, development and production functions now also has its own dedicated global support functions (including Finance, People & Culture, Legal, Ethics, Business Integrity & Global Security, Information Solutions & Technology, Global Business Services, etc.); and (ii) organizational changes to Sanofi’s Manufacturing & Supply global function (previously known as Industrial Affairs).
SANOFI FORM 20-F 2023 | 57 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
Consequently, with effect from January 1, 2023, Sanofi reports two operating segments: Biopharma and CHC (3).
The Biopharma operating segment comprises commercial operations and research, development and production activities relating to the Specialty Care, General Medicines and Vaccines franchises, for all geographical territories. The segment’s results include the costs of global support functions that are not within the managerial responsibility of the CHC GBU.
The CHC operating segment comprises commercial operations relating to CHC products, and research, development and production activities and global support functions (as listed above) dedicated to the segment, for all geographical territories. The CHC GBU segment’s results reflect all incurred costs of global support functions attributable to its business.
The “Other” category comprises reconciling items, primarily but not limited to (i) gains and losses on centralized foreign exchange risk hedging transactions that cannot be allocated to the operating segments and (ii) gains and losses on retained commitments in respect of previously divested operations.
2/ Business operating income (non-IFRS financial measure)
We report segment results on the basis of “Business operating income.” This non-IFRS indicator is used internally by Sanofi’s chief operating decision maker to measure the performance of each operating segment and to allocate resources. For a definition of “Business operating income,” and a reconciliation between that indicator and IFRS Income before tax and investments accounted for using the equity method, refer to Note D.35. to our consolidated financial statements included at Item 18. of this annual report.
At Group level, “Business operating income” is a non-IFRS financial measure which is reconciled with IFRS Operating income. IFRS Operating income for 2023 amounted to €7,875 million versus €10,656 million for 2022; refer to Note D.35. to our consolidated financial statements included at Item 18. of this annual report. Our ratio of Operating Income to Net Sales was 18.3% in 2023 versus 24.8% in 2022.
Our “Business operating income” for 2023 amounted to €12,670 million, versus €13,040 million for 2022, while our “Business operating income margin” was 29.4%, versus 30.3% for 2022. “Business operating income margin” is a non-IFRS financial measure, which we define as the ratio of our “Business operating income” to IFRS Net sales.
Because our “Business operating income” and “Business operating income margin” are not standardized measures, they may not be directly comparable with the non-IFRS financial measures of other companies using the same or similar non-IFRS financial measures. Although management uses those non-IFRS measures to set goals and measure performance, they have no standardized meaning prescribed by IFRS. These non-IFRS measures are presented solely to permit investors to more fully understand how Sanofi’s management assesses underlying performance. These non-IFRS measures are not, and should not be viewed as, a substitute for IFRS measures, and should be viewed in conjunction with our IFRS financials and performance measures. As a result, such measures have limits in their usefulness to investors.
3/ Business net income (non-IFRS financial measure)
We believe that the understanding of our operational performance by our management and our investors is enhanced by reporting “Business net income.” This non-IFRS financial measure represents “Business operating income,” less (i) net financial expenses (except those related to financial liabilities accounted for at amortized cost and subject to periodic remeasurement in accordance with paragraph B5.4.6 of IFRS 9) and (ii) income tax expense related to “Business operating income”.
“Business net income” is a non-IFRS financial measure; it is reconciled with IFRS Net income attributable to equity holders of Sanofi, which amounted to €5,400 million for 2023 versus €8,371 million for 2022.
Our “Business net income” for 2023 was €10,155 million, 1.8% lower than in 2022 (€10,341 million). That represents 23.6% of our net sales, compared with 24.1 % in 2022.
We also report “Business earnings per share” (“Business EPS”), a non-IFRS financial measure we define as “Business net income” divided by the weighted average number of shares outstanding. “Business EPS” was €8.11 for 2023, 1.8% lower than the 2022 figure of €8.26, based on an average number of shares outstanding of 1,251.7 million for 2023 and 1,251.9 million for 2022.
The table below reconciles our “Business operating income” to our “Business net income”:
| (€ million) | December 31, 2023 | December 31, 2022 | ||||||
| Business operating income | 12,670 | 13,040 | ||||||
Financial income and expenses (except those related to financial liabilities accounted for at amortized cost and subject to periodic remeasurement in accordance with paragraph B5.4.6 of IFRS 9) | (181) | (234) | ||||||
Income tax expense on business operating income | (2,334) | (2,465) | ||||||
| Business net income | 10,155 | 10,341 | ||||||
We define “Business net income” as Net income attributable to equity holders of Sanofi determined under IFRS, excluding the following items:
•amortization and impairment losses charged against intangible assets (other than software and other rights of an industrial or operational nature);
•fair value remeasurements of contingent consideration relating to business combinations (IFRS 3), or to business divestments;
(3) Due to a lack of available data and the complex adjustments that would be required (particularly for our reporting tools), the 2021 figures have not been restated to reflect changes arising from our new organizational structure.
58 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
•expenses arising from the remeasurement of inventories following business combinations (IFRS 3) or acquisitions of groups of assets that do not constitute a business within the meaning of paragraph 2b of IFRS 3;
•restructuring costs and similar items (presented within the line item Restructuring costs and similar items);
•other gains and losses (including gains and losses on major divestments, presented within the line item Other gains and losses, and litigation);
•other costs and provisions related to litigation (presented within the line item Other gains and losses, and litigation);
•upfront payments and regulatory milestone payments received and recognized with the line item Other operating income and arising from transactions outside the scope of Sanofi’s ordinary activities;
•(income)/expenses related to financial liabilities accounted for at amortized cost and subject to periodic remeasurement in accordance with paragraph B5.4.6 of IFRS 9 (Financial Instruments);
•the tax effects of the items listed above, the effects of major tax disputes, and the effects of the deferred tax liability arising on investments in consolidated entities following the announcement on October 27, 2023 of Sanofi’s intention to proceed with the separation of its Consumer Health Care business;
•the share of profits/losses from investments accounted for using the equity method, except for joint ventures and associates with which Sanofi has a strategic alliance; and
•the portion attributable to non-controlling interests of the items listed above.
The table below reconciles our “Business net income” to Net income attributable to equity holders of Sanofi:
| (€ million) | 2023 | 2022 | ||||||
| Net income attributable to equity holders of Sanofi (IFRS) | 5,400 | 8,371 | ||||||
Amortization of intangible assets | 2,172 | 2,053 | ||||||
Impairment of intangible assets (a) | 896 | (454) | ||||||
Fair value remeasurement of contingent consideration | 93 | 53 | ||||||
| Expenses arising from the impact of acquisitions on inventories | 20 | 3 | ||||||
Income from out-licensing (b) | — | (952) | ||||||
| Restructuring costs and similar items | 1,490 | 1,336 | ||||||
Other gains and losses, and litigation | 38 | 370 | ||||||
Financial (income)/expenses relating to financial liabilities accounted for at amortized cost and subject to periodic remeasurement (c) | 541 | — | ||||||
| Tax effects of the items listed above: | (1,097) | (459) | ||||||
•amortization and impairment of intangible assets | (567) | (267) | ||||||
•fair value remeasurement of contingent consideration | (13) | (9) | ||||||
•expenses arising from the impact of acquisitions on inventories | (3) | — | ||||||
•restructuring costs and similar items | (397) | (231) | ||||||
•other items | (117) | 48 | ||||||
Other tax effects (d) | 365 | — | ||||||
Other items (e) | 237 | 20 | ||||||
Business net income (non-IFRS) | 10,155 | 10,341 | ||||||
| Average number of shares outstanding (million) | 1,251.7 | 1,251.9 | ||||||
| Basic earnings per share (€) | 4.31 | 6.69 | ||||||
| Reconciling items per share (€) | 3.80 | 1.57 | ||||||
| Business earnings per share (€) | 8.11 | 8.26 | ||||||
(a) For 2023, this amount mainly comprises an impairment loss of €833 million, reflecting the impact of the strategic decision to de-prioritize certain R&D programs, in particular those related to the NK Cell and PRO-XTEN technology platforms.
For 2022, this line includes a reversal of €2,154 million on ELOCTATE franchise products following FDA approval of ALTUVIIIO dated February 22, 2023, partly offset by an impairment loss of €1,586 million on intangible assets relating to SAR444245 (non-alpha interleukin-2) based on revised cash flow projections reflecting unfavorable developments in the launch schedule in key indications.
(b) For 2022, this line includes an upfront payment of $900 million and a regulatory milestone payment of $100 million in connection with the out-licensing of LIBTAYO following the restructuring of the Immuno-Oncology collaboration agreement with Regeneron (see Note C.1. to our consolidated financial statements, included at Item 18. of this annual report).
(c) For 2023, this amount corresponds to the financial expense related to the financial liability recognized in the balance sheet to reflect estimated future royalties on sales of BEYFORTUS in the United States, which has been remeasured to reflect the strong success of the product launch.
(d) For 2023, this amount corresponds to the deferred tax liability recognized in respect of investments in consolidated entities in light of the proposed separation of the Consumer Healthcare business in the fourth quarter of 2024 at the earliest.
(e) In 2023, an impairment loss of €231 million was recognized on the equity-accounted investment in EUROAPI in view of the fall in the share price since March 2023. The amount of the loss recognized was based on the share price as of December 31, 2023 (€5.73), as used in determining the recoverable amount of the equity interest as of that date.
SANOFI FORM 20-F 2023 | 59 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
The most significant reconciling items between “Business net income” and Net income attributable to equity holders of Sanofi relate to (i) the purchase accounting effects of our acquisitions of groups of assets and business combinations, particularly the amortization and impairment of intangible assets (other than software and other rights of an industrial or operational nature); (ii) the impacts of restructurings or transactions regarded as non-recurring, where the amounts involved are particularly significant; and (iii) remeasurements recognized through profit or loss in respect of (a) amounts receivable in respect of business divestments and accounted for at fair value, (b) liabilities arising from business combinations (IFRS 3) and accounted for at fair value, and (c) liabilities accounted for at amortized cost and subject to periodic remeasurement under IFRS 9. We believe that excluding those impacts enhances an investor’s understanding of our underlying economic performance, because it gives a better representation of our recurring operating performance.
We believe that eliminating charges related to the purchase accounting effects of our acquisitions and business combinations (particularly amortization and impairment of some intangible assets) enhances comparability of our ongoing operating performance relative to our peers. Those intangible assets (principally rights relating to research and development, technology platforms and commercialization of products) are accounted for in accordance with IAS 38 (Intangible Assets) and IFRS 3 (Business Combinations).
We also believe that eliminating the other effects of business combinations (such as the incremental cost of sales arising from the workdown of acquired inventories remeasured at fair value in business combinations) gives a better understanding of our recurring operating performance.
Eliminating restructuring costs and similar items enhances comparability with our peers because those costs are incurred in connection with reorganization and transformation processes intended to optimize our operations.
We believe that eliminating the effects of transactions that we regard as non-recurring and that involve particularly significant amounts (such as major gains and losses on disposals, and costs and provisions associated with major litigation and other major non-recurring items) improves comparability from one period to the next.
Finally, remeasurements recognized in profit or loss during the period in respect of (i) assets or liabilities accounted for at fair value and recognized in the balance sheet in connection with business acquisitions or divestments or (ii) liabilities accounted for at amortized cost and subject to periodic remeasurement, generally determined on the basis of revised sales forecasts, are not reflective of our operating performance.
We remind investors, however, that “Business net income” should not be considered in isolation from, or as a substitute for, Net income attributable to equity holders of Sanofi reported in accordance with IFRS. In addition, we strongly encourage investors and potential investors not to rely on any single financial measure but to review our financial statements, including the notes thereto, carefully and in their entirety.
We compensate for the material limitations described above by using “Business net income” only to supplement our IFRS financial reporting and by ensuring that our disclosures provide sufficient information for a full understanding of all adjustments included in “Business net income.”
Because our “Business net income” and “Business EPS” are not standardized measures, they may not be directly comparable with the non-IFRS financial measures of other companies using the same or similar non-IFRS financial measures.
A.1.6. Presentation of net sales
In the discussion below, we present our consolidated net sales for 2023 and 2022. We analyze our net sales by various categories including segment, franchise, product, and geographical region. In addition to reported net sales, we analyze non-IFRS financial measures designed to isolate the impact on our net sales of currency exchange rates and changes in the structure of our group.
When we refer to changes in our net sales at constant exchange rates (CER), that means that we have excluded the effect of exchange rates by recalculating net sales for the relevant period using the exchange rates that were used for the previous period.
A presentation of consolidated net sales for 2022 compared with 2021 is available in our annual report filed on February 24, 2023, in “Item 5. Operating and Financial Review and Prospects — A. Operating Results — A.2.1. Net Sales.”
A.1.7. Financial presentation of alliances
We have entered into a number of alliances for the development, co-promotion and/or co-marketing of our products. We believe that a presentation of our two principal alliances is useful to an understanding of our financial statements.
1/ Alliance arrangements with Regeneron Pharmaceuticals Inc. (Regeneron)
Collaboration agreements on human therapeutic antibodies
In November 2007, Sanofi and Regeneron signed two agreements (amended in November 2009) relating to human therapeutic antibodies: (i) the Discovery and Preclinical Development Agreement, and (ii) the License and Collaboration Agreement, relating to clinical development and commercialization. Under the License and Collaboration Agreement, Sanofi had an option to develop and commercialize antibodies discovered by Regeneron under the Discovery and Preclinical Development Agreement.
60 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
Discovery and development
Because Sanofi decided not to exercise its option to extend the Discovery and Preclinical Development Agreement, that agreement expired on December 31, 2017.
As a result of Sanofi’s exercise of an option with respect to an antibody under the Discovery and Preclinical Development Agreement, such antibody became a “Licensed Product” under the License and Collaboration Agreement, pursuant to which Sanofi and Regeneron co-develop the antibody with Sanofi initially being wholly responsible for funding the development program. On receipt of the first positive Phase 3 trial results for any antibody being developed under the License and Collaboration Agreement, the subsequent development costs for that antibody are split 80% Sanofi, 20% Regeneron. Amounts received from Regeneron under the License and Collaboration Agreement are recognized by Sanofi as a reduction in the line item Research and development expenses. Co-development with Regeneron of the antibodies DUPIXENT, KEVZARA and REGN3500 (SAR440340 - itepekimab) is ongoing under the License and Collaboration Agreement as of December 31, 2023.
Once a product begins to be commercialized, and provided that the share of quarterly results under the agreement represents a profit, Sanofi is entitled to an additional portion of Regeneron’s profit-share (capped at 20% of Regeneron’s share of quarterly profits since April 1, 2022, and at 10% until March 31, 2022) until Regeneron has paid 50% of the cumulative development costs incurred by the parties in the collaboration (see Note D.21.1.).
On the later of (i) 24 months before the scheduled launch date or (ii) the first positive Phase 3 trial results, Sanofi and Regeneron share the commercial expenses of the antibodies co-developed under the License and Collaboration Agreement.
Commercialization
Sanofi is the lead party with respect to the commercialization of all co-developed antibodies, and Regeneron has certain option rights to co-promote the antibodies. Regeneron has exercised its co-promotion rights in the United States and in certain other countries. Sanofi recognizes all sales of the antibodies. Profits and losses arising from commercial operations in the United States are split 50/50. Outside the United States, Sanofi is entitled to between 55% and 65% of profits depending on sales of the antibodies, and bears 55% of any losses. The share of profits and losses due to or from Regeneron under the agreement is recognized within the line items Other operating income or Other operating expenses, which are components of Operating income.
In addition, Regeneron is entitled to receive payments contingent on the attainment of specified levels of aggregate sales on all antibodies outside the United States, on a rolling twelve-month basis. A liability for those payments is recognized on the balance sheet when it is probable that the specified level of aggregate sales will be met. The opposite entry for that liability is capitalized within Other intangible assets on the balance sheet. Two payments of $50 million each were made in 2022, following attainment first of $2.0 billion and then of $2.5 billion in sales of all antibodies outside the United States on a rolling twelve-month basis. The final milestone payment of $50 million, payable to Regeneron in the event that $3.0 billion in sales on a rolling twelve-month basis is attained, was made in 2023.
Amendments to the collaboration agreements
In January 2018, Sanofi and Regeneron signed a set of amendments to their collaboration agreements, including an amendment that allowed for the funding of additional programs on DUPIXENT and REGN3500 (SAR440340 – itepekimab) with an intended focus on extending the current range of indications, finding new indications, and improving co-morbidity between multiple pathologies.
Effective April 1, 2020, Sanofi and Regeneron signed a Cross License and Commercialization Agreement for PRALUENT, whereby Sanofi obtained sole ex-US rights to PRALUENT, and Regeneron obtained sole US rights to PRALUENT along with a right to 5% royalties on Sanofi’s sales of PRALUENT outside the United States. Each party is solely responsible for funding the development, manufacturing and commercialization of PRALUENT in their respective territories. Although each party has sole responsibility for supplying PRALUENT in its respective territory, Sanofi and Regeneron entered into agreements to support manufacturing needs for each other.
Effective September 30, 2021, Sanofi and Regeneron signed an amendment to their collaboration agreement in order to specify allocations of responsibilities and associated resources between the two parties in connection with the co-promotion of DUPIXENT in certain countries. The terms of the collaboration relating to REGN3500 (SAR440340 – itepekimab) are unchanged.
Effective July 1, 2022, Sanofi and Regeneron signed an amendment to their collaboration agreement in order to increase the additional portion of Regeneron’s quarterly profit-share attributable to Sanofi from 10% to 20% with retroactive impact as of April 1, 2022.
Immuno-oncology (IO) collaboration agreements
On July 1, 2015, Sanofi and Regeneron signed two agreements – the IO Discovery and Development Agreement and the IO License and Collaboration Agreement (IO LCA) – relating to new antibody cancer treatments in the field of immuno-oncology.
The Amended IO Discovery Agreement, effective from December 31, 2018, was terminated through a Letter Amendment dated March 16, 2021 in which Sanofi formalized its opt-out from the BCMAxCD3 and MUC16xCD3 programs.
SANOFI FORM 20-F 2023 | 61 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
LIBTAYO (cemiplimab)
Under the 2015 IO LCA as amended in January 2018, Sanofi and Regeneron committed funding of no more than $1,640 million, split on a 50/50 basis ($820 million per company), for the development of REGN2810 (cemiplimab, trademark LIBTAYO), a PD-1 inhibitor antibody. The funding was raised to $1,840 million by way of amendment effective on September 30, 2021. Regeneron was responsible for the commercialization of LIBTAYO in the United States, and Sanofi in all other territories. Sanofi has exercised its option to co-promote LIBTAYO in the United States. In 2021, Regeneron exercised its option to co-promote LIBTAYO in certain other countries.
The IO LCA also provided for a one-time milestone payment of $375 million by Sanofi to Regeneron in the event that sales of a PD-1 product were to exceed, in the aggregate, $2 billion in any consecutive 12-month period.
Under the IO LCA Sanofi and Regeneron shared equally in profits and losses generated by the commercialization of collaboration products, except that Sanofi was entitled to an additional portion of Regeneron’s profit-share (capped at 10% of Regeneron’s share of quarterly profits) until Regeneron had paid 50% of the cumulative development costs incurred by the parties under the IO Discovery Agreement, as amended.
LIBTAYO is approved in the United States and Europe for the treatment of two types of locally advanced or metastatic skin cancer (cutaneous squamous cell carcinoma and basal cell carcinoma) and non-small cell lung cancer (NSCLC). It is also approved in Brazil and Canada as a second line treatment for recurring or metastatic cervical cancer. In the fourth quarter of 2022, it was approved in the United States in association with chemotherapy for the treatment of NSCLC, and in Europe and Japan as a second line treatment for recurring or metastatic cervical cancer. LIBTAYO is currently approved in more than 30 countries.
In June 2022, Sanofi and Regeneron restructured their IO LCA. Under the terms of the Amended and Restated IO LCA, Regeneron holds exclusive worldwide licensing rights to LIBTAYO with effect from July 1, 2022.
In July 2022, Sanofi received as consideration an upfront payment of $900 million (€856 million), which was recognized within Other operating income on the date of receipt. The same line item also includes a regulatory milestone payment of $100 million (€96 million) following the US FDA approval in November 2022 of LIBTAYO in combination with chemotherapy as a first line treatment for NSCLC. In addition, Sanofi is entitled to royalties of 11% and to milestone payments (€116 million in 2023, €111 million in 2022) linked to global net sales of LIBTAYO; those royalties are recognized within Other operating income in line with the pattern of sales. All of the cash inflows relating to the above items (€196 million in 2023, €952 million in 2022) are presented within Net cash provided by/(used in) operating activities in the consolidated statement of cash flows.
The amendment to the terms of the IO LCA resulted in Sanofi recognizing an accelerated amortization charge of €226 million in 2022; this was allocated to the LIBTAYO product rights included within the residual carrying amount of the intangible asset recognized in July 2015 to reflect rights to an antibody targeting the immune checkpoint receptor PD-1 (programmed cell death protein-1) under the Sanofi/Regeneron alliance.
The transaction also includes a time-limited transitional services agreement with Regeneron which includes manufacturing, distribution (for which Sanofi acts as agent), and promotion.
Investor agreement
In 2014 and 2020, Sanofi and Regeneron amended the investor agreement entered into by the two companies in 2007. Under the terms of the amendments, Sanofi accepted various restrictions, including “standstill” provisions that contractually prohibit Sanofi from seeking to directly or indirectly exert control of Regeneron or acquiring more than 30% of Regeneron’s capital stock (consisting of the outstanding shares of common stock and the shares of Class A stock). This prohibition remains in place until the earlier of (i) the later of the fifth anniversaries of the expiration or earlier termination of the ZALTRAP collaboration agreement with Regeneron (related to the development and commercialization of ZALTRAP) or the collaboration agreement with Regeneron on monoclonal antibodies (see “Collaboration agreements on human therapeutic antibodies” above), each as amended or (ii) other specified events.
Starting in 2018 Sanofi began to sell shares of Regeneron stock and announced on May 29, 2020 the closing of its sale of 13 million shares of Regeneron common stock in a registered offering and a private sale to Regeneron (see Note D.2.).
Pursuant to subsequent sales in 2022, Sanofi no longer holds any shares of Regeneron stock, as of December 31, 2023.
2/ Agreements on the commercialization of BEYFORTUS (nirsevimab, previously MEDI8897) in the US
On March 1, 2017, Sanofi and AstraZeneca entered into an agreement to develop and commercialize a monoclonal antibody (MEDI8897, nirsevimab) for the prevention of Respiratory Syncytial Virus (RSV) associated illness in newborns and infants.
Under the terms of the agreement, Sanofi made an upfront payment of €120 million in March 2017, a development milestone payment of €30 million in the third quarter of 2019, a regulatory milestone payment of €25 million associated with the approval of BEYFORTUS (nirsevimab) by the EMA in Europe in November 2022, and a regulatory milestone payment of €65 million associated with the approval of BEYFORTUS (nirsevimab) by the US FDA in July 2023. In addition, Sanofi could pay up to €375 million if sales objectives are met. Those amounts are recognized as a component of the value of the intangible asset when payment becomes probable. During 2023, an amount of €25 million was recognized as an accrued expense further to a contractual threshold being passed.
62 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
The agreement also specifies that AstraZeneca is responsible for development and manufacturing, and Sanofi for commercialization. Sanofi recognizes the sales and cost of sales (purchases of finished products from AstraZeneca) and shares the Alliance’s commercial profits (i) 50/50 in major territories and (ii) based on 25% of net sales in other territories. The share of commercial profits and losses due to or from AstraZeneca is recognized as a component of operating income, within the line items Other operating income or Other operating expenses. In addition, Sanofi and AstraZeneca share development costs 50/50, with Sanofi’s portion recognized within the income statement line item Research and development expenses.
On April 9, 2023, Sanofi and AstraZeneca simplified their contractual agreements for the development and commercialization of BEYFORTUS (nirsevimab) in the US. Sanofi thereby obtained control of all commercial rights to BEYFORTUS (nirsevimab) in the US, and ended the sharing of commercial profits between the two partners in that territory. In line with the terms of the revised agreements and in accordance with IAS 38, Sanofi recognized an intangible asset of €1.6 billion for the fair value of the additional US rights. On the same date, AstraZeneca and Sobi ended their participation agreement, signed in 2018, which transferred the economic rights for the US territory to Sobi.
Sanofi simultaneously entered into an agreement with Sobi relating to direct royalties on US net sales of BEYFORTUS (nirsevimab). In line with the terms of that agreement, on April 9, 2023 Sanofi recognized a financial liability amounting to €1.6 billion. That liability is classified as a financial liability at amortized cost under IFRS 9. Other than royalty payments, subsequent movements in the liability comprise (i) the unwinding of discount and (ii) changes in estimates of future cash outflows for royalty payments. Those movements will be recognized in the income statement within Net financial income/(expenses) in accordance with paragraph B.5.4.6 of IFRS 9.
As of December 31, 2023 the liability was remeasured by an amount of €541 million, reflecting the strong success of the US launch of BEYFORTUS (nirsevimab), which led to sales forecasts being revised upward from the initial estimate. The resulting adjustment was recognized within Financial expenses.
For territories other than the US (except for China, which is now considered a “major market,” with profits/losses shared 50/50 with AstraZeneca), the existing agreement between AstraZeneca and Sanofi continues to govern the principal terms of the collaboration: Sanofi recognizes the sales and cost of sales and shares the Alliance’s commercial profits with AstraZeneca.
In May 2023, data from the HARMONIE Phase 3b study confirmed that nirsevimab prevents infant hospitalizations due to RSV with consistent and high efficacy.
BEYFORTUS (nirsevimab) was approved in Europe in November 2022, and in the United States on July 17, 2023.
A.1.8. Impact of exchange rates
We report our consolidated financial statements in euros. Because we earn a significant portion of our revenues in countries where the euro is not the local currency, our results of operations can be significantly affected by exchange rate movements between the euro and other currencies, primarily the US dollar and, to a lesser extent, the Japanese yen, and currencies in emerging countries. We experience these effects even though certain of these countries do not account for a large portion of our net sales. In 2023, we earned 43.0% of our net sales in the United States. An increase in the value of the US dollar against the euro has a positive impact on both our revenues and our operating income. A decrease in the value of the US dollar against the euro has a negative impact on our revenues, which is not offset by an equal reduction in our costs and therefore negatively affects our operating income. A variation in the value of the US dollar has a particularly significant impact on our operating income, which is higher in the United States than elsewhere.
For a description of arrangements entered into to manage operating foreign exchange risks as well as our hedging policy, see “Item 11. Quantitative and Qualitative Disclosures about Market Risk,” and “Item 3. Key Information — D. Risk Factors — Risks Related to Financial Markets — Fluctuations in currency exchange rates could adversely affect our results of operations and financial condition.”
A.1.9. Divestments
Reserved.
A.1.10. Acquisitions
On July 28, 2023, Sanofi agreed to acquire QRIB Intermediate Holdings, LLC (QRIB), the owner of QUNOL, a market-leading US-based health & wellness brand. The acquisition strengthened Sanofi’s CHC operations in the Vitamin, Mineral and Supplements (VMS) category. The acquisition of QRIB by Sanofi was completed on September 29, 2023, at a purchase price of $1,419 million. The impact of this acquisition is reflected in Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows and represents a net cash outflow of $1,410 million.
On March 13, 2023, Sanofi entered into a merger agreement with Provention Bio, Inc. (Provention), a US-based publicly traded biopharmaceutical company developing therapies to prevent and intercept immune-mediated diseases including type 1 diabetes. Under the terms of the agreement, Sanofi acquired the outstanding shares of Provention common stock for $25.00 per share in an all-cash transaction valued at approximately $2.8 billion. The acquisition of Provention was completed on April 27, 2023, with Sanofi holding all of the shares of Provention on expiration of the tender offer. The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of $2,722 million.
SANOFI FORM 20-F 2023 | 63 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
On February 8, 2022, Sanofi acquired the entire share capital of the immuno-oncology company Amunix Pharmaceuticals, Inc. (Amunix), thereby gaining access to Amunix’s innovative PRO-XTEN technology and a promising pipeline of immunotherapies. The acquisition price of Amunix comprised a fixed payment of €970 million, plus contingent consideration in the form of milestone payments based on attainment of certain future development objectives of up to $225 million, the fair value of which as of the acquisition date was €156 million. The impact of this acquisition was reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of €852 million.
For further information about the acquisitions mentioned above, see Notes D.1. and D.2. to our consolidated financial statements included at Item 18. of this annual report.
A.1.11. Critical accounting and reporting policies
Our consolidated financial statements are affected by the accounting and reporting policies that we use. Certain of our accounting and reporting policies are critical to an understanding of our results of operations and financial condition, and in some cases the application of these critical policies can be significantly affected by the estimates, judgments and assumptions made by management during the preparation of our consolidated financial statements. The accounting and reporting policies that we have identified as fundamental to a full understanding of our results of operations and financial condition are the following:
1/ Revenue recognition
Our policies with respect to revenue recognition are discussed in Note B.13. to our consolidated financial statements included at Item 18. of this annual report. Revenue arising from the sale of goods is presented in the income statement within Net sales. Net sales comprise revenue from sales of pharmaceutical products, consumer healthcare products, active ingredients and vaccines, net of sales returns, of customer incentives and discounts, and of certain sales-based payments paid or payable to the healthcare authorities. In accordance with IFRS 15 (Revenue from Contracts with Customers), such revenue is recognized when Sanofi transfers control over the product to the customer. Control refers to the ability to direct the use of, and obtain substantially all of the remaining benefits from, the products. For the vast majority of contracts, revenue is recognized when the product is physically transferred, in accordance with the delivery and acceptance terms agreed with the customer.
For contracts entered into by Sanofi Pasteur, transfer of control is usually determined by reference to the terms of release (immediate or deferred) and acceptance of batches of vaccine.
As regards contracts with distributors, Sanofi does not recognize revenue when the product is physically transferred to the distributor in case of products sold on consignment, or if the distributor acts as an agent. In such cases, revenue is recognized when control is transferred to the end customer, and the distributor’s commission is presented within the line item Selling and general expenses in the income statement.
We offer various types of price reductions on our products. In particular, products sold in the United States are covered by various programs (such as Medicare and Medicaid) under which products are sold at a discount. Rebates are granted to healthcare authorities, and under contractual arrangements with certain customers. Some wholesalers are entitled to chargeback incentives based on the selling price to the end customer, under specific contractual arrangements. Cash discounts may also be granted for prompt payment. The discounts, incentives and rebates described above are estimated on the basis of specific contractual arrangements with our customers or of specific terms of the relevant regulations and/or agreements applicable for transactions with healthcare authorities, and of assumptions about the attainment of sales targets. We also estimate the amount of sales returns, on the basis of contractual sales terms and reliable historical data. Discounts, incentives, rebates and sales returns are recognized in the period in which the underlying sales are recognized within Net Sales, as a reduction of gross sales. For additional details regarding the financial impact of discounts, incentives, rebates and sales returns, see Note D.23. to our consolidated financial statements included at Item 18. of this annual report.
Revenues from non-Sanofi products, mainly comprising royalty income from license arrangements and sales of non-Sanofi products by our US-based entity VaxServe, are presented within Other revenues. This line item also includes revenues arising from the distribution of ELOCTATE and ALPROLIX under Sanofi’s agreements with Swedish Orphan Biovitrum AB (Sobi) and revenue received under agreements for Sanofi to provide manufacturing services to third parties.
2/ Business combinations
As discussed in Note B.3. “Business combinations and transactions with non-controlling interests” to our consolidated financial statements included at Item 18. of this annual report, business combinations are accounted for by the acquisition method. The acquiree’s identifiable assets and liabilities that satisfy the recognition criteria of IFRS 3 (Business Combinations) are measured initially at their fair values as at the acquisition date, except for (i) non-current assets classified as held for sale, which are measured at fair value less costs to sell and (ii) assets and liabilities that fall within the scope of IAS 12 (Income Taxes) and IAS 19 (Employee Benefits). Business combinations completed on or after January 1, 2010 are accounted for in accordance with the revised IFRS 3 and IFRS 10 (Consolidated Financial Statements). In particular, contingent consideration payable to former owners agreed in a business combination, e.g. in the form of payments upon the achievement of certain R&D milestones, is recognized as a liability at fair value as of the acquisition date irrespective of the probability of payment. If the contingent consideration was originally recognized as a liability, subsequent adjustments to the liability are recognized in profit or loss (see Note D.18. “Liabilities related to business combinations and non-controlling interests” to our consolidated financial statements included at Item 18. of this annual report).
64 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
3/ Impairment of goodwill and intangible assets
As discussed in Note B.6. “Impairment of property, plant and equipment, intangible assets, and investments accounted for using the equity method” and in Note D.5. “Impairment of intangible assets and property, plant and equipment” to our consolidated financial statements included at Item 18. of this annual report, we test our intangible assets for impairment periodically or when there is any internal or external indication of impairment. Such indicators could include primarily but not exclusively (i) increased market competition resulting from (for example) the introduction of a competitor’s product; (ii) earlier than expected loss of exclusivity; (iii) increased pricing pressure; (iv) restrictions imposed by regulatory authorities on the manufacture or sale of a product; (v) delay in the projected launch of a product; (vi) different from expected clinical trial results; (vii) higher than expected development costs or (viii) lower than expected economic performance.
We test for impairment on the basis of the same objective criteria that were used for the initial valuation. Our initial valuation and ongoing tests are based on the relationship of the value of our projected future cash flows associated with the asset to either the purchase price of the asset (for its initial valuation) or the carrying amount of the asset (for ongoing tests for impairment).
Significant underlying assumptions requiring the exercise of considerable judgement are applied in the future cash flow projections used to determine the recoverability of intangible assets, including primarily but not exclusively (i) therapeutic class market growth drivers; (ii) expected impacts from competing products (including but not exclusively generics and biosimilars); (iii) projected pricing and operating margin levels; (iv) likely changes in the regulatory, legal or tax environment; and (v) management’s estimates of terminal growth or attrition rates.
The recoverable amounts of intangible assets related to research and development projects are determined based on future net cash flows, which reflect the development stage of the project and the associated probability of success of marketization of the compound.
The projected cash flows are discounted to present value using a discount rate, which factors in the risks inherent in cash flow projections.
Changes in facts and circumstances, assumptions and/or estimates may lead to future additional impairment losses or reversal of impairment previously recorded.
Key assumptions relating to goodwill impairment are the perpetual growth rate and the post-tax discount rate. A sensitivity analysis to the key assumptions is disclosed in Note D.5. “Impairment of intangible assets and property, plant and equipment” to our consolidated financial statements included at Item 18. of this annual report.
4/ Pensions and post-retirement benefits
As described in Note B.23. “Employee benefit obligations” to our consolidated financial statements included at Item 18. of this annual report, we recognize our pension and retirement benefit commitments as liabilities on the basis of an actuarial estimate of the rights vested in employees and retirees at the end of the reporting period, net of the fair value of plan assets held to meet those obligations. We prepare this estimate at least on an annual basis taking into account financial assumptions (such as discount rates) and demographic assumptions (such as life expectancy, retirement age, employee turnover, and the rate of salary increases).
We recognize all actuarial gains and losses (including the impact of a change in discount rate) immediately through equity.
Depending on the key assumptions used, the pension and post-retirement benefit expense could vary within a range of outcomes and have a material effect on reported earnings. A sensitivity analysis to these key assumptions is set forth in Note D.19.1. “Provisions for pensions and other benefits” to our consolidated financial statements included at Item 18. of this annual report.
5/ Taxes
As discussed in Note B.22. “Income tax expense” to our consolidated financial statements included at Item 18. of this annual report, we recognize deferred income taxes on tax loss carry-forwards and on temporary differences between the tax base and carrying amount of assets and liabilities. We calculate our deferred tax assets and liabilities using enacted tax rates applicable for the years during which we estimate that the temporary differences are expected to reverse. We do not recognize deferred tax assets when it is more likely than not that the deferred tax assets will not be realized. The recognition of deferred tax assets is determined on the basis of profit forecasts for each tax group, and of the tax consequences of the strategic opportunities available to Sanofi.
The positions adopted by Sanofi in tax matters are based on its interpretation of tax laws and regulations. Some of those positions may be subject to uncertainty. In such cases, Sanofi assesses the amount of the tax liability on the basis of the following assumptions: that its position will be examined by one or more tax authorities on the basis of all relevant information; that a technical assessment is carried out with reference to legislation, case law, regulations, and established practice; and that each position is assessed individually (or collectively where appropriate), with no offset or aggregation between positions. Those assumptions are assessed on the basis of facts and circumstances existing at the end of the reporting period. When an uncertain tax liability is regarded as probable, it is measured on the basis of Sanofi’s best estimate and recognized as a liability; uncertain tax assets are not recognized.
SANOFI FORM 20-F 2023 | 65 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
6/ Provisions for risks
Sanofi and its subsidiaries and affiliates may be involved in litigation, arbitration or other legal proceedings. These proceedings typically are related to product liability claims, intellectual property rights, compliance and trade practices, commercial claims, employment and wrongful discharge claims, tax assessment claims, waste disposal and pollution claims, and claims under warranties or indemnification arrangements relating to business divestitures. As discussed in Note B.12. “Provisions for risks” to our consolidated financial statements included at Item 18. of this annual report, we record a provision where we have a present obligation, whether legal or constructive, as a result of a past event; it is probable that an outflow of resources embodying economic benefits will be required to settle the obligation; and a reliable estimate can be made of the amount of the outflow of resources. We also disclose a contingent liability in circumstances where we are unable to make a reasonable estimate of the expected financial effect that will result from the ultimate resolution of the proceeding, or a cash outflow is not probable.
For additional details regarding the financial impact of provisions for risks see Notes D.19.3. “Other provisions” and D.22. “Legal and Arbitral Proceedings” to our consolidated financial statements included at Item 18. of this annual report.
7/ Provisions for restructuring costs
Provisions for restructuring costs include collective redundancy or early retirement benefits, compensation for early termination of contracts, and rationalization costs relating to restructured sites. Refer to Note D.19.2. to our consolidated financial statements included at Item 18. of this annual report.
Provisions are estimated on the basis of events and circumstances related to present obligations at the end of the reporting period and of past experience, and to the best of management’s knowledge at the date of preparation of the financial statements. The assessment of provisions can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions. Given the inherent uncertainties related to these estimates and assumptions, the actual outflows resulting from the realization of those risks could differ from our estimates.
A.2. Results of operations – Year ended December 31, 2023 compared with year ended December 31, 2022
Consolidated income statements
| (€ million) | 2023 | as % of net sales | 2022 | as % of net sales | ||||||||||
| Net sales | 43,070 | 100.0 | % | 42,997 | 100.0 | % | ||||||||
| Other revenues | 3,374 | 7.8 | % | 2,392 | 5.6 | % | ||||||||
| Cost of sales | (14,236) | -33.1 | % | (13,695) | -31.9 | % | ||||||||
| Gross profit | 32,208 | 74.8 | % | 31,694 | 73.7 | % | ||||||||
| Research and development expenses | (6,728) | -15.6 | % | (6,706) | -15.6 | % | ||||||||
| Selling and general expenses | (10,692) | -24.8 | % | (10,492) | -24.4 | % | ||||||||
| Other operating income | 1,292 | 1,969 | ||||||||||||
| Other operating expenses | (3,516) | (2,531) | ||||||||||||
| Amortization of intangible assets | (2,172) | (2,053) | ||||||||||||
| Impairment of intangible assets | (896) | 454 | ||||||||||||
| Fair value remeasurement of contingent consideration | (93) | 27 | ||||||||||||
| Restructuring costs and similar items | (1,490) | (1,336) | ||||||||||||
| Other gains and losses, and litigation | (38) | (370) | ||||||||||||
| Operating income | 7,875 | 18.3 | % | 10,656 | 24.8 | % | ||||||||
| Financial expenses | (1,313) | (440) | ||||||||||||
| Financial income | 591 | 206 | ||||||||||||
| Income before tax and investments accounted for using the equity method | 7,153 | 16.6 | % | 10,422 | 24.2 | % | ||||||||
| Income tax expense | (1,602) | (2,006) | ||||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | (115) | 68 | ||||||||||||
| Net income | 5,436 | 12.6 | % | 8,484 | 19.7 | % | ||||||||
| Net income attributable to non-controlling interests | 36 | 113 | ||||||||||||
| Net income attributable to equity holders of Sanofi | 5,400 | 12.5 | % | 8,371 | 19.5 | % | ||||||||
| Average number of shares outstanding (million) | 1,251.7 | 1,251.9 | ||||||||||||
| Average number of shares after dilution (million) | 1,256.4 | 1,256.9 | ||||||||||||
•Basic earnings per share (€) | 4.31 | 6.69 | ||||||||||||
•Diluted earnings per share (€) | 4.30 | 6.66 | ||||||||||||
66 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
A.2.1. Net sales
Consolidated net sales for the year ended December 31, 2023 amounted to €43,070 million, 0.2% higher than in 2022 on a reported basis. Exchange rate fluctuations had a negative effect of 5.1 percentage points overall, due mainly to adverse trends in the US dollar and Argentine peso against the euro. At CER(1), net sales rose by 5.3%, mainly reflecting strong growth for DUPIXENT and increased sales for our Vaccines business, more than offsetting lower sales for of Non-Core Assets within the General Medicines GBU.
Reconciliation of Net sales (IFRS) to net sales at CER (non-IFRS)
| (€ million) | 2023 | 2022 | Change | ||||||||
| Net sales (IFRS) | 43,070 | 42,997 | +0.2 | % | |||||||
| Effect of exchange rates | 2,189 | ||||||||||
Net sales at constant exchange rates (non-IFRS) | 45,259 | 42,997 | +5.3 | % | |||||||
To facilitate analysis and comparisons with prior periods, some figures are given at CER.
We calculate net sales at CER by recalculating net sales for the relevant period using the exchange rates that were used for the previous period.
1/ Net sales by operating segment
Our net sales comprise the net sales generated by our Biopharma and Consumer Healthcare segments.
(€ million) | 2023 | 2022 | Change on a reported basis | Change at constant exchange rates | ||||||||||
Biopharma segment | 37,890 | 37,812 | +0.2 | % | +5.1 | % | ||||||||
Consumer Healthcare segment | 5,180 | 5,185 | -0.1 | % | +6.3 | % | ||||||||
| Total net sales | 43,070 | 42,997 | +0.2 | % | +5.3 | % | ||||||||
(1) Non-IFRS financial measure: see definition in “— Presentation of Net Sales.”
SANOFI FORM 20-F 2023 | 67 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
2/ Net sales by franchise, geographical region and product
(€ million) | Net sales | Change (CER) | Change (reported) | United States | Change (CER) | Europe | Change (CER) | Rest of the world | Change (CER) | ||||||||||||||||||||
| DUPIXENT | 10,715 | +34.0 | % | +29.2 | % | 8,145 | +32.6 | % | 1,224 | +30.9 | % | 1,346 | +46.1 | % | |||||||||||||||
| AUBAGIO | 955 | -52.6 | % | -53.0 | % | 460 | -67.8 | % | 437 | -14.3 | % | 58 | -33.0 | % | |||||||||||||||
| MYOZYME | 783 | -15.1 | % | -18.3 | % | 254 | -17.9 | % | 341 | -16.4 | % | 188 | -9.1 | % | |||||||||||||||
| FABRAZYME | 991 | +11.2 | % | +5.7 | % | 503 | +9.8 | % | 241 | +6.1 | % | 247 | +18.8 | % | |||||||||||||||
| CEREZYME | 687 | +9.1 | % | -2.8 | % | 189 | +0.5 | % | 229 | -3.3 | % | 269 | +25.9 | % | |||||||||||||||
| ELOCTATE | 471 | -15.5 | % | -18.8 | % | 341 | -22.0 | % | — | — | % | 130 | +6.9 | % | |||||||||||||||
| ALPROLIX | 540 | +11.3 | % | +7.1 | % | 440 | +11.6 | % | — | — | % | 100 | +10.2 | % | |||||||||||||||
| NEXVIAZYME | 425 | +126.0 | % | +116.8 | % | 272 | +77.8 | % | 100 | +494.1 | % | 53 | +190.5 | % | |||||||||||||||
| JEVTANA | 320 | -14.8 | % | -18.2 | % | 230 | -14.2 | % | 12 | -63.6 | % | 78 | +2.4 | % | |||||||||||||||
| SARCLISA | 381 | +37.1 | % | +29.6 | % | 165 | +33.9 | % | 111 | +27.3 | % | 105 | +53.2 | % | |||||||||||||||
| KEVZARA | 357 | +9.7 | % | +5.3 | % | 195 | +8.6 | % | 115 | +8.5 | % | 47 | +17.0 | % | |||||||||||||||
| CERDELGA | 298 | +6.9 | % | +3.5 | % | 164 | +5.6 | % | 118 | +6.3 | % | 16 | +23.5 | % | |||||||||||||||
| ALDURAZYME | 279 | +12.0 | % | +4.5 | % | 67 | +13.1 | % | 82 | -4.7 | % | 130 | +23.3 | % | |||||||||||||||
| CABLIVI | 227 | +10.0 | % | +7.6 | % | 112 | +4.5 | % | 98 | +4.3 | % | 17 | +171.4 | % | |||||||||||||||
| FASTURTEC | 170 | -1.1 | % | -4.0 | % | 110 | — | % | 43 | -8.3 | % | 17 | +12.5 | % | |||||||||||||||
| ENJAYMO | 72 | +240.9 | % | +227.3 | % | 42 | +152.9 | % | 6 | — | % | 24 | +420.0 | % | |||||||||||||||
| XENPOZYME | 91 | +347.6 | % | +333.3 | % | 52 | +980.0 | % | 31 | +106.7 | % | 8 | +800.0 | % | |||||||||||||||
ALTUVIIIO | 159 | — | % | — | % | 155 | — | % | — | — | % | 4 | — | % | |||||||||||||||
Other | 119 | -46.3 | % | -50.4 | % | 21 | -32.3 | % | 18 | -80.4 | % | 80 | -23.1 | % | |||||||||||||||
| Total Specialty Care | 18,040 | +14.2 | % | +9.6 | % | 11,917 | +13.2 | % | 3,206 | +6.7 | % | 2,917 | +26.6 | % | |||||||||||||||
| TOUJEO | 1,123 | +6.2 | % | +0.5 | % | 213 | -23.0 | % | 441 | +5.5 | % | 469 | +26.9 | % | |||||||||||||||
| LOVENOX | 1,125 | -8.7 | % | -14.1 | % | 7 | -58.8 | % | 622 | -5.5 | % | 496 | -10.7 | % | |||||||||||||||
| PLAVIX | 948 | +4.4 | % | -3.6 | % | 8 | -11.1 | % | 96 | -5.0 | % | 844 | +5.6 | % | |||||||||||||||
| THYMOGLOBULIN | 478 | +14.1 | % | +7.2 | % | 292 | +11.9 | % | 37 | +8.8 | % | 149 | +19.6 | % | |||||||||||||||
| MULTAQ | 344 | -7.6 | % | -10.2 | % | 310 | -8.1 | % | 12 | -25.0 | % | 22 | +15.0 | % | |||||||||||||||
| PRALUENT | 422 | +15.2 | % | +12.2 | % | (1) | -101.8 | % | 296 | +30.6 | % | 127 | +46.7 | % | |||||||||||||||
REZUROCK | 310 | +54.6 | % | +49.8 | % | 303 | +51.9 | % | 5 | +400.0 | % | 2 | — | % | |||||||||||||||
| MOZOBIL | 220 | -14.6 | % | -15.7 | % | 119 | -22.1 | % | 70 | +6.0 | % | 31 | -20.0 | % | |||||||||||||||
| SOLIQUA/SULIQUA | 217 | +5.6 | % | +0.9 | % | 95 | -18.5 | % | 35 | +24.1 | % | 87 | +40.3 | % | |||||||||||||||
| Other Core Assets | 1,083 | +3.5 | % | -0.7 | % | 139 | -25.8 | % | 374 | +3.9 | % | 570 | +13.8 | % | |||||||||||||||
| Total Core Assets | 6,270 | +3.3 | % | -1.9 | % | 1,485 | -7.7 | % | 1,988 | +4.2 | % | 2,797 | +9.3 | % | |||||||||||||||
| LANTUS | 1,420 | -32.3 | % | -37.1 | % | 281 | -62.6 | % | 357 | -15.7 | % | 782 | -17.5 | % | |||||||||||||||
| APROVEL/AVAPRO | 417 | -8.8 | % | -12.8 | % | 9 | +28.6 | % | 78 | -4.9 | % | 330 | -10.3 | % | |||||||||||||||
| Other Non-Core Assets | 3,687 | -9.2 | % | -15.8 | % | 302 | -24.5 | % | 961 | -14.6 | % | 2,424 | -4.9 | % | |||||||||||||||
| Total Non-Core Assets | 5,524 | -16.5 | % | -22.4 | % | 592 | -48.7 | % | 1,396 | -14.4 | % | 3,536 | -8.5 | % | |||||||||||||||
| Industrial Sales | 582 | -5.5 | % | -6.1 | % | 7 | -58.8 | % | 548 | -6.1 | % | 27 | +75.0 | % | |||||||||||||||
Total General Medicines | 12,376 | -7.1 | % | -12.4 | % | 2,084 | -24.9 | % | 3,932 | -4.6 | % | 6,360 | -1.3 | % | |||||||||||||||
| Influenza vaccines | 2,669 | -5.5 | % | -10.3 | % | 1,406 | -12.8 | % | 694 | +1.9 | % | 569 | +8.2 | % | |||||||||||||||
| Polio/Pertussis/Hib vaccines | 2,165 | -0.1 | % | -5.3 | % | 398 | -10.5 | % | 297 | -8.6 | % | 1,470 | +4.9 | % | |||||||||||||||
| Meningitis, travel and endemics vaccines | 1,170 | +0.5 | % | -3.5 | % | 650 | -0.7 | % | 154 | +41.3 | % | 366 | -8.1 | % | |||||||||||||||
| Booster vaccines | 598 | +5.1 | % | +1.9 | % | 323 | +1.2 | % | 180 | +16.9 | % | 95 | — | % | |||||||||||||||
| Respiratory Syncytial Virus | 547 | — | % | — | % | 407 | — | % | 140 | — | % | — | — | % | |||||||||||||||
| Other vaccines | 325 | +96.4 | % | +94.6 | % | 80 | -2.4 | % | 232 | +223.6 | % | 13 | +18.2 | % | |||||||||||||||
| Total Vaccines | 7,474 | +8.3 | % | +3.4 | % | 3,264 | +4.9 | % | 1,697 | +26.6 | % | 2,513 | +3.3 | % | |||||||||||||||
| Total Biopharma | 37,890 | +5.1 | % | +0.2 | % | 17,265 | +5.2 | % | 8,835 | +4.3 | % | 11,790 | +5.5 | % | |||||||||||||||
| Allergy | 769 | +4.3 | % | -0.1 | % | 412 | -4.6 | % | 70 | +29.1 | % | 287 | +13.4 | % | |||||||||||||||
| Cough, Cold and Flu | 512 | +11.1 | % | +7.1 | % | — | — | % | 300 | +14.1 | % | 212 | +7.4 | % | |||||||||||||||
| Pain Care | 1,106 | +0.6 | % | -3.0 | % | 180 | -12.7 | % | 502 | -0.6 | % | 424 | +8.7 | % | |||||||||||||||
| Digestive Wellness | 1,502 | +15.6 | % | +3.7 | % | 138 | -2.1 | % | 520 | +8.1 | % | 844 | +23.1 | % | |||||||||||||||
Physical and Mental Wellness | 606 | +12.5 | % | +6.9 | % | 118 | +139.2 | % | 126 | -3.1 | % | 362 | +1.0 | % | |||||||||||||||
| Personal Care | 550 | -3.2 | % | -6.1 | % | 409 | -7.3 | % | 1 | — | % | 140 | +10.6 | % | |||||||||||||||
| Non-Core/Other | 135 | -23.1 | % | -30.8 | % | (10) | — | % | 38 | -41.5 | % | 107 | -12.9 | % | |||||||||||||||
| Total Consumer Healthcare | 5,180 | +6.3 | % | -0.1 | % | 1,247 | -0.9 | % | 1,557 | +3.9 | % | 2,376 | +11.7 | % | |||||||||||||||
| Total Sanofi | 43,070 | +5.3 | % | +0.2 | % | 18,512 | +4.8 | % | 10,392 | +4.3 | % | 14,166 | +6.5 | % | |||||||||||||||
68 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
3/ Net sales – Biopharma segment
Since 2023, our new Biopharma segment comprises our Specialty Care, General Medicines and Vaccines businesses (see “Item 5.A.1.5, Segment Information” for detailed disclosures about our operating segments and Note D.35. to our consolidated financial statements included at Item 18. of this annual report).
In 2023, net sales for the Biopharma segment amounted to €37,890 million, up 0.2% on a reported basis and 5.1% at CER. The year-on-year reported-basis increase of €78 million reflects adverse exchange rate effects amounting to €1,858 million, and the following principal effects at CER:
•a solid performance from DUPIXENT (+€2,822 million) and the launches of NEXVIAZYME (+ €247 million) and ALTUVIIIO
(+€168 million), which more than offset a drop in sales of AUBAGIO (-€1,069 million);
(+€168 million), which more than offset a drop in sales of AUBAGIO (-€1,069 million);
•a 16.5% decrease in sales of Non-Core Assets within the General Medicines franchise (-€1,176 million);
•the launch of BEYFORTUS (+€572 million).
Comments on the performances of our major Biopharma segment products are provided below.
Specialty Care
DUPIXENT (developed in collaboration with Regeneron) generated net sales of €10,715 million in 2023, up 29.2% on a reported basis and 34.0% at CER. In the United States, sales of DUPIXENT reached €8,145 million in 2023, up 32.6% CER, boosted by continuing strong demand in the product's approved indications: atopic dermatitis, asthma, nasal polyps, eosinophilic esophagitis, and prurigo nodularis. In Europe, the product posted 2023 net sales of €1,224 million, up 30.9% CER, driven by continuing growth in atopic dermatitis, asthma and nasal polyps. In the Rest of the World region, DUPIXENT posted net sales of €1,346 million (+46.1% CER), driven mainly by Japan and China.
Net sales of AUBAGIO fell by 52.6% CER in 2023 to €955 million, mainly due to the arrival of generics. In the United States, where generics came on the market on March 12, 2023, AUBAGIO fell by -67.8% CER at €460 million. In Europe, generic competition for AUBAGIO began at the end of September 2023.
Net sales of the Pompe disease franchise (MYOZYME/LUMIZYME and NEXVIAZYME/NEXVIADYME) were up 8.8% CER in 2023 at €1,208 million. Sales of NEXVIAZYME/NEXVIADYME reached €425 million, including €272 million in the United States, reflecting switches of eligible Pompe patients (advanced stage) from MYOZYME/LUMIZYME and increased uptake by new patients. Sales of MYOZYME/LUMIZYME were down year-on-year (-15.1% CER at €783 million) as patients switched to NEXVIAZYME. In 2023, sales of NEXVIAZYME/NEXVIADYME represented 35.2% of total sales for the Pompe disease franchise.
Net sales of the Fabry disease treatment FABRAZYME in 2023 were €991 million (+11.2% CER), driven by the Rest of the World region (+18.8% CER at €247 million) followed by the United States (+9.8% CER at €503 million). The year-on-year increase reflects more patients adopting the product across all three regions.
Net sales for the Gaucher disease franchise (CEREZYME and CERDELGA) reached €985 million in 2023, up 8.4% CER. CEREZYME sales were up 9.1% CER at €687 million on a solid performance in the Rest of the World region (+25.9% CER at €269 million), driven by new patients on therapy and favorable pricing. In parallel, sales of CERDELGA rose by 6.9% CER to €298 million, with growth reported in the United States (+5.6% CER at €164 million), Europe (+6.3% CER at €118 million), and the Rest of the World region (+23.5% CER at €16 million) as new patients adopted the product or switched treatment.
ELOCTATE generated net sales of €471 million in 2023, down 15.5% CER, due to the adoption of ALTUVIIIO and competitive pressures.
ALTUVIIIO , a first-in-class, once-weekly factor VIII replacement therapy that confers significant protection against bleeds for hemophilia A patients, was launched in the United States at the end of March 2023 and generated sales of €159 million in 2023.
In 2023, net sales of ALPROLIX were €540 million, up 11.3% CER, driven by the United States where sales of the product reached €440 million, up 11.6% CER.
Net sales of SARCLISA in 2023 were €381 million, up 37.1% CER, with good performances in all three regions: €165 million (+33.9% CER) in the United States, €111 million (+27.3% CER) in Europe, and in Japan (€77 million, up 28.8% CER).
JEVTANA posted net sales of €320 million in 2023 (-14.8% CER), reflecting the launch of generics in Europe in March 2021 and competitive pressures in the United States.
CABLIVI posted net sales of €227 million in 2023, up 10.0% CER, reflecting increased awareness of acquired thrombotic thrombo-cytopenic purpura (aTTP), and treatment in line with guidelines from the International Society on Thrombosis and Haemostasis (ISTH) recommending first-line use of CABLIVI for all aTTP patients. Sales reached €112 million in the United States (+4.5% CER), and in Europe net sales were up 4.3% CER at €98 million, mainly due to greater market penetration as a result of increased product awareness.
XENPOZYME reported net sales of €91 million, mainly in the United States (€52 million) and Europe (€31 million).
Net sales of ENJAYMO reached €72 million, with sales being generated primarily in the United States and Japan.
SANOFI FORM 20-F 2023 | 69 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
General Medicines
In 2023, General Medicines GBU net sales reached €12,376 million, down 12.4% on a reported basis and 7.1 % at CER. Industrial sales were €582 million, down 6.1% o reported basis and 5.5% at CER.
Core Assets
In 2023, Core Assets sales were €6,270 million, down 1.9% on a reported basis but up 3.3% at CER. The main drivers were a solid performance from REZUROCK and strong contributions from TOUJEO, THYMOGLOBULIN, PRALUENT and PLAVIX, partly offset by lower sales of LOVENOX (due to strong competition from biosimilars) and MOZOBIL (due to loss of US exclusivity). Core Assets accounted for 50.7% of total General Medicines sales in 2023, compared with 45.2% in 2022.
By geography, Core Assets sales reported growth in the Rest of the World region (+9.3% CER) and Europe (+4.2% CER), though the impact was partly offset by the effect of insulin price erosion in the United States, where sales were down 7.7% CER.
Net sales of REZUROCK reached €310 million, a significant increase of 54.6% CER. Since its launch, approximately 4,000 patients have been treated, with strong persistency rates. Sanofi recently reacquired rights to be the sole marketing authorization holder for REZUROCK in China, where in August 2023 the China National Medical Products Administration (NMPA) approved belumosudil (REZUROCK) for the treatment of patients aged 12 years and older with chronic Graft Versus Host Disease (cGVHD) who have an inadequate response to corticosteroids or other systemic treatments.
In the second quarter of 2023, Sanofi acquired Provention, adding TZIELD , an innovative first-in-class treatment for people with type 1 diabetes, to the Core Assets portfolio. In 2023, TZIELD sales were €25 million, in line with the expected gradual ramp-up as a result of early patient identification programs.
TOUJEO net sales were €1,123 million in 2023, up 6.2% CER. Growth was driven mainly by the Rest of the World region (+26.9% CER), due to the Value Based Procurement program in China and the associated acceleration in sales volumes. The impact was partly offset by lower sales in the United States (-23.0% CER) due to price erosion.
Net sales of SOLIQUA amounted to €217 million (+5.6% CER), driven by growth in the Rest of the World region (+40.3% CER) and Europe (+24.3% CER). SOLIQUA was launched in China in May 2023, where it was added to the National Reimbursement Drug List (NRDL) in December 2023.
PRALUENT posted net sales of €422 million, up 15.2% CER. Growth was reported in Europe (+30.6% CER) and the Rest of the World region (+46.7% CER, due mainly due to China), though the effect was partly offset by lower sales in the United States following the release of a glyceryl trinitrate.
PLAVIX net sales reached €948 million in 2023, up 4.4% CER, in line with consistent volume growth in China.
Net sales of LOVENOX were €1,125 million in 2023, down 8.7% CER, reflecting strong biosimilar competition across all geographies.
MOZOBIL sales were €220 million in 2023, down 14.6% CER, due to loss of exclusivity in the United States and China. In Europe, the European Patent Office Technical Board of Appeal invalidated the SPC (Supplementary Protection Certificate) which protected MOZOBIL until August 2024.
Non-Core Assets
In 2023, net sales of Non-Core Assets were €5,524 million, down 22.4% on a reported basis and 16.5% at CER, due mainly to divestments (negative impact 1.3% on total General Medicines net sales) as portfolio streamlining gathered pace. The General Medicines GBU has achieved its objective of reducing its Non-Core Assets portfolio from around 300 products to 100, generating total divestment proceeds of approximately €2 billion between 2020 and the end of 2023 (two years ahead of schedule).
The other main factor in the decrease in Non-Core Assets sales was a decline in sales of LANTUS to €1,420 million in 2023 (-32.3% CER), mainly in the United States (-62.6% CER, due to lower net selling prices reflecting a higher proportion of sales through government channels) and China (due to the Value Based Procurement rollout).
Vaccines
In 2023, the Vaccines segment posted net sales of €7,474 million, up 3.4% on a reported basis and 8.3% CER. The main driver was the launch of BEYFORTUS, which more than offset slow sales of influenza vaccines.
The BEYFORTUS launch began in late September 2023, in the United States and Europe. Sales of the product reached €547 million in 2023, reflecting strong demand through All Infant Protection Programs rolled out in United States, Spain and France.
Sales of influenza vaccines decreased by 5.5% CER in 2023 to €2,669 million, due to a slight reduction in vaccine uptake and increased competition in the United States.
Polio/Pertussis/Hib (PPH) vaccines posted net sales of €2,165 million in 2023 (-0.1% CER), reflecting the ongoing expansion of VAXELIS in the United States at the expense of pentavalent vaccines in the first series of infant vaccinations. In the US, VAXELIS became market leader at the end of 2023 in the three-dose primary series market. As a reminder, sales of VAXELIS in the United States are not consolidated, and the profits are shared equally between Sanofi and Merck & Co.
70 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
Net sales of Meningitis, Travel and Endemics vaccines for 2023 reached €1,170 million, up 0.5% CER, with 41% growth in Europe more than offsetting lower sales in the Rest of the World region (-8.1 %) and the United States (-0.7% CER at €650 million), reflecting a favorable pattern in the US while the divestment of the Japanese Encephalitis vaccine in 2022 impacted the Rest of the World region.
In 2023, sales of Booster vaccines advanced by 5.1% CER to €598 million, driven by strong growth in Europe.
Net sales of other vaccines were €325 million (+96.4% CER), and include sales of the monovalent recombinant-protein COVID-19 booster vaccine VIDPREVTYN Beta.
4/ Net sales – Consumer Healthcare segment/GBU
In 2023, net sales for the CHC segment fell by 0.1% to €5,180 million on a reported basis but rose by 6.3% at CER, driven by double-digit growth in the Rest of the World region. Divestments of non-core products had a negative impact of 1.7 percentage point. CHC therefore achieved organic growth of 9.6% in 2023 excluding divestments.
Sanofi announced its intention to separate the CHC Business as it increases its focus on innovative medicines and vaccines. The intended separation will seek to create two entities, each better equipped to pursue its own business strategy, resourcing and capital allocation and enabling each to focus on long-term growth in its respective markets. Sanofi believes that the separation will unlock further opportunities for CHC to leverage its portfolio of leading brands and continue to drive growth and shareholder value. Sanofi is reviewing potential separation scenarios, but believes that the most likely path would be through a capital markets transaction, by creating a listed entity headquartered in France. The timing is driven by the desire to maximize value creation and reward Sanofi shareholders. Subject to market conditions, the intended separation could be achieved at the earliest in the fourth quarter of 2024, following consultation with social partners.
In the United States, CHC net sales amounted to €1,247 million in 2023, down 0.9% CER, mainly on lower sales in the Personal Care, Pain Care and Allergy categories.
In Europe, CHC net sales were up 3.9% CER in 2023 at €1,557 million, mainly reflecting growth in the Digestive Wellness and Cough & Cold categories.
In the Rest of the World region, CHC net sales were up 11.7% CER at €2,376 million in 2023, driven by growth in Digestive Wellness, Allergy, and Pain Care.
On September 29, 2023, Sanofi completed the acquisition of QRIB Intermediate Holdings, LLC (QRIB), the owner of QUNOL, a leading US health and wellness brand. Sales of QUNOL are consolidated within the Physical Wellness category.
5/ Net sales by geographical region
The table below sets forth our net sales for 2023 and 2022 by geographical region:
(€ million) | 2023 | 2022 | Change on a reported basis | Change at constant exchange rates | ||||||||||
| United States | 18,512 | 18,275 | +1.3 | % | +4.8 | % | ||||||||
| Europe | 10,392 | 9,999 | +3.9 | % | +4.3 | % | ||||||||
| Rest of the World | 14,166 | 14,723 | -3.8 | % | +6.5 | % | ||||||||
| of which China | 2,912 | 3,123 | -6.8 | % | +0.4 | % | ||||||||
| Total net sales | 43,070 | 42,997 | +0.2 | % | +5.3 | % | ||||||||
In 2023, net sales in the United States reached €18,512 million, up 1.3% on a reported basis and 4.8% at CER, reflecting a strong performance from DUPIXENT (+32.6% CER at €8,145 million) and the launches of BEYFORTUS (€407 million) and ALTUVIIOTM, (€155 million) partly offsetting by the impact of generics of AUBAGIO and lower sales of LANTUS and influenza vaccines .
In Europe, net sales advanced by 3.9% on a reported basis and 4.3% at CER in 2023 to €10,392 million. The performance of DUPIXENT (+30.9% CER at €1,224 million) and the launch of BEYFORTUS (€140 million) more than offset lower sales for the Non-Core Assets franchise (-14.4% CER).
In the Rest of the World region, net sales for 2023 decreased by 3.8% on a reported basis but rose by 6.5% at CER to €14,166 million, due to exceptional performances from DUPIXENT (+46.1% CER at €1,346 million) and CHC (+11.7% CER at €2,376 million).
A.2.2. Other income statement items
1/ Other revenues
Other revenues increased by 41.1% to €3,374 million in 2023 (versus €2,392 million in 2022). This line item mainly comprises VaxServe sales of non-Sanofi vaccines (€2,167 million in 2023 versus €1,567 million in 2022, recorded within the Biopharma segment). The year-on-year increase also reflects higher revenues from manufacturing services contracts and revenues from the COVID-19 vaccine (in particular, €411 million received from the US government in connection with the supply contract for the recombinant COVID-19 vaccine candidate).
SANOFI FORM 20-F 2023 | 71 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
2/ Gross profit
Gross profit for 2023 amounted to €32,208 million compared with €31,694 million in 2022, an increase of 1.6%. Gross margin (the ratio of gross profit to net sales) also rose, reaching 74.8% in 2023, versus 73.7% in 2022. The year-on-year increase in gross margin reflects in particular stronger gross margin for the Biopharma segment, which reached 76.4% in 2023 versus 75.0% in 2022, driven largely by a favorable product mix in Specialty Care and revenues related to the COVID-19 vaccine, which more than offset the effects of generic competition for AUBAGIO and unfavorable pricing effects for LANTUS in the United States.
3/ Research and development expenses
Research and development (R&D) expenses amounted to €6,728 million in 2023, versus €6,706 million in 2022, an increase of 0.3%, as investment stabilized. R&D expenses represented 15.6% of net sales in 2023, the same as in 2022.
4/ Selling and general expenses
Selling and general expenses amounted to €10,692 million in 2023 (24.8% of net sales), versus €10,492 million in 2022 (24.4% of net sales); the 1.9% increase was a result of increased marketing spend in the Biopharma segment and commercial spend in CHC.
5/ Other operating income and expenses
Other operating income amounted to €1,292 million in 2023 (versus €1,969 million in 2022), and other operating expenses to €3,516 million (versus €2,531 million in 2022).
Overall, this represented a net expense of €2,224 million in 2023, compared with a net expense of €562 million in 2022.
| (€ million) | 2023 | 2022 | Change | |||||||||||
| Other operating income | 1,292 | 1,969 | (677) | |||||||||||
| Other operating expenses | (3,516) | (2,531) | (985) | |||||||||||
| Other operating income/(expenses), net | (2,224) | (562) | (1,662) | |||||||||||
The increase of €1,662 million mainly reflects an increase in the share of profits generated by the monoclonal antibody alliance with Regeneron under the collaboration agreement (see Note C.1. to our consolidated financial statements, included at Item 18 of this annual report), the principal factors being (i) increased sales of DUPIXENT and (ii) the impact in 2022 of the recognition of the proceeds arising from the restructuring of the immuno-oncology (IO) collaboration agreement between Sanofi and Regeneron (see Note C.1. to our consolidated financial statements, included at Item 18. of this annual report).
The net contribution of items related to Regeneron to this line item is as follows:
| (€ million) | 2023 | 2022 | ||||||
Income & expense related to (profit)/loss sharing under the Monoclonal Antibody Alliance | (3,321) | (2,325) | ||||||
Additional share of profit paid by Regeneron towards development costs(a) | 668 | 434 | ||||||
Reimbursement to Regeneron of selling expenses incurred | (543) | (476) | ||||||
| Total: Monoclonal Antibody Alliance | (3,196) | (2,367) | ||||||
| Immuno-Oncology Alliance | — | 16 | ||||||
Other (mainly ZALTRAP and LIBTAYO)(b) | 217 | 1,120 | ||||||
| Other operating income/(expenses), net related to Regeneron | (2,979) | (1,231) | ||||||
(a) As of December 31, 2023, the commitment received by Sanofi in respect of the additional profit share payable by Regeneron towards development costs amounted to €2.1 billion, compared with €2.7 billion as of December 31, 2022 (see note D.21.to our consolidated financial statements included at Item 18. of this annual report).
(b) Following the restructuring of the Immuno-Oncology collaboration agreement between Sanofi and Regeneron effective July 1, 2022 (see Note C.1. to our consolidated financial statements, included at Item 18. of this annual report).
6/ Amortization of intangible assets
Amortization charged against intangible assets amounted to €2,172 million in 2023, compared with €2,053 million in 2022.
This €119 million increase was mainly due to (i) increased amortization expense in 2023 against ELOCTATE franchise assets further to FDA approval for ALTUVIIIO (€206 million) and (ii) the acquisition of Provention Bio, Inc., which led to €144 million of amortization being charged from the acquisition date against the intangible asset related to TZIELD product; those effects were partly offset by the non-recurrence of the €226 million accelerated amortization charge taken in 2022 against LIBTAYO rights following the restructuring of the IO LCA with Regeneron (see Note C.1. to our consolidated financial statements, included at Item 18 of this annual report).
7/ Impairment of intangible assets, net of reversals
For 2023, this line shows a net loss of €896 million, mainly comprising an impairment loss of €833 million reflecting the impact of the strategic decision to de-prioritize certain R&D programs, in particular those related to the NK Cell and PRO-XTEN technology platforms.
72 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
For 2022, this line item shows a net gain of €454 million, mainly comprising:
•a reversal of €2,154 million relating to ELOCTATE franchise assets, following FDA approval of ALTUVIIIO (the commercial name of efanesoctocog alpha, corresponding to the BIVV001 project); and
•an impairment loss of €1,586 million relating to the development project for SAR444245 (non-alpha interleukin-2), based on revised cash flow projections reflecting unfavorable developments in the launch schedule.
8/ Fair value remeasurement of contingent consideration
Fair value remeasurements of contingent consideration assets and liabilities recognized in business combinations represented a net expense of €93 million in 2023, versus a net gain of €27 million in 2022. For 2023, this line item mainly comprises a change in the amount of contingent consideration payable to Shire as a result of a transaction carried out by Translate Bio, Inc. prior to the acquisition of that entity by Sanofi (expense of €74 million in 2023, versus €2 million in 2022).
9/ Restructuring costs and similar items
Restructuring costs and similar items represented a total charge of €1,490 million in 2023, versus a charge of €1,336 million in 2022.
Restructuring costs and similar items increased by €154 million year-on-year. For 2023 they include the impact of French pension reforms on future annuities under the rules of each severance plan, while for 2022 they mainly comprised severance costs recognized further to the announcements made during that period. Also included in restructuring costs are the impacts of ongoing transformational projects, primarily those associated with the creation of the standalone CHC entity and the implementation of Sanofi’s new digital strategy.
10/ Other gains and losses, and litigation
For 2023, this line item showed an expense of €38 million, comprising costs arising on the settlement of litigation with Bioverativ former shareholders.
For 2022, this line item showed an expense of €370 million, comprising (i) the pre-tax loss arising on the deconsolidation of EUROAPI (see Note D.2. to our consolidated financial statements, included at Item 18 of this annual report) and (ii) costs associated with major litigation.
11/ Operating income
Operating income amounted to €7,875 million in 2023, versus €10,656 million in 2022.
The year-on-year decrease was largely due to the movements in impairment allowances against intangible assets.
12/ Financial income and expenses
Net financial expenses were €722 million in 2023, versus €234 million in 2022, a increase of €488 million.
The cost of our net debt (see the definition in “— Liquidity and Capital Resources” below and Note D.29. to our consolidated financial statements, included at Item 18. of this annual report) was €22 million in 2023, compared with €124 million in 2022; the reduction of €102 million was largely due to an increased return on cash, cash equivalents and associated derivatives (€533 million in 2023 versus €241 million in 2022, an increase of €292 million).
In addition, a financial expense of €541 million was recognized in 2023 in respect of the liability recognized in the balance sheet for estimated future royalties on US sales of BEYFORTUS, which was remeasured as of December 31, 2023 to reflect the very successful US launch of the product (see Notes C.2. and D.29. to our consolidated financial statements, included at Item 18. of this annual report).
13/ Income before tax and investments accounted for using the equity method
Income before tax and investments accounted for using the equity method reached €7,153 million in 2023, versus €10,422 million in 2022.
14/ Income tax expense
Income tax expense represented €1,602 million in 2023, versus €2,006 million in 2022, giving an effective tax rate based on consolidated net income of 22.4% in 2023, compared with 19.2% in 2022. The reduction in income tax expense was mainly due to a year-on-year increase in net amortization and impairment losses charged against intangible assets (impact of €563 million in 2023 and €268 million in 2022). In addition, a deferred tax asset of €133 million was recognized on the remeasurement of the financial liability recognized in the balance sheet to reflect estimated future royalties on US sales of BEYFORTUS. Those effects were partly offset by the deferred tax expense arising on investments in consolidated subsidiaries, which Sanofi expects will reverse as a result of the proposed separation of the CHC business as announced in October 2023 (€365 million impact in 2023).
In 2022, income tax expense included taxation of income from the out-licensing of LIBTAYO (€246 million impact in 2022) and the effect of the reversal of impairment losses relating to ALTUVIIIO (€503 million impact in 2022) following FDA approval.
The effective tax rate based on business net income is a non-IFRS financial measure (see definition under “— Segment information — Business Net Income” above). It is calculated on the basis of business operating income, minus net financial expenses and before (i) the share of profit/loss from investments accounted for using the equity method and (ii) net income attributable to non-controlling interests. We believe the presentation of this measure, used by our management, is also useful for investors as it provides a means to analyze the effective tax cost of our current business activities. It should not be seen as a substitute for the effective tax rate based on consolidated net income.
SANOFI FORM 20-F 2023 | 73 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
When calculated on business net income, our effective tax rate was 18.8% in 2023, compared with 19.3% in 2022.
The table below reconciles our effective tax rate based on consolidated net income to our effective tax rate based on business net income:
| (as a percentage) | 2023 | 2022 | ||||||
Effective tax rate based on consolidated net income (IFRS) | 22.4 | % | 19.2 | % | ||||
| Tax effects: | ||||||||
| Amortization and impairment of intangible assets | (0.1) | (0.4) | ||||||
| Restructuring costs and similar items | 1.5 | (0.3) | ||||||
Other tax effects | (4.9) | 0.8 | ||||||
Effective tax rate based on business net income (non-IFRS) | 18.8 | % | 19.3 | % | ||||
15/ Share of profit/(loss) from investments accounted for using the equity method
The line item Share of profit/(loss) from investments accounted for using the equity method was a net loss of €115 million in 2023 (including an impairment loss of €231 million on the equity-accounted investment in EUROAPI – see Note D.6.), compared with net income of €68 million for 2022
16/ Net income
Net income amounted to €5,436 million in 2023, compared with €8,484 million in 2022.
17/ Net income attributable to non-controlling interests
Net income attributable to non-controlling interests was €36 million in 2023, versus €113 million in 2022.
18/ Net income attributable to equity holders of Sanofi
Net income attributable to equity holders of Sanofi amounted to €5,400 million in 2023, compared with €8,371 million in 2022.
Basic earnings per share for 2023 was €4.31 versus €6.69 for 2022, based on an average number of shares outstanding of 1,251.7 million in 2023 and 1,251.9 million in 2022. Diluted earnings per share for 2023 was €4.30 versus €6.66 for 2022, based on an average number of shares after dilution of 1,256.4 million in 2023 and 1,256.9 million in 2022.
A.2.3. Segment results
Our business operating income, as defined in Note D.35. (“Segment information”) to our consolidated financial statements included at Item 18. of this annual report, amounted to €12,670 million in 2023, compared with €13,040 million in 2022, a decrease of 2.8%. That represents 29.4% of our net sales, compared with 30.3% in 2022.
The table below sets forth our business operating income for the years ended December 31, 2023 and 2022:
| (€ million) | December 31, 2023 | December 31, 2022 | Change | |||||||||||
Biopharma | 11,247 | 11,490 | -2.1 | % | ||||||||||
As percentage of sales | 29.7 | % | 30.4 | % | ||||||||||
Consumer Healthcare | 1,438 | 1,522 | -5.5 | % | ||||||||||
| As percentage of sales | 27.8 | % | 29.4 | % | ||||||||||
| Other | (15) | 28 | -153.6 | % | ||||||||||
Business operating income (non-IFRS) | 12,670 | 13,040 | -2.8 | % | ||||||||||
B. Liquidity and capital resources
Our operations generate significant positive cash flows. We fund our day-to-day investments (with the exception of significant acquisitions) primarily with operating cash flow, and pay regular dividends on our shares.
“Net debt” is a non-IFRS financial indicator which is reviewed by our management, and which we believe provides useful information to measure our overall liquidity and capital resources. We define “net debt” as (i) the sum total of long-term debt, short-term debt and current portion of long-term debt, and interest rate and currency derivatives used to manage debt, minus (ii) the sum total of cash and cash equivalents and interest rate and currency derivatives used to manage cash and cash equivalents. Lease liabilities are not included in net debt.
As of December 31, 2023 our net debt was €7,793 million, compared with €6,437 million as of December 31, 2022. For an explanation of the increase in our net debt, refer to section “B.2. Consolidated Balance Sheet and Debt” below.
In order to assess our financing risk, we also use the “gearing ratio", a non-IFRS financial measure (see table in section “— B.2. Consolidated Balance Sheet and Debt” below). We define the gearing ratio as the ratio of net debt to total equity. As of December 31, 2023, our gearing ratio was 10.5%, compared with 8.6% as of December 31, 2022.
Because our net debt and gearing ratio are not standardized measures, they may not be directly comparable with the non-IFRS financial measures of other companies using the same or similar non-IFRS financial measures. Despite the use of non-IFRS measures by management in setting goals and measuring performance, these are non-IFRS measures that have no standardized meaning prescribed by IFRS.
74 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
B.1. Consolidated statement of cash flows
Generally, factors that affect our earnings – for example, pricing, volume, costs and exchange rates – flow through to cash from operations. The most significant source of cash from operations is sales of our branded pharmaceutical products and vaccines. Receipts of royalty payments also contribute to cash from operations.
Summarized consolidated statements of cash flows
| (€ million) | 2023 | 2022 | ||||||
| Net cash provided by/(used in) operating activities | 10,258 | 10,526 | ||||||
| Net cash provided by/(used in) investing activities | (6,200) | (2,075) | ||||||
| Net cash provided by/(used in) financing activities | (8,052) | (5,821) | ||||||
| Impact of exchange rates on cash and cash equivalents | (32) | 8 | ||||||
| Net change in cash and cash equivalents | (4,026) | 2,638 | ||||||
Net cash provided by/used in operating activities represented a net cash inflow of €10,258 million in 2023, compared with €10,526 million in 2022. The year-on-year decrease was due mainly to a lower level of operating cash flow before changes in working capital (€9,494 million in 2023, versus €11,233 million in 2022) and a net increase of €764 million in the working capital requirement in 2023 (versus a net decrease of €707 million in 2022).
Net cash provided by/used in investing activities represented a net cash outflow of €6,200 million in 2023, compared with a net outflow of €2,075 million in 2022. The net outflow in 2023 was mainly a result of the acquisitions of Provention Bio, Inc. ($2,722 million) and QRIB Intermediate Holdings, LLC ($1,410 million). For 2022, the net cash outflow was mainly due to the acquisition of Amunix Pharmaceuticals, Inc (€852 million), partly offset by the proceeds of €150 million from the sale of a 12% equity interest in EUROAPI to EPIC Bpifrance.
Acquisitions of property, plant and equipment and intangible assets amounted to €3,024 million, versus €2,201 million in 2022. There were €1,719 million of acquisitions of property, plant and equipment (versus €1,606 million in 2022), most of which (€1,619 million) related to the Biopharma segment, primarily in industrial facilities. Acquisitions of intangible assets (€1,305 million, versus €595 million in 2022) mainly comprised contractual payments for intangible rights under license and collaboration agreements.
After-tax proceeds from disposals (€1,015 million in 2023, €1,488 million in 2022) exclude proceeds from divestments of investments in consolidated undertakings and investments accounted for using the equity method, and mainly comprised divestments of assets and activities related to the streamlining of the portfolio, and disposals of equity and debt instruments.
Net cash provided by/used in financing activities represented a net cash outflow of €8,052 million in 2023, compared with a net cash outflow of €5,821 million in 2022. The 2023 figure includes the redemption of bond issues totaling €3,664 million. Other movements included (i) the dividend payout to our shareholders of €4,454 million (versus €4,168 million in 2022; and (ii) the effect of changes in our share capital (repurchases of our own shares, net of capital increases), representing a net cash outflow of €398 million in 2023 versus a net cash outflow of €309 million in 2022.
The net change in cash and cash equivalents in 2023 was a decrease of €4,026 million, versus an increase of €2,638 million in 2022.
“Free cash flow,” a non-IFRS measure, for the year ended December 31, 2023 was €8,478 million, virtually unchanged from the 2022 figure of €8,483 million. This reflects our operational performance (including the effect of cost containment measures), and proceeds from asset divestments during the period.
For details of the arrangements in place to manage our liquidity needs for current operations as of December 31, 2023, refer to Note 17.1.(b) to our consolidated financial statements, included at Item 18. of this annual report.
“Free cash flow” is a non-IFRS financial indicator which is reviewed by our management, and which we believe provides useful information to measure the net cash generated from our operations that is available for strategic investments(1) (net of divestments(1)), for debt repayment, and for payments to shareholders. “Free cash flow” is determined from our “Business net income”(2) after adding back (in the case of expenses and losses) or deducting (in the case of income and gains) the following items: depreciation, amortization and impairment, share of undistributed earnings from investments accounted for using the equity method, gains & losses on disposals, net change in provisions including pensions and other post-employment benefits, deferred taxes, share-based payment expense and other non-cash items. It also includes net changes in working capital, capital expenditures and other asset acquisitions(3) net of disposal proceeds(3), and payments related to restructuring and similar items. “Free cash flow” is not defined by IFRS, and is not a substitute for Net cash provided by operating activities as reported under IFRS. Management recognizes that the term “Free cash flow” may be interpreted differently by other companies and under different circumstances.
(1) Above a cap of €500 million per transaction.
(2) Non-IFRS financial measure, as defined in “— Segment Information — Business Net income” above.
(3) Not exceeding a cap of €500 million per transaction.
SANOFI FORM 20-F 2023 | 75 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
The table below sets forth a reconciliation between Net cash provided by operating activities and “Free cash flow”:
| (€ million) | 2023 | 2022 | ||||||
| Net cash provided by operating activities (IFRS) | 10,258 | 10,526 | ||||||
| Acquisitions of property, plant and equipment and software | (1,771) | (1,656) | ||||||
Acquisitions of intangible assets, equity interests and other non-current financial assets(a) | (1,113) | (824) | ||||||
Proceeds from disposals of property, plant and equipment, intangible assets and other non-current assets, net of tax(a) | 997 | 1,531 | ||||||
Repayments of lease liabilities(b) | (265) | (291) | ||||||
Other items(c) | 372 | (803) | ||||||
Free cash flow (non-IFRS) | 8,478 | 8,483 | ||||||
(a) Free cash flow includes investments and divestments not exceeding a cap of €500 million per transaction.
(b) Cash outflows relating to repayments of the principal portion of lease liabilities (IFRS 16) are included in free cash flow.
(c) In 2022, includes an upfront payment of $900 million and a regulatory milestone payment of $100 million related to the granting of the LIBTAYO license.
B.2. Consolidated balance sheet and debt
Total assets were €126,464 million as of December 31, 2023, compared with €126,722 million as of December 31, 2022, a decrease of €258 million.
Total equity was €74,353 million as of December 31, 2023, versus €75,152 million as of December 31, 2022. The year-on-year net change reflects the following principal factors:
•increases: our net income for 2023 (€5,436 million); and
•decreases: the dividend paid to our shareholders in respect of the 2022 financial year (€4,454 million), repurchases of our own shares (€593 million), and negative currency translation differences (€1,540 million).
Net debt was €7,793 million as of December 31, 2023, compared with €6,437 million as of December 31, 2022. The increase in 2023 mainly reflects cash outflows of €3,915 million on the acquisitions of the newly-consolidated entities Provention Bio, Inc. and QRIB Intermediate Holdings, LLC and of €4,454 million for the dividend payout to our shareholders, less the €8,478 million of free cash flow generated in the year.
“Net debt” is a non-IFRS financial measure which is reviewed by our management, and which we believe provides useful information to measure our overall liquidity and capital resources. We define “net debt” as (i) the sum total of long-term debt, short-term debt and current portion of long-term debt and interest rate and currency derivatives used to manage debt, minus (ii) the sum total of cash and cash equivalents and interest rate and currency derivatives used to manage cash and cash equivalents.
| (€ million) | 2023 | 2022 | ||||||
| Long-term debt | 14,347 | 14,857 | ||||||
| Short-term debt and current portion of long-term debt | 2,045 | 4,174 | ||||||
| Interest rate and currency derivatives used to manage debt | 139 | 187 | ||||||
| Total debt | 16,531 | 19,218 | ||||||
| Cash and cash equivalents | (8,710) | (12,736) | ||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | (28) | (45) | ||||||
Net debt(a) (non- IFRS) | 7,793 | 6,437 | ||||||
| Total equity | 74,353 | 75,152 | ||||||
Gearing ratio (non-IFRS) | 10.5 | % | 8.6 | % | ||||
(a)Net debt does not include lease liabilities, which amounted to €2,030 million as of December 31, 2023 and €2,181 million as of December 31, 2022.
“Net debt” is a non-IFRS financial measure used by management and investors to measure Sanofi’s overall net indebtedness.
To assess our financing risk, we use the “gearing ratio”, a non-IFRS financial measure. This ratio (which we define as the ratio of net debt to total equity) increased from 8.6% as of December 31, 2022 to 10.5% as of December 31, 2023. Analyses of debt as of December 31, 2023 and December 31, 2022, by type, maturity, interest rate and currency, are provided in Note D.17.1. to our consolidated financial statements, included at Item 18. of this annual report.
We expect that the future cash flows generated by our operating activities will be sufficient to repay our debt. The financing arrangements in place as of December 31, 2023 at the Sanofi parent company level are not subject to covenants regarding financial ratios and do not contain any clauses linking fees to Sanofi’s credit rating.
As of December 31, 2023, we held 13.5 million of our own shares, recorded as a deduction from equity and representing 1.06% of our share capital.
76 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
Goodwill and Other intangible assets (€73,723 million in total) rose by €2,191 million. The year-on-year increase takes account of (i) the purchase price allocations relating to the acquisitions during the year of Provention Bio, Inc. and QRIB Intermediate Holdings, LLC on April 27, 2023 and September 29, 2023, respectively (see Note D.1. to our consolidated financial statements, included at Item 18. of this annual report on Form 20-F) and (ii) the recognition of an asset corresponding to the additional US rights to BEYFORTUS (nirsevimab) acquired under the terms of the agreement reached between Sanofi and AstraZeneca in April 2023 (see Note C.2. to our consolidated financial statements, included at Item 18. of this annual report on Form 20-F). Those upward movements were partly offset by amortization and impairment charged during the period, and by movements in currency translation differences.
Investments accounted for using the equity method (€424 million) decreased by €253 million, mainly reflecting an impairment loss taken against the equity-accounted investment in EUROAPI to reflect the drop in the quoted market price of EUROAPI shares since March 2023.
Other non-current assets amounted to €3,218 million, a year-on-year increase of €123 million.
Net deferred tax assets amounted to €4,570 million as of December 31, 2023, versus €3,540 million as of December 31, 2022, a year-on-year increase of €1,030 million. This mainly reflects deferred taxes arising on the spread tax deduction of R&D expenses, partly offset by deferred taxes arising from temporary differences related to investments in consolidated entities dedicated to our CHC business.
Non-current provisions and other non-current liabilities (€7,602 million) showed an increase of €1,261 million, mainly due to the recognition of the financial liability in respect of royalties payable to Sobi on net sales of BEYFORTUS (nirsevimab) in the United-States (see Note C.2. to our consolidated financial statements).
Liabilities related to business combinations and to non-controlling interests were €70 million lower year-on-year, at €709 million.
B.3. Liquidity
We expect that our existing cash resources and cash from operations will be sufficient to finance our foreseeable working capital requirements, in both the short term (i.e. the 12 months following the year ended December 31, 2023) and the long term (i.e. beyond such additional 12-month period). At year-end 2023, we held cash and cash equivalents amounting to €8,710 million, substantially all of which were held in euros (see Note D.13. to our consolidated financial statements, included at Item 18. of this annual report). As at December 31, 2023, €476 million of our cash and cash equivalents were held by captive insurance and reinsurance companies in accordance with insurance regulations.
We run the risk of delayed payments or even non-payment by our customers, who consist principally of wholesalers, distributors, pharmacies, hospitals, clinics and government agencies (see “Item 3. Key information — D. Risk Factors — 2. Risks Relating to Our Business — We are subject to the risk of non-payment by our customers”). Deteriorating credit and economic conditions and other factors in some countries have resulted in, and may continue to result in an increase in the average length of time taken to collect our accounts receivable in these countries. Should these factors continue, it may require us to re-evaluate the collectability of these receivables in future periods. We carefully monitor sovereign debt issues and economic conditions and evaluate accounts receivable in these countries for potential collection risks. We have been conducting an active recovery policy, adapted to each country and including intense communication with customers, negotiations of payment plans, charging of interest for late payments, and legal action. Over our business as a whole, the amount of trade receivables overdue by more than 12 months (which primarily consists of amounts due from public sector bodies) increased from €51 million as of December 31, 2022 to €81 million as of December 31, 2023 (see Note D.10. to our consolidated financial statements included at Item 18. of this annual report).
At year-end 2023, we had no commitments for capital expenditures that we consider to be material to our consolidated financial position. Undrawn confirmed credit facilities amounted to a total of €8,000 million at December 31, 2023. For a discussion of our treasury policies, see “Item 11. Quantitative and Qualitative Disclosures about Market Risk.”
We expect that cash from our operations will be sufficient to repay our debt. For a discussion of our liquidity risks, see “Item 11. Quantitative and Qualitative Disclosures about Market Risk.”
B.4. Off balance sheet arrangements/Contractual obligations and other commercial commitments
We have various contractual obligations and other commercial commitments arising from our operations. Our contractual obligations and our other commercial commitments as of December 31, 2023 are shown in Notes D.3., D.17., D.18., and D.21. to our consolidated financial statements, included at Item 18. of this annual report. Note D.21. to our consolidated financial statements included at Item 18. of this annual report discloses details of commitments under our principal research and development collaboration agreements. For a description of the principal contingencies arising from certain business divestitures, refer to Note D.22.d.) to our 2023 consolidated financial statements included at Item 18. of this annual report.
Sanofi’s contractual obligations and other commercial commitments are set forth in the table below.
SANOFI FORM 20-F 2023 | 77 | ||||
| PART I | ||
ITEM 5. Operating and financial review and prospects | ||
| December 31, 2023 | Payments due by period | ||||||||||||||||
| (€ million) | Total | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | ||||||||||||
Future contractual cash flows relating to debt and debt hedging instruments(a) | 17,853 | 2,200 | 6,154 | 3,044 | 6,455 | ||||||||||||
Principal payments related to lease liabilities(b) | 2,088 | 291 | 448 | 360 | 989 | ||||||||||||
Other lease obligations (with a term of less than 12 months, low value asset leases and lease contracts committed but not yet commenced)(c) | 221 | 24 | 26 | 25 | 146 | ||||||||||||
Irrevocable purchase commitments(d) | |||||||||||||||||
•given | 6,141 | 2,446 | 2,090 | 778 | 827 | ||||||||||||
•received | (550) | (443) | (96) | (11) | — | ||||||||||||
| Research & development license agreements | |||||||||||||||||
•Commitments related to R&D and other commitments | 381 | 256 | 101 | 15 | 9 | ||||||||||||
•Potential milestone payments(e) | 4,886 | 280 | 1,757 | 818 | 2,031 | ||||||||||||
Obligations relating to business combinations(f) | 133 | 69 | 64 | — | — | ||||||||||||
Estimated benefit payments on unfunded pensions and post employment benefits(g) | 1,238 | 86 | 117 | 144 | 891 | ||||||||||||
| Total contractual obligations and other commitments | 32,391 | 5,209 | 10,661 | 5,173 | 11,348 | ||||||||||||
| Undrawn general-purpose credit facilities | 8,000 | — | — | 4,000 | 4,000 | ||||||||||||
(a) See Note D.17.1. to our consolidated financial statements, included at Item 18. of this annual report.
(b) See Note D.17.2. to our consolidated financial statements, included at Item 18. of this annual report.
(c) See Note D.21.1. to our consolidated financial statements, included at Item 18. of this annual report.
(d) These comprise irrevocable commitments to suppliers of (i) property, plant and equipment, net of down payments (see Note D.3. to our consolidated financial statements, included at Item 18. of this annual report) and (ii) goods and services.
(e) This line includes all milestone payments on projects regarded as reasonably possible, i.e. on projects in the development phase.
(f) See Note D.18. to our consolidated financial statements, included at Item 18. of this annual report.
(g) See Note D.19.1. to our consolidated financial statements, included at Item 18. of this annual report. The table above does not include ongoing annual employer’s contributions to plan assets, estimated at €86 million for 2023.
We may have payments due to our current or former research and development partners under collaboration agreements. These agreements typically cover multiple products, and give us the option to participate in development on a product-by-product basis. When we exercise our option with respect to a product, we pay our collaboration partner a fee and receive intellectual property rights to the product in exchange. We are also generally required to fund some or all of the development costs for the products that we select, and to make payments to our partners when those products reach development milestones.
We have entered into collaboration agreements under which we have rights to acquire products or technology from third parties through the acquisition of shares, loans, license agreements, joint development, co-marketing and other contractual arrangements. In addition to upfront payments on signature of the agreement, our contracts frequently require us to make payments contingent upon the completion of development milestones by our alliance partner or upon the granting of approvals or licenses.
Because of the uncertain nature of development work, it is impossible to predict (i) whether Sanofi will exercise further options for products, or (ii) whether the expected milestones will be achieved, or (iii) the number of compounds that will reach the relevant milestones. It is therefore impossible to estimate the maximum aggregate amount that Sanofi will actually pay in the future under existing collaboration agreements.
Given the nature of its business, it is highly unlikely that Sanofi will exercise all options for all products or that all milestones will be achieved.
The main collaboration agreements relating to development projects are described in Note D.21.1. to our consolidated financial statements, included at Item 18. of this annual report. Milestone payments relating to development projects under these agreements included in the table above exclude projects still in the research phase (€16.8 billion in 2023, and €18.0 billion in 2022) and payments contingent upon the attainment of sales targets once a product is on the market (€17.9 billion in 2023, and €18.5 billion in 2022).
C. Research and development, patents and licenses, etc.
Our research and development teams utilize our deep expertise to contribute to the growth of our business. As of December 31, 2023, we had 11,660 employees engaged in research and development activities. In the years ended December 31, 2021, 2022 and 2023 we spent €5,692, €2022 and €6,728 respectively, on research and development. For a discussion of our research and development activities, see “Item 4. Information on the Company — B. Business Overview” and section “— Operating Results” above.
D. Trend information
For a discussion of trends, see “Item 4. Information on the Company — Business Overview” and sections “— Operating Results” and “— Liquidity and Capital Resources” above.
E. Critical accounting estimates
For a discussion of our critical accounting estimates, see Note A.3 of our consolidated financial statements included in Item 18 of this annual report.
78 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management
Since January 1, 2007, Sanofi has separated the offices of Chairman and Chief Executive Officer. Annual evaluations conducted since that date have indicated that this governance structure is appropriate to Sanofi’s current configuration. When the term of office of Serge Weinberg as Chairman ended, our Board of Directors decided to continue separating the offices of Chairman and Chief Executive Officer. The Board believes this governance structure is still appropriate to the current context in which Sanofi operates and its share ownership structure, as well as protecting the rights of all of its stakeholders.
The Chairman organizes and directs the work of the Board, and is responsible for ensuring the proper functioning of the corporate decision-making bodies in compliance with good governance principles. The Chairman coordinates the work of the Board of Directors with that of its Committees. He ensures that the Company’s management bodies function properly, and in particular that the directors are able to fulfil their duties. The Chairman is accountable to the Shareholders’ General Meeting, which he chairs.
In addition to these roles conferred by law, the Chairman:
•in coordination with the Chief Executive Officer, liaises between the Board of Directors and the shareholders of the Company;
•is kept regularly informed by the Chief Executive Officer of significant events and situations affecting the affairs of the Company, and may request from the Chief Executive Officer any information useful to the Board of Directors;
•may, in close collaboration with the Chief Executive Officer, represent the Company in high-level dealings with governmental bodies and with key partners of the Company and/or of its subsidiaries, both nationally and internationally;
•seeks to prevent any conflict of interest and manages any situation that might give rise to a conflict of interest. He also gives rulings, in the name of the Board, on requests to take up external directorships of which he may become aware or that may be submitted to him by a director;
•may interview the statutory auditors in preparation for the work of the Board of Directors and the Audit Committee; and
•strives to promote in all circumstances the values and image of the Company.
The Chairman is also required to develop and maintain a proper relationship of trust between the Board and the Chief Executive Officer, so as to ensure that the latter consistently and continuously implements the orientations determined by the Board.
In fulfilling his remit, the Chairman may meet with any individual, including senior executives of the Company, while avoiding any involvement in directing the Company or managing its operations, which are exclusively the responsibility of the Chief Executive Officer.
Finally, the Chairman reports to the Board on the fulfilment of his remit.
The Chairman carries out his duties during the entire period of his term of office, subject to the caveat that a director who is a natural person may not be appointed or reappointed once that director has reached the age of 70.
The Chief Executive Officer manages the Company, and represents it in dealings with third parties within the limit of the corporate purpose. The Chief Executive Officer has the broadest powers to act in all circumstances in the name of the Company, subject to the powers that are attributed by law to the Board of Directors and to the Shareholders’ General Meeting and within the limits set by the Board of Directors.
The Chief Executive Officer must be less than 65 years old.
SANOFI FORM 20-F 2023 | 79 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Limitations on the powers of the Chief Executive Officer set by the Board
The limitations on the powers of the Chief Executive Officer are specified in the Board Charter. Without prejudice to legal provisions regarding authorizations that must be granted by the Board (regulated agreements, guarantees, divestments of equity holdings or real estate, etc.), prior approval from the Board of Directors is required for transactions or decisions resulting in an investment or divestment, or an expenditure or guarantee commitment, made by the Company and its subsidiaries, in excess of:
•a cap of €500 million (per transaction) for transactions, decisions or commitments pertaining to a previously approved strategy; and
•a cap of €150 million (per transaction) for transactions, decisions or commitments not pertaining to a previously approved strategy.
When such transactions, decisions or commitments give rise to installment payments to the contracting third party (or parties) that are contingent upon future results or objectives, such as the registration of one or more products, attainment of the caps is calculated by aggregating the various payments due from the signing of the contract until (and including) the filing of the first application for marketing authorization in the United States or in Europe.
Attainment of the above caps is also assessed after taking into account all commitments to make payments upon exercising a firm or conditional option with immediate or deferred effect, and all guarantees or collateral to be provided to third parties over the duration of such commitments.
The prior approval procedure does not apply to transactions and decisions that result in the signature of agreements that solely involve subsidiaries and the Company itself.
Remit of the Board of Directors
The Board of Directors establishes the orientation of the Company’s activities and ensures that they are implemented, paying due consideration to social and environmental issues. Subject to those powers expressly attributed to Shareholders’ General Meetings and within the limits set by the corporate purpose, the Board addresses any issue of relevance to the proper conduct of the Company’s affairs and, through its deliberations, settles matters concerning the Company.
French law, Articles of Association and Board Charter
The rules and operating procedures of our Board of Directors are defined by French law, by our Articles of Association, and by our Board Charter (English language versions of which are reproduced in full as Exhibit 1.1 and Exhibit 1.2 to this annual report).
Our Board Charter describes the rights and obligations of Board members; the composition, role and operating procedures of the Board of Directors and Board Committees; and the roles and powers of the Chairman and the Chief Executive Officer. It is prepared in accordance with the French Commercial Code and our Articles of Association.
80 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Composition of the Board of Directors
As of December 31, 2023 our Board of Directors had 16 directors, including 11 independent directors and two directors representing employees. 43% of the directors (excluding directors representing employees, in accordance with regulations) are women, and 50% of the directors (including directors representing employees) are non-French nationals.
| Director | Age | Gender | Nationality | Number of shares | Number of directorships in listed companies(a) | Independent | First appointed | Term expires | Years of Board service | AC | AGC | CC | SC | SciC | ||||||||||||||||||||||||||||||
Frédéric Oudéa, Chairman of the Board | 60 | M | French | 1,000 | 2 | Yes | 2023(b) | 2027 AGM | 1 | M | C | M | ||||||||||||||||||||||||||||||||
| Paul Hudson, Chief Executive Officer | 56 | M | British | 70,805 (c) | 1 | No | 2019 | 2026 AGM | 4 | M | ||||||||||||||||||||||||||||||||||
Christophe Babule(d) | 58 | M | French | 1,000 | 2 | No (d) | 2019 | 2026 AGM | 4 | M | ||||||||||||||||||||||||||||||||||
| Rachel Duan | 53 | F | Chinese | 1,000 | 4 | Yes | 2020 | 2024 AGM | 3 | M | ||||||||||||||||||||||||||||||||||
| Carole Ferrand | 53 | F | French | 1,000 | 2 | Yes | 2022 | 2025 AGM | 1 | |||||||||||||||||||||||||||||||||||
| Lise Kingo | 62 | F | Danish | 1,000 | 3 | Yes | 2020 | 2024 AGM | 3 | M | ||||||||||||||||||||||||||||||||||
| Patrick Kron | 70 | M | French | 1,000 | 3 | Yes | 2014 | 2026 AGM | 9 | M | C | M | ||||||||||||||||||||||||||||||||
Wolfgang Laux(e) | 56 | M | German | 3,835 | 1 | No | 2021 | 2025 AGM | 2 | M | ||||||||||||||||||||||||||||||||||
| Barbara Lavernos | 55 | F | French | 1,000 | 2 | No | 2021 | 2025 AGM | 2 | M | ||||||||||||||||||||||||||||||||||
| Fabienne Lecorvaisier | 61 | F | French | 1,000 | 3 | Yes | 2013 | 2025 AGM | 10 | C | ||||||||||||||||||||||||||||||||||
| Gilles Schnepp | 65 | M | French | 1,000 | 3 | Yes | 2020 | 2026 AGM | 3 | C | M | |||||||||||||||||||||||||||||||||
| Diane Souza | 71 | F | American | 1,244 | 1 | Yes | 2016 | 2024 AGM | 7 | M | M | |||||||||||||||||||||||||||||||||
| Thomas Südhof | 68 | M | American/ German | 1,242 | 1 | Yes | 2016 | 2024 AGM | 7 | C | ||||||||||||||||||||||||||||||||||
Yann Tran(e) | 58 | M | French | 1,385 | 1 | No | 2021 | 2025 AGM | 2 | |||||||||||||||||||||||||||||||||||
| Emile Voest | 63 | M | Dutch | 1,000 | 1 | Yes | 2022 | 2025 AGM | 1 | M | ||||||||||||||||||||||||||||||||||
| Antoine Yver | 66 | M | French/American/Swiss | 1,000 | 2 | Yes | 2022 | 2025 AGM | 1 | M | ||||||||||||||||||||||||||||||||||
Independent directors(f) | Female directors(e) | Non-French directors | ||||||||||||||||||||||||||||||||||||||||||
| 79% | 43% | 50% | ||||||||||||||||||||||||||||||||||||||||||
AC: Audit Committee.
AGC: Appointments, Governance and Corporate Social Responsability (CSR) Committee.
CC: Compensation Committee.
SC: Strategy Committee.
SciC: Scientific Committee.
C: Chairman/Chairwoman.
M: Member.
(a) Includes all non-executive and executive (and equivalent) directorships held in listed companies. The office held within Sanofi is included.
(b) Frédéric Oudéa was initially appointed as a non-voting director by the Board on September 2, 2022, and then appointed as a director by the Annual General Meeting on May 25, 2023.
(c) Amount including shares definitively granted to Paul Hudson in May 2023 pursuant to the long-term incentive plan dated April 28, 2020
(d) This table only refers to independence as defined under the AFEP-MEDEF Code. However, Christophe Babule is independent for the purposes of the NASDAQ Listing Rules and Rule 10A-3 under the Exchange Act.
(e) Director representing employees.
(f) Directors representing employees are not taken into consideration for the calculation of these percentages, in accordance with regulation.
In line with current legislation and given that less than 3% of our share capital is owned by our employees, Sanofi does not have a director representing its employee shareholders.
SANOFI FORM 20-F 2023 | 81 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Term of Office
The term of office of directors is four years. Directors are required to seek reappointment by rotation, such that members of the Board are required to seek reappointment on a regular basis in the most equal proportions possible. Exceptionally, the Shareholders’ Ordinary General Meeting may appoint a director to serve for a term of one, two or three years, in order to ensure an adequate rotation of Board members. Each director standing down is eligible for reappointment. Should one or more directorships fall vacant as a result of death or resignation, the Board of Directors may make provisional appointments in the period between two Shareholders’ General Meetings, in accordance with applicable laws.
Directors may be removed from office at any time by a Shareholders’ General Meeting.
A natural person cannot be appointed or reappointed as a director once he or she reaches the age of 70. As soon as the number of directors over the age of 70 represents more than one-third of the directors in office, the oldest director shall be deemed to have resigned; his or her term of office shall end at the date of the next Shareholders’ Ordinary General Meeting.
Changes in the composition of the Board of Directors during 2022 and 2023
The table below shows changes in the composition of the Board of Directors during 2022 and 2023:
| Annual General Meeting of May 3, 2022 | Annual General Meeting of May 25, 2023 | |||||||
| End of term of office | Melanie Lee(a) Carole Piwnica(a) | Serge Weinberg(b) | ||||||
| Renewal of term of office | Paul Hudson Christophe Babule Patrick Kron Gilles Schnepp | None | ||||||
| Proposed new appointments | Carole Ferrand Emile Voest Antoine Yver | Frédéric Oudéa(b) | ||||||
| Co-opted | None | None | ||||||
| Other | None | None | ||||||
(a) Melanie Lee and Carole Piwnica left the Board of Directors before the General Meeting of May 3, 2022.
(b) The term of office of Serge Weinberg expired at the Annual General Meeting held to approve the financial statements for the year ended December 31, 2022, and could not be renewed (see below). Acting on a recommendation from the Appointments, Governance and CSR Committee, the Board of Directors appointed Frédéric Oudéa as a non-voting member of the Board on September 2, 2022. The Board meeting of February 22, 2023 decided to ask the Annual General Meeting of May 25, 2023 to approve the appointment of Frédéric Oudéa as an independent director, to replace Serge Weinberg.
The term of office of Serge Weinberg expired at the Annual General Meeting of May 25, 2023, which approved the appointment of Frédéric Oudéa as his successor. The Board then appointed Frédéric Oudéa as Chairman.
That appointment brought to the Board Frédéric Oudéa’s:
•experience from senior executive roles in international groups;
•experience from Board membership in international groups; and
•competencies in finance and accounting.
Following his appointment, the proportion of independent directors increased from 71% to 79%.
For further information about the career of Frédéric Oudéa, refer to his biographical details later in this section.
Changes in Board membership to be submitted for shareholder approval at the Annual General Meeting on April 30, 2024
Expiry of term of office | Diane Souza(a) Thomas Südhof | ||||
Reappointments | Rachel Duan Lise Kingo | ||||
Proposed appointments | Clotilde Delbos Anne-Françoise Nesmes John Sundy | ||||
Co-opted | None | ||||
Other | None | ||||
(a) Diane Souza's term of office cannot be renewed because she will be over the age limit set in our Articles of Association.
82 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
The terms of office of Rachel Duan, Lise Kingo, Diane Souza and Thomas Sudhöf will expire at the Annual General Meeting to be held on April 30, 2024.
The Annual General Meeting will be asked to:
•renew the terms of office of:
–Rachel Duan – refer to “— Detailed Information about Members of the Board of Directors” for her biographical details, and to “— Competencies of Board Members” below for details of what she brings to the Board,
–Lise Kingo – refer to “— Detailed Information about Members of the Board of Directors” for her biographical details, and to “— Competencies of Board Members” below for details of what she brings to the Board;
•appoint three independent directors, who would bring the following competencies to the Board:
–Clotilde Delbos: Senior executive role in international groups, Board membership in international groups, International experience, Mergers & acquisitions, Finance & accounting;
–Anne-Françoise Nesmes: Healthcare/pharmaceutical industry experience, Senior executive role in international groups, Board membership in international groups, International experience, Mergers & acquisitions, Finance & accounting; and
–John Sundy: Scientific training, Board membership in international groups.
In order to optimize preparations for the expiry in 2025 of the term of office of Fabienne Lecorvaisier, Chair of the Audit Committee, our Board of Directors would temporarily have 17 members with effect from the Annual General Meeting of April 30, 2024.
Rules relating to the composition of the Board and its Committees
Each year, the Board of Directors conducts a review to ensure that there is an appropriate balance in its composition and in the composition of its Committees. In particular, the Board seeks gender balance and a broad diversity of competencies, experiences, nationalities and ages, reflecting our status as a diversified global business. The Board investigates and evaluates not only potential candidates, but also whether existing directors should seek reappointment. Above all, the Board seeks directors who show independence of mind and are competent, dedicated and committed, with compatible and complementary personalities.
Acting on proposals from the Chief Executive Officer and in liaison with the Appointments, Governance and CSR Committee, the Board sets objectives for gender balance on Sanofi’s executive bodies, and more generally ensures that an inclusion (non-discrimination) and diversity policy is applied within the Company. That policy is fully embedded in our Play to Win strategy. As of December 31, 2023, 25% of the 12 Executive Committee members were women, and 67% were non-French nationals.
The Board of Directors is also kept informed, in particular on the occasion of its annual discussion on its equal opportunity and equal pay policy, on how Sanofi’s inclusion and diversity policy is cascaded down to “Senior Leaders” and “Executives” (the positions in Sanofi with the highest level of responsibility).
SANOFI FORM 20-F 2023 | 83 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Competencies of Board members
The Board of Directors, in liaison with the Appointments, Governance and CSR Committee, must ensure that the composition of the Board is balanced, diverse and fit for purpose.
In assessing its composition, the Board takes account of the new challenges facing Sanofi and our corporate strategy, and determines whether the qualities and skills of serving directors are sufficient for the Board to deliver on its remit.
In recent years, the Board has adapted its composition in line with its roadmap by bringing additional scientific expertise onto the Board and maintaining the level of other key competencies, especially in finance and accounting.
The Board has completed an overview of the Board’s current competencies. The matrix below(a) shows a comprehensive, balanced spread of the types of competencies required, both in general terms and by reference to our strategic ambitions (the matrix shows the number of directors possessing each of those competencies)(b):
Scientific training | Healthcare/pharmaceutical industry experience | Senior executive role in international group | Board membership in international group | International experience | Mergers & Acquisitions | Finance/Accounting | |||||||||||||||||
 |  |  |  |  |  |  | |||||||||||||||||
| Frédéric Oudéa | l | l | l | ||||||||||||||||||||
| Paul Hudson | l | l | l | l | |||||||||||||||||||
| Christophe Babule | l | l | l | l | |||||||||||||||||||
| Rachel Duan | l | l | l | l | |||||||||||||||||||
| Carole Ferrand | l | l | l | ||||||||||||||||||||
| Lise Kingo | l | l | l | l | |||||||||||||||||||
| Patrick Kron | l | l | l | l | |||||||||||||||||||
| Barbara Lavernos | l | l | |||||||||||||||||||||
| Fabienne Lecorvaisier | l | l | l | l | l | ||||||||||||||||||
| Gilles Schnepp | l | l | l | l | l | ||||||||||||||||||
| Diane Souza | l | l | l | l | |||||||||||||||||||
| Thomas Südhof | l | ||||||||||||||||||||||
| Emile Vœst | l | ||||||||||||||||||||||
| Antoine Yver | l | l | l | l | |||||||||||||||||||
% coverage for each competency | 21% | 36% | 79% | 50% | 71% | 43% | 43% | ||||||||||||||||
a) Based on the composition of the Board as of February 22, 2024.
(b) The information shown excludes directors representing employees.
It was not considered appropriate to include CSR as a specific component of the competencies matrix. CSR is a broad field that encompasses a wide variety of knowledge, competencies and experience, some of which is highly technical; thus it was not practicable to attribute overall competence in CSR to one or more of our directors. All members of our Board are in practice engaged with CSR issues, and have complementary skills and experience in the field, for example (non-exhaustive list):
•Christophe Babule, as CFO of L’Oréal, is in charge of financing the L’Oréal group’s sustainable transition. He is also a director of the L’Oréal for Women endowment fund;
•Lise Kingo holds a Master's degree in Responsibility & Business Practice from the University of Bath in the United Kingdom. She was Professor of Sustainability and Innovation at the Vrije Universiteit Amsterdam (The Netherlands) from 2006 to 2015, and in parallel held various CSR-related positions including Director of Environmental Affairs at Novozymes and Executive Vice President, Corporate Relations at Novo Nordisk, before becoming CEO & Executive Director of the United Nations Global Compact from 2015 to 2020 – a key role at the Paris COP21 climate change conference in 2015;
•Barbara Lavernos was appointed Vice-Chair of the L'Oréal Climate Emergency Fund, to support vulnerable communities to develop greater resilience in the face of climate disasters created in September 2023;
•Fabienne Lecorvaisier has experience as Executive Vice President of Air Liquide with responsibility for sustainable development, public and international affairs, along with experience acquired through her involvement in societal programs such as the Air Liquide Foundation and Inclusive Business; and
•Gilles Schnepp led Legrand’s CSR policy as Chairman and CEO from 2006 to 2018, and since March 2021 has been Chairman of the Board of Directors of Danone, a société à mission (social purpose company). He also chaired the Ecological and Economic Transition Commission of MEDEF (the French employer’s federation) from 2018 to 2021.
84 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Since 2018, when the remit of our Appointments and Governance Committee was extended to include CSR, our in-house CSR team has been updating Committee members on specific CSR issues on an as-needed basis. In addition, a progress report on our CSR strategy (established in 2021) is included as a Board agenda item on an annual basis. The report is presented by our Head of CSR, who is available to answer questions – including on technical issues – and inform the Board about each of the key CSR issues specific to Sanofi.
The number of meetings of the Appointments, Governance and CSR Committee has increased from 2023 onwards (five meetings in the year, versus three in 2022), so that the Committee can look in greater depth at each pillar of our CSR strategy; a CSR update is included on the agenda for every meeting. There are also regular updates on extra-financial ratings and regulatory changes.
Director Training
No training was provided to Board members during 2023.
The Board meeting of October 26, 2023 considered the training needs of directors and agreed on a training plan for 2024 that will include modules on CSR, cybersecurity, and AI.
Independence of Board Members
Under the terms of the AFEP-MEDEF Code, a director is independent when he or she has no relationship of any kind whatsoever with the Company, its group or its senior management that may color his or her judgment. More specifically, a director can only be regarded as independent if he or she:
•is not (and has not been during the past five years):
–an employee or executive officer of the Company,
–an employee, executive officer or director of an entity consolidated by the Company, or
–an employee, executive officer or director of the Company’s parent, or of an entity consolidated by that parent (criterion 1);
•is not an executive officer of an entity in which (i) the Company directly or indirectly holds a directorship or (ii) an employee of the Company is designated as a director or (iii) an executive officer of the Company (currently, or who has held office within the past five years) holds a directorship (criterion 2);
•is not a customer, supplier, investment banker or corporate banker that is material to the Company or its group, or for whom the Company or its group represents a significant proportion of its business (criterion 3);
•has no close family ties with a corporate officer of the Company (criterion 4);
•has not acted as an auditor for the Company over the course of the past five years (criterion 5);
•has not been a director of the Company for more than 12 years (criterion 6);
•does not receive variable compensation in cash or in the form of shares or any compensation linked to the performance of the Company or its group (criterion 7); or
•does not represent a shareholder that has a significant or controlling interest in the Company (criterion 8).
The influence of other factors such as the ability to understand challenges and risks, and the courage to express ideas and form a judgment, is also evaluated before it is decided whether a director can be regarded as independent.
In accordance with our Board Charter and pursuant to the AFEP-MEDEF Code, the Board of Directors’ meeting of February 22, 2024 discussed the independence of the current directors. Of the 16 directors in office on that date, 11 were deemed to be independent directors by reference to the independence criteria used by the Board of Directors pursuant to the AFEP-MEDEF Code: Frédéric Oudéa, Rachel Duan, Carole Ferrand, Lise Kingo, Patrick Kron, Fabienne Lecorvaisier, Gilles Schnepp, Diane Souza, Thomas Südhof, Emile Voest and Antoine Yver.
In accordance with the rules described above, Paul Hudson (who is an executive director of Sanofi), and Barbara Lavernos and Christophe Babule (who were appointed on the recommendation of L’Oréal, a major shareholder of Sanofi), are not deemed independent.
Consequently, the proportion of independent directors is 79%. This complies with the AFEP-MEDEF recommendation of at least 50% in companies with dispersed ownership and no controlling shareholder (which is the case for Sanofi). In accordance with the recommendations of the AFEP-MEDEF Code, directors representing employees and elected by trade unions are excluded when calculating the proportion of independent directors.
SANOFI FORM 20-F 2023 | 85 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Frédéric Oudéa | Paul Hudson | Christophe Babule | Rachel Duan | Carole Ferrand | Lise Kingo | Patrick Kron | Barbara Lavernos | Fabienne Lecorvaisier | Gilles Schnepp | Diane Souza | Thomas Südhof | Emile Voest | Antoine Yver | |||||||||||||||||||||||||||||||
Criterion 1: not an employee/executive officer in past 5 years | YES | NO | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 2: no cross-directorships | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 3: no significant business relationship | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 4: no close family ties | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 5: not an auditor | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 6: not held office for > 12 years | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 7: no variable or performance-linked compensation | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
Criterion 8: not a significant shareholder | YES | YES | NO(a) | YES | YES | YES | YES | NO | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
| Deemed independent | YES | NO | NO | YES | YES | YES | YES | NO | YES | YES | YES | YES | YES | YES | ||||||||||||||||||||||||||||||
(a) This table only refers to independence as defined under the AFEP-MEDEF Code. However, Christophe Babule is independent for the purposes of the NASDAQ Listing Rules and Rule 10A-3 under the Exchange Act.
Failure to fulfil one of the criteria does not automatically disqualify a director from being independent.
In assessing the criterion related to significant business relationships (criterion 3), the Board of Directors took into account the various relationships between directors and Sanofi and concluded that no relationships were of a kind that might undermine their independence. The Board of Directors noted that the Company and its subsidiaries had, in the normal course of business, over the past three years, sold products and provided services to, and/or purchased products and received services from, companies in which certain of the Company’s directors who are classified as independent (or their close family members) were senior executives or employees during 2023. In each case, the amounts paid to or received from such companies over the past three years were determined on an arm’s length basis and not at amounts that the Board regarded as undermining the independence of the directors in question.
86 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Selection Process for Board members
The Appointments, Governance and CSR Committee has a remit to organize a procedure for selecting future independent directors. Once the desired profile and skillset for a new director has been defined, a search for potential candidates is conducted by external consultants.
Once a shortlist has been established, the Committee interviews two or three candidates. The candidates also meet with the Chairs of the other Board committees, and in some cases the other Committee members as well. In all cases, they meet with the Chairman of the Board of Directors and the Chief Executive Officer. After completing the interviews, the Committee makes a recommendation to the Board on the candidate with the best fit for the profile, supporting that recommendation with an explanation of how the interviews were conducted and giving reasons why a candidate was selected. Before recommending a candidate to the Board, the Committee obtains assurance as to their availability, in particular as regards any other executive posts or offices the candidate may hold.
Overview of selection process for Board members
| Definition of profile and skillset | Pre-selection | Selection | Appointment | |||||||||||||||||||||||||||||||||||
| Independent directors | Appointments, Governance & CSR Committee defines the profile and skillset | Appointments, Governance & CSR Committee pre-selects three potential candidates from a long-list suggested by external consultants | Some or all Committee members interviews two or three short-listed candidates | Appointments, Governance & CSR Committee recommends a candidate, and explains the reasons for its recommendation | ||||||||||||||||||||||||||||||||||
| Directors representing employees | •One Director representing employees is designated by the trade union body which is the most representative, in the Company and those of its direct or indirect subsidiaries that have their registered office in French territory, •One Director representing employees is designated by the European Works Council | |||||||||||||||||||||||||||||||||||||
Succession planning
General principles
The remit of the Appointments, Governance and CSR Committee includes preparing for the future of the Company’s executive bodies, in particular through the establishment of a succession plan for executive officers.
The succession plan, which is reviewed at meetings of the Appointments, Governance and CSR Committee, addresses various scenarios:
•unplanned vacancy due to prohibition, resignation or death;
•forced vacancy due to poor performance, mismanagement or misconduct; and
•planned vacancy due to retirement or expiration of term of office.
Through its work and discussions, the Committee seeks to devise a succession plan that is adaptable to situations arising in the short, medium or long term, but which also builds in diversity – in all its facets – as a key factor.
To fulfill its remit, the Appointments, Governance and CSR Committee:
•provides the Board with progress reports, in particular at executive sessions;
•co-ordinates with the Compensation Committee. In that regard, having a director that sits on both Committees is a great advantage;
•works closely with the Chief Executive Officer to (i) ensure the succession plan is consistent with the Company’s own practices and market practices, (ii) ensure high-potential internal prospects receive appropriate support and training, and (iii) check there is adequate monitoring of key posts likely to fall vacant;
•meets with key executives as needed; and
•involves the Chairman and the Chief Executive Officer insofar as each has a key role in planning for his own successor, though without them directing the process.
SANOFI FORM 20-F 2023 | 87 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
In fulfilling their remit, Committee members are acutely conscious of confidentiality issues.
Although aware that separating the offices of Chairman and Chief Executive Officer provides continuity of power, the Committee nonetheless assesses the situation of the Chairman as well as that of the executive team.
Succession planning for the Chief Executive Officer is also reviewed regularly by the Appointments, Governance and CSR Committee.
Evaluation of the Board and its Committees
Under the terms of the Board Charter, and in accordance with the AFEP-MEDEF code, a discussion of the operating procedures of the Board and its committees must be included on the agenda of one Board meeting every year. The Charter also requires a formal evaluation to be performed at least every three years under the direction of the Appointments, Governance and CSR Committee, with assistance from an independent consultant.
In practice, even in years when the three-yearly formal evaluation procedure (assisted by an independent consultant) is not conducted, an annual internal evaluation is conducted using a detailed questionnaire sent to directors by the Secretary to the Board, and covering the composition and the operation of the Board and its committees. Each director is allowed a few weeks to complete the questionnaire using a secure digital platform. At the end of that period, the responses (which are confidential) are analyzed by the Secretary to the Board. The results are then presented and discussed at a meeting of the Appointments, Governance and CSR Committee; the detailed report prepared for that meeting is then submitted to a Board meeting at the start of the following year.
In addition, as reported in our annual report for 2022, from 2023 onwards the evaluation procedure also includes one-on-one interviews with each director (including the Chief Executive Officer), intended to measure the contribution of each director to the work of the Board and its committees and to record any suggestions they may have. The interviews are conducted by the Chairman of the Board, and a summary of the key points is provided to each director.
The most recent “formal” evaluation conducted with the assistance of an independent consultant took place in 2021 under the direction of the Appointments, Governance and CSR Committee.
In 2023, the evaluation was conducted internally via questionnaire (as described above).
The results of the 2023 evaluation were presented and discussed at a meeting of the Appointments, Governance and CSR Committee. The detailed report prepared for that meeting was then submitted to the Board meeting of February 22, 2024.
Detailed information about Board members
The following pages provide key information about each director individually:
•directorships and appointments held during 2023 (directorships in listed companies are indicated by an asterisk, and each director’s principal position is indicated in bold);
•other directorships held during the last five years;
•training and professional experience; and
•competencies.
88 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Frédéric Oudéa | |||||
 | Date of birth: July 3, 1963 (aged 60) | ||||
| Nationality: French | |||||
First appointed: May 2023 | |||||
Term expires: 2027 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
Chairman of the Board of Directors | In French companies | |||||||
•Chairman of the Strategy Committee •Member of the Appointments, Governance and CSR Committee •Member of the Scientific Committee | •Lead independent Director of Capgemini* •Member of the Supervisory Board of Sonic Topco, non-listed simplified joint stock company (société par actions simplifiée) registered in France - since February 1, 2024 | |||||||
Chairman of Foundation S | ||||||||
In Foreign companies | ||||||||
•Director of Sienna Investment managers, non-listed company incorporated under Luxembourg law, since December 11, 2023 | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•Board member of ALD Automotive* | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Graduate of ENA (École Nationale d'Administration) •Degree from École Polytechnique | ||||||||
Since November 2023 | Senior Executive Advisor of Bruxelles Lambert Group* | |||||||
Since May 2023 | Chairman of the Board of Directors of Sanofi* | |||||||
Since February 2022 | Board member of École Polytechnique | |||||||
Since January 2022 | Chairman of the École Polytechnique Foundation | |||||||
2015-2023 | Chief Executive Officer of Société Générale* | |||||||
| 2009-2015 | Chief Executive Officer and Chairman of the Board of Société Générale* | |||||||
| 2008-2009 | Chief Executive Officer of Société Générale* | |||||||
| 2003-2008 | Group Chief Financial Officer of Société Générale* | |||||||
| 2002-2003 | Deputy Group Chief Financial Officer of Société Générale* | |||||||
| 1998-2002 | Head of global supervision and development of the Equity Department of Société Générale* | |||||||
| 1995-1998 | Assistant Manager, then Manager of the Corporate Banking department in London at Société Générale* | |||||||
1987-1995 | Various positions within the Administration (General Inspectorate of Finance Service, Ministry of the Economy and Finance, Ministry of the Budget and Office of the Minister of Budget and Communication) | |||||||
| Competencies | ||||||||||||||
| Senior executive role in international groups, Board membership in international groups, Finance/Accounting | ||||||||||||||
SANOFI FORM 20-F 2023 | 89 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Paul Hudson | |||||
 | Date of birth: October 14, 1967 (aged 56) | ||||
| Nationality: British | |||||
| First appointed: September 2019 | |||||
| Last reappointment: May 2022 | |||||
| Term expires: 2026 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 70,805 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Chief Executive Officer | In French companies | |||||||
•Chairman of the Executive Committee •Director •Member of the Strategy Committee | •None | |||||||
In foreign companies | ||||||||
•None | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Degree in economics from Manchester Metropolitan University, UK | ||||||||
•Diploma in marketing from the Chartered Institute of Marketing, UK | ||||||||
•Honorary Doctorate in Business Administration, Manchester Metropolitan University, UK | ||||||||
| From September 1, 2019 | Chief Executive Officer of Sanofi* | |||||||
| 2016-2019 | CEO of Novartis Pharmaceuticals*, member of Executive Committee | |||||||
| 2006-2016 | Various operational and managerial positions at AstraZeneca* (including President, AstraZeneca US; Executive Vice President, North America; Representative Director & President, AstraZeneca KK, Japan; President of AstraZeneca Spain; and Vice-President and head of Primary Care United Kingdom) | |||||||
| Before 2006 | Various operational and managerial positions at Schering-Plough, including Head of Global Marketing for biologicals. Various sales and marketing positions at GlaxoSmithKline* UK and Sanofi-Synthélabo UK | |||||||
| Competencies | ||||||||||||||
| Healthcare/pharmaceutical industry experience, Senior executive role in international group, International experience, Mergers & acquisitions | ||||||||||||||
90 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Christophe Babule | |||||
 | Date of birth: September 20, 1965 (aged 58) | ||||
| Nationality: French | |||||
| First appointed: February 2019 | |||||
| Last reappointment: May 2022 | |||||
| Term expires: 2026 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Director | In French companies | |||||||
•Member of the Audit Committee | •Director of the “L'Oréal Fund for Women” charitable endowment fund | |||||||
| In foreign companies | ||||||||
•None | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
L'Oréal* Group: | ||||||||
•Director of L'Oréal US Inc. (United States) | ||||||||
| Education and professional experience | ||||||||
•MBA, HEC School of Management | ||||||||
| Since February 2019 | Chief Financial Officer at L'Oréal* | |||||||
| Since 1988 | Various positions within the L’Oréal* Group, including as Director of Administration & Finance for China, then Mexico; Director of Internal Audit; and Administration & Financial Director for the Asia Pacific Zone | |||||||
| Competencies | ||||||||||||||
| Senior executive role in international groups, International experience, Mergers & acquisitions, Finance/Accounting, CSR | ||||||||||||||
SANOFI FORM 20-F 2023 | 91 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Rachel Duan | |||||
 | Date of birth: July 25, 1970 (aged 53) | ||||
| Nationality: Chinese | |||||
| First appointed: April 2020 | |||||
| Term expires: 2024 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Member of the Compensation Committee | •Director of AXA* | |||||||
| In foreign companies | ||||||||
•Director of HSBC* | ||||||||
•Director of Adecco Group* | ||||||||
Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•MBA, University of Wisconsin-Madison (United States) | ||||||||
•Bachelor’s degree in Economics and International Trade, Shanghai International Studies University (China) | ||||||||
| Since September 2021 | Independent Director, HSBC* | |||||||
| Since April 2020 | Independent Director, Adecco Group* | |||||||
| Since April 2018 | Independent Director, AXA* | |||||||
| 1996-2020 | Senior Vice President of General Electric* (United States) and President & CEO of GE Global Markets (China) | |||||||
| Competencies | ||||||||||||||
Healthcare/pharmaceutical industry experience, Senior executive role in international groups, Board membership in international groups, International experience | ||||||||||||||
92 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Carole Ferrand | |||||
 | Date of birth: April 2, 1970 (aged 53) | ||||
| Nationality: French | |||||
| First appointed: May 2022 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Member of the Audit Committee | •Honorary President and Director of Terra Nova (non-profit association) •President of Capgemini Ventures SAS, non-listed company | |||||||
| In foreign companies | ||||||||
•Director of Capgemini Solutions Canada Inc, non-listed company •Director of Capgemini UK plc, non-listed company incorporated •Director of CGS Holdings Ltd (United Kingdom), non-listed company | ||||||||
Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•Director and Chair of the Audit Committee of Fnac Darty* •Member of the Executive Committee of June 21 SAS •Director of Capgemini* •Director of Sebdo, Le Point •Director of Archer Obligations (previously Artemis 21) •Director of Editions Tallandier •Member of the Audit Committee of Capgemini* •Director of Collection Pinault-Paris | ||||||||
| In foreign companies | ||||||||
•Director of June 21 SAS •Substitute of Alain de Marcellus, Capgemini Brasil SA (Brazil) •Director of Pallazzo Grassi (Italy) •Director of Capgemini Espana SL (Spain) •Director of Altran Innovacion S.L.U (Spain) | ||||||||
| Education and professional experience | ||||||||
•HEC School of Management, Master's degree | ||||||||
2018 - 2023 | Chief Financial Officer of Capgemini* | |||||||
| 2013-2018 | Financing Operations Director of Groupe Artémis | |||||||
| 2011-2012 | Chief Financial Officer of EuropaCorp | |||||||
| 2000-2011 | Chief Financial Officer and General Counsel of Sony France | |||||||
| 1992-2000 | Audit and Transaction Services at PricewaterhouseCoopers (PwC) | |||||||
| Competencies | ||||||||||||||
Senior executive role in international groups, Board membership in international groups, Finance/Accounting | ||||||||||||||
SANOFI FORM 20-F 2023 | 93 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Lise Kingo | |||||
 | Date of birth: August 3, 1961 (aged 62) | ||||
| Nationality: Danish | |||||
| First appointed: April 2020 | |||||
| Term expires: 2024 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Member of the Appointments, Governance & CSR Committee | •Director of Danone* | |||||||
| In foreign companies | ||||||||
•Member of the Supervisory Board of Covestro AG* (Germany) | ||||||||
Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | |||||||||||
•Master’s degree in Responsibility & Business, University of Bath (United Kingdom) | |||||||||||
•Bachelor’s degree in Marketing and Economics, Copenhagen Business School (Denmark) | |||||||||||
•Bachelor’s degree in Religions and Ancient Greek Art, University of Aarhus (Denmark) | |||||||||||
•Director Certification, INSEAD (France) | |||||||||||
| Since 2022 | Independent director of Danone* | ||||||||||
| Since 2021 | Independent director of Covestro AG* (Germany) | ||||||||||
| Since 2021 | Independent Director, Aker Horizons ASA* (Norway) | ||||||||||
| Since 2020 | Member of the Advisory Panel for Humanitarian and Development Aid Coordination, Novo Nordisk Foundation (Denmark) | ||||||||||
| 2015-2020 | CEO & Executive Director of United Nations Global Compact (US) | ||||||||||
| 2002-2014 | Executive Vice President Corporate Relations & Chief of Staff at Novo Nordisk A/S (Denmark) | ||||||||||
| 1999-2002 | Senior Vice President, Stakeholder Relations at Novo Holding (Denmark) | ||||||||||
| 1988-1999 | Director, Environmental Affairs of Novozymes (Denmark) | ||||||||||
| Competencies | ||||||||||||||
Healthcare/pharmaceutical industry experience, Senior executive role in international groups, Board membership in international groups, International experience | ||||||||||||||
94 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Patrick Kron | |||||
 | Date of birth: September 26, 1953 (aged 70) | ||||
| Nationality: French | |||||
| First appointed: May 2014 | |||||
Last reappointment: May 2022 | |||||
| Term expires: 2026 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Chairman of the Compensation Committee •Member of the Appointments, Governance and CSR Committee •Member of the Strategy Committee | •Chairman of Imerys* •Chairman of Truffle Capital SAS (non-listed company) •Chairman of PKC&I SAS (non-listed company): –Permanent representative of PKC&I on the Supervisory Board of Segula Technologies | |||||||
| In foreign companies | ||||||||
•Director of Viohalco* (Belgium) | ||||||||
Past directorships expiring within the last five years | ||||||||
| WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•Interim Chief Executive Officer of Imerys* | ||||||||
| In foreign companies | ||||||||
•ElvalHalcor* (Greece) | ||||||||
•Director of Holcim* (Switzerland) | ||||||||
| Education and professional experience | ||||||||
•Degree from École Polytechnique and École Nationale Supérieure des Mines de Paris | ||||||||
| Since 2019 | Chairman of Imerys* (and Interim Chief Executive Officer from October 2019 to February 2020) | |||||||
| Since 2016 | Chairman of Truffle Capital SAS | |||||||
| Since 2016 | Chairman of PKC&I SAS | |||||||
| 2003-2016 | Chief Executive Officer, then Chairman and Chief Executive Officer of Alstom* | |||||||
| 1998-2002 | Chairman of the Managing Board of Imerys | |||||||
| 1995-1997 | Manager of the Food and Health Care Packaging Sector at Pechiney, and Chief Operating Officer of American National Can Company in Chicago (United States) | |||||||
| 1993-1997 | Chairman and Chief Executive Officer of Carbone Lorraine | |||||||
| 1993 | Member of the Executive Committee of the Pechiney Group | |||||||
| 1988-1993 | Various senior operational and financial positions within the Pechiney Group | |||||||
| 1984-1988 | Operational responsibilities in one of the Pechiney Group’s biggest factories in Greece, then manager of the Greek subsidiary of Pechiney | |||||||
| 1979-1984 | Various positions at the French Ministry of Industry, including as project officer at the Direction régionale de l’Industrie, de la Recherche et de l’Environnement (DRIRE) and in the Ministry’s general directorate | |||||||
| Competencies | ||||||||||||||
| Senior executive role in international groups, Board membership in international group, International experience, Mergers & acquisitions | ||||||||||||||
SANOFI FORM 20-F 2023 | 95 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Wolfgang Laux | |||||
 | Date of birth: January 24, 1968 (aged 56) | ||||
| Nationality: German | |||||
| First appointed: April 2021 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 2,277 FCPE shares and 1,558 Performance shares | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
Director representing employees | In French companies | |||||||
•Member of the Compensation Committee | •None | |||||||
| In foreign companies | ||||||||
•None | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Post-doctoral research fellow at the State University of New York at Stony Brook (1998-2000) and at the University of Montpellier (1996-1997) •Ph.D. in organic chemistry from the University of Frankfurt am Main •Corporate Director’s Certificate from SciencesPo/IFA (Certificat Administrateur de Sociétés) •European Board Diploma by ecoDa | ||||||||
| Since 2006 | Industrialization Coordinator at Sanofi Chimie and Sanofi Winthrop Industries, Croix-de-Berny and Gentilly (France) | |||||||
| Since 2014 | Staff representative on the CFE-CGC ticket | |||||||
| 2016-2021 | Union delegate | |||||||
| 2014-2021 | Member of the Works Council, Sanofi Chimie headquarters | |||||||
| 2016-2019 | Member of the Committee on health, safety and working conditions (CHSCT) | |||||||
| 2000-2006 | Senior scientist in Process Development at the Frankfurt site of Höchst AG | |||||||
| Competencies | ||||||||||||||
| Scientific training, Healthcare/pharmaceutical industry experience, International experience. | ||||||||||||||
96 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Barbara Lavernos | |||||
 | Date of birth: April 22, 1968 (aged 55) | ||||
| Nationality: French | |||||
| First appointed: April 2021 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
Director | In French companies | |||||||
•Member of the Appointments, Governance and CSR Committee | •Aucun | |||||||
•Member of the Strategy Committee | In foreign companies | |||||||
L'Oréal Group* | ||||||||
•Board member of Lactobio A/S (Denmark) | ||||||||
•Board member of Bak Skincare ApS (Denmark) | ||||||||
Past directorships expiring within the last five years | ||||||||
| WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•Director of Bpifrance Investment and Bpifrance Participations | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Graduate of the HEI chemical engineering school at Lille (HEI France) | ||||||||
Since September 2023 | Vice-Chair of the L'Oréal Climate Emergency Fund | |||||||
| Since May 2021 | Deputy CEO of L'Oréal* in charge of Research, Innovation and Technology | |||||||
| February 2021-May 2021 | President Research, Innovation and Technologies at L’Oréal*– Member of the Executive Committee at L’Oréal* | |||||||
| 2018-2021 | Chief Technology and Operations Officer at L’Oréal* – Member of the Executive Committee at L’Oréal* | |||||||
| 2014-2018 | Executive Vice-President Operations at L’Oréal* – Member of the Executive Committee at L’Oréal* | |||||||
| 2011-2014 | Managing Director of Travel Retail at L'Oréal* | |||||||
| 2004-2011 | Global Chief Procurement Officer at L'Oréal* | |||||||
| Competencies | ||||||||||||||
| Senior executive role in international groups, International experience, Scientific training | ||||||||||||||
SANOFI FORM 20-F 2023 | 97 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Fabienne Lecorvaisier | |||||
 | Date of birth: August 27, 1962 (aged 61) | ||||
| Nationality: French | |||||
| First appointed: May 2013 | |||||
| Last reappointment: April 2021 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Chairwoman of the Audit Committee | •Director of Safran * (Member of the Audit and Risk Committee) | |||||||
•Member of the Supervisory Board of Wendel (Member of the Audit Committee) | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
Air Liquide Group*: | ||||||||
•Director of Air Liquide International | ||||||||
•Director of The Hydrogen Company | ||||||||
•Director of Air Liquide Finance | ||||||||
•Director of ANSA (Association Nationale des Sociétés par Actions) | ||||||||
•Director of Rexecode (economic research institute) | ||||||||
| In foreign companies | ||||||||
Air Liquide Group*: •Chairwoman of Air Liquide US LLC •Executive Vice President of Air Liquide International Corporation | ||||||||
•Director of American Air Liquide Holdings, Inc. | ||||||||
| Education and professional experience | ||||||||
•Civil engineer, graduate of École Nationale des Ponts et Chaussées | ||||||||
2021- May 2023 | Executive Vice President in charge of Sustainable Development, Public and International Affairs, Social Programs and General Secretariat of Air Liquide* | |||||||
| July 2017-July 2021 | Executive Vice President of Air Liquide* | |||||||
2008-2023 | Executive Committee member of Air Liquide* | |||||||
| 2008-2021 | Chief Financial Officer of Air Liquide* | |||||||
| 1993-2008 | Various positions within Essilor* including Group Chief Financial Officer (2001-2007) and Chief Strategy and Acquisitions Officer (2007-2008) | |||||||
| 1990-1993 | Assistant General Manager of Banque du Louvre, Taittinger Group | |||||||
| 1989-1990 | Senior Banking Executive in charge of the LBO Department (Paris)/Corporate Finance Department (Paris and London) at Barclays | |||||||
| 1985-1989 | Member of the Corporate Finance Department, then Mergers and Acquisitions Department of Société Générale* | |||||||
| Competencies | ||||||||||||||
Senior executive role in international groups, Board membership in international groups, International experience, Mergers & acquisitions, Finance/Accounting. | ||||||||||||||
98 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Gilles Schnepp | |||||
 | Date of birth: October 16, 1958 (aged 65) | ||||
| Nationality: French | |||||
| First appointed: May 2020 | |||||
| Last reappointment: May 2022 | |||||
| Term expires: 2026 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Chairman of the Appointments, Governance and CSR Committee •Member of the Strategy Committee | •Chairman of the Board of Directors of Danone* •Director of Saint Gobain* •Director of Socotec, non-listed company | |||||||
| In foreign companies | ||||||||
•None | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•Vice-Chairman of the Supervisory Board of PSA* | ||||||||
•Director of Legrand* | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Graduate of HEC business school | ||||||||
| Since 2021 | Chairman of Danone* | |||||||
| 2006-2018 | Chairman & CEO of Legrand | |||||||
| 2004-2006 | CEO of Legrand | |||||||
| 2001-2004 | Deputy CEO of Legrand | |||||||
| 1989-2001 | Various positions within the Legrand Group | |||||||
| 1983 | Merrill Lynch | |||||||
| Competencies | ||||||||||||||
| Senior executive role in international groups, Board membership in international groups, International experience, Mergers & acquisitions, Finance/Accounting | ||||||||||||||
SANOFI FORM 20-F 2023 | 99 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Diane Souza | |||||
 | Date of birth: July 3, 1952 (aged 71) | ||||
| Nationality: American | |||||
| First appointed: May 2016 | |||||
| Last reappointment: April 2020 | |||||
| Term expires: 2024 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 2,489 American Depositary Receipts, equivalent to 1,244 shares | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Member of the Compensation Committee •Member of the Audit Committee | •None | |||||||
| In foreign companies | ||||||||
Amica Insurance Companies (United States), non-listed company: •Director (Member of the Compensation and Investment Committees) | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
UnitedHealth Group: •Director of Unimerica Insurance Company, Unimerica Life Insurance Company of New York, National Pacific Dental, Inc., Nevada Pacific Dental, DBP Services of New York, IPA, Dental Benefits Providers of California, Inc., Dental Benefit Providers of Illinois, Inc., Dental Benefit Providers, Inc., Spectera, Inc. and Spectera of New York, IPA, Inc. United States Farm Credit East (United States) •Director | ||||||||
| Education and professional experience | ||||||||
•Degree in Accounting from University of Massachusetts | ||||||||
•Honorary doctorate in Business Administration from University of Massachusetts Dartmouth | ||||||||
•Certified Public Accountant | ||||||||
•Diploma in Dental Hygiene from Northeastern University, Forsyth School for Dental Hygienists | ||||||||
| 2008-2014 | Chief Operating Officer of OptumHealth Specialty Benefits (2008), then Chief Executive Officer of UnitedHealthcare Specialty Benefits (2009-2014) (United States) | |||||||
| 2007-2008 | Principal consultant at Strategic Business Solutions, LLC (United States) | |||||||
| 1994-2006 | Various positions at Aetna Inc. including Deputy Vice President Federal and State Taxes; Vice President and Chief Financial Officer, Large Case Pensions; Vice President and Head of Global Internal Audit Services; Vice President, National Customer Operations; and finally Vice President, Strategic Systems & Processes (United States) | |||||||
| 1988-1994 | Various positions at Price Waterhouse from Senior Tax Manager to Head of the Northeast Insurance Tax Region (United States) | |||||||
| 1980-1988 | Various positions at Deloitte Haskins & Sells, from Audit Staff Accountant to Senior Tax Manager-in-Charge (United States) | |||||||
| 1979 | Audit Staff Accountant at Price Waterhouse (United States) | |||||||
| Competencies | ||||||||||||||
| Healthcare/pharmaceutical industry experience, International experience, Mergers & acquisitions, Finance/Accounting | ||||||||||||||
100 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Thomas Südhof | |||||
 | Date of birth: December 22, 1955 (aged 68) | ||||
| Nationality: German and American | |||||
| First appointed: May 2016 | |||||
| Last reappointment: April 2020 | |||||
| Term expires: 2024 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 2,485 American Depositary Receipts, equivalent to 1,242 shares | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Chairman of the Scientific Committee | •None | |||||||
| In foreign companies | ||||||||
•Director of CytoDel Inc. (United States), non-listed company •Member of the Scientific Advisory Committee of NeuroCentria (United States) | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•Director of Abide Therapeutics (United States) | ||||||||
| Education and professional experience | ||||||||
•Degree in medicine from the Faculty of Medicine of the University of Göttingen (Germany) | ||||||||
•Elected member of the National Academy of Sciences of the US (2002) | ||||||||
•Elected member of the National Academy of Medicine (2007) | ||||||||
•Bernard Katz Prize of the Biophysical Society, jointly with Reinhard Jahn (2008) | ||||||||
•Elected member of the American Academy of Arts and Sciences (2010) | ||||||||
•Nobel Prize for Physiology or Medicine, jointly with James Rothman and Randy Schekman (2013) | ||||||||
•Albert Lasker Prize for Basic Medical Research, jointly with Richard Scheller (2013) | ||||||||
•Elected foreign member of the German Academy Leopoldina (2015) | ||||||||
•Elected foreign member of the Royal Society of London for Improving Natural Knowledge (2017) | ||||||||
•Elected member of the Norwegian Society of Sciences | ||||||||
| Since 2020 | Member of the Scientific Advisory Board of Danaher Corporation (United States) | |||||||
| Since 2020 | Co-founder and member of the Scientific Advisory Board of Boost, Inc. and Recognify, Inc. (United States) | |||||||
| Since 2020 | Member of the Scientific Advisory Board of NeuroCure, Charite, Berlin (Germany) | |||||||
| Since 2019 | Member of the Scientific Advisory Board of the Neuroscience Department at the Institut Pasteur (France) | |||||||
| Since 2019 | Member of the Scientific Advisory Board of the Chinese Institute for Brain Research, Beijing (China) | |||||||
| Since 2019 | Advisor to Camden Venture Partners (United States) | |||||||
| Since 2018 | Member of the Scientific Advisory Board of Jupiter, Inc. (United States) | |||||||
| Since 2018 | Chairman of the Scientific Advisory Board of Capital Medical University, Beijing (China) | |||||||
| Since 2018 | Member of the Scientific Advisory Board of Alector, Inc. (United States) | |||||||
| Since 2017 | Member of the Scientific Advisory Board of Cytodel, Inc. (United States) | |||||||
| Since 2017 | Member of the Scientific Advisory Board of the Chinese Academy of Sciences Institute of Guangzhou (China) | |||||||
| Since 2016 | Member of the Scientific Advisory Board of the Picower Institute, MIT Boston (United States) | |||||||
| Since 2016 | Member of the Scientific Advisory Board of Simcere, Inc. (China) | |||||||
| Since 2014 | Member of the Scientific Advisory Board of Elysium, Inc. (United States) | |||||||
| Since 2013 | Member of the Scientific Advisory Board of the Shemyakin-Ovchinnikov Institute of Bio-Organic Chemistry (Russia) | |||||||
| Since 2008 | Avram Goldstein Professor in the Molecular & Cellular Physiology, Neurosurgery, Psychiatry, and Neurology Department in the School of Medicine at Stanford University (United States) | |||||||
| Since 2002 | Co-founder and member of the Scientific Advisory Board of REATA Pharmaceuticals (United States) | |||||||
| Since 1986 | Investigator at the Howard Hughes Medical Institute (United States) | |||||||
SANOFI FORM 20-F 2023 | 101 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| 2017-2019 | Member of the Scientific Advisory Board of C-Bridge Everest Medical (China) | |||||||
| 2017-2018 | Member of the Scientific Advisory Board of Abide (United States) | |||||||
| 2014-2018 | Member of the Scientific Advisory Committee of the Institute of Cellular and Molecular Biology of A*Star (China) | |||||||
| 2014-2018 | Member of the Scientific Advisory Board of the Chinese Academy Institute of Biophysics (China) | |||||||
| 2014-2018 | Member of the Scientific Advisory Board of the Singapore National Research Foundation (Singapore) | |||||||
| 2014-2017 | Co-founder and member of the Scientific Advisory Board of Bluenobel, Inc. (China) | |||||||
| 2013-2016 | Member of the Review Board of Genentech Neuroscience (United States) | |||||||
| 2011-2019 | Co-founder and member of the Scientific Advisory Board of Circuit Therapeutics, Inc. (United States) | |||||||
| 1986-2008 | Professor and subsequently Chair of the Neuroscience Department at the University of Texas Southwestern Medical School (United States) | |||||||
| 1983-1986 | Postdoctoral Fellow, Dept. of Molecular Genetics, UT Southwestern Medical School (United States) | |||||||
| 1981-1982 | Intern at the University Hospital of Göttingen (Germany) | |||||||
| 1979 | Student on exchange clerkship program at Harvard Medical School (United States) | |||||||
| 1978-1981 | Research assistant at the Max Planck Institute for Biophysical Chemistry (Germany) | |||||||
| Competencies | ||||||||||||||
| Scientific training | ||||||||||||||
102 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Yann Tran | |||||
 | Date of birth: December 5, 1965 (aged 58) | ||||
| Nationality: French | |||||
| First appointed: May 2021 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,385 FCPE shares | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
Director representing employees | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•Coordinator for Industrial Europe on the Sanofi European Works Council | In French companies | |||||||
•Member of the French Strategy Committee for the Healthcare Industries and Technologies Sector | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•IFA Company Director Certificate from Sciences Po (2022) | ||||||||
•DEA in Biochemistry: Integrative Protein Biology from the University of Paris VII (France) | ||||||||
•Master's degree in Biochemical and Biological Engineering Sciences and Techniques from the University of Paris XII (France) | ||||||||
| Since 2010 | Head of Labor Relations, France at Sanofi | |||||||
| 2021 | Coordinator for Industrial Europe on the Sanofi European Works Council | |||||||
| 2014-2021 | Federation delegate for the Pharmaceuticals industry, in charge of negotiating and monitoring of industry agreements and national collective agreements | |||||||
| 2014-2021 | FCE-CFDT federation delegate for social welfare | |||||||
| 2010-2021 | Trade union leader in labor relations in the Sanofi Group | |||||||
| 2010-2014 | Member of the Supervisory Board of Sanofi employee savings plans (PEG and PERCO) and member of the Sanofi Group Committee | |||||||
| 2006-2010 | Bioinformatics researcher at Sanofi R&D | |||||||
| 1995-2006 | Researcher in molecular biology at Sanofi and Aventis | |||||||
| Competencies | ||||||||||||||
| Scientific training, Healthcare/pharmaceutical industry experience | ||||||||||||||
SANOFI FORM 20-F 2023 | 103 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Emile Voest | |||||
 | Date of birth: August 20, 1959 (aged 64) | ||||
| Nationality: Dutch | |||||
| First appointed: May 2022 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 1,000 | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Member of the Scientific Committee | •None | |||||||
| In foreign companies | ||||||||
•Chairman of the Board of Cancer Core Europe •Board Member of the Center for Personalized Cancer Treatment •Member of the Supervisory Board of Hartwig Medical Foundation | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Ph.D. in Medicine, cum laude, University of Utrecht | ||||||||
| Since 2021 | Founder of Mosaic Therapeutics and Strategic Advisor | |||||||
| Since 2019 | Senior Group Leader of the Oncode Institute | |||||||
| Since 2016 | Director of Cancer Core Europe | |||||||
| Since 2015 | Founder and Member of Supervisory Board of the Hartwig Medical Foundation | |||||||
| 2015-2020 | ESMO (European Society for Medical Oncology) •Chair of the Publications Committee (2016-2020) •Member of the Executive Board (2015-2020) | |||||||
| Since 2014 | The Netherlands Cancer Institute •Medical Oncologist (since 2014) •Executive Medical Director (2014-2020) and senior group leader | |||||||
| 2013-2016 | Co-founder and Non-Executive Medical Director of Hubrecht Organoid Technology | |||||||
| Since 2010 | Co-founder and Member of the Executive Board of the Center for Personalized Cancer Treatment (CPCT) | |||||||
| Since 1999 | Professor of Medical Oncology at UMC Utrecht | |||||||
| Competencies | ||||||||||||||
| Scientific training | ||||||||||||||
104 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Antoine Yver | |||||
 | Date of birth: January 31, 1958 (aged 66) | ||||
| Nationality: French, American, Swiss | |||||
| First appointed: May 2022 | |||||
| Term expires: 2025 | |||||
Business address: Sanofi – 46, avenue de la Grande Armée – 75017 Paris – France | |||||
Number of shares held: 2,000 American Depositary Receipts, equivalent to 1,000 shares | |||||
| Current directorships and appointments | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
| Independent director | In French companies | |||||||
•Member of the Scientific Committee | •None | |||||||
| In foreign companies | ||||||||
Director of Spotlight Therapeutics * | ||||||||
| Past directorships expiring within the last five years | ||||||||
WITHIN THE SANOFI GROUP | OUTSIDE THE SANOFI GROUP | |||||||
•None | In French companies | |||||||
•None | ||||||||
| In foreign companies | ||||||||
•None | ||||||||
| Education and professional experience | ||||||||
•Doctor of Medicine and Pediatrics, University of Paris-Sud 11 | ||||||||
| 2021-2022 | Chairman of Development of Centessa Pharmaceuticals | |||||||
| 2016-2021 | EVP Global Head Oncology R&D at Daiichi Sankyo, Inc. | |||||||
| 2009-2016 | AstraZeneca* •SVP Head Oncology Global Medicines Development & Lead China GMD (2013-2016) •VP Head Oncology Global Medicines Development & Lead China GMD (2012-2013) •VP Clinical Oncology & New Opportunities (2011-2012) •VP Clinical Oncology & Infection (2009-2011) | |||||||
| 2006-2009 | Executive Director in Oncology at the Schering-Plough Research Institute | |||||||
| 2005-2006 | Senior Director Oncology at Johnson & Johnson* | |||||||
| 1990-2005 | Senior Director Clinical Research at Aventis | |||||||
| 1981-1990 | Medical doctor at the Assistance Publique des Hôpitaux de Paris | |||||||
| Competencies | ||||||||||||||
| Scientific training, Healthcare/pharmaceutical industry experience, Senior executive role in international groups, International experience | ||||||||||||||
SANOFI FORM 20-F 2023 | 105 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Attendance Rate of Board members
| Director | Attendance rate at Board meetings | Attendance rate at Committee meetings | ||||||
Serge Weinberg* | 100 | % | 100 | % | ||||
Frédéric Oudéa** | 100 | % | 100 | % | ||||
Paul Hudson | 100 | % | 100 | % | ||||
| Christophe Babule | 91 | % | 100 | % | ||||
| Rachel Duan | 91 | % | 100 | % | ||||
Carole Ferrand | 100 | % | 100 | % | ||||
| Lise Kingo | 100 | % | 100 | % | ||||
| Patrick Kron | 100 | % | 100 | % | ||||
| Wolfgang Laux | 100 | % | 100 | % | ||||
Barbara Lavernos | 64 | % | 100 | % | ||||
| Fabienne Lecorvaisier | 100 | % | 100 | % | ||||
| Gilles Schnepp | 100 | % | 100 | % | ||||
| Diane Souza | 100 | % | 100 | % | ||||
| Thomas Südhof | 100 | % | 100 | % | ||||
| Yann Tran | 100 | % | — | % | ||||
Emile Voest | 100 | % | 100 | % | ||||
Antoine Yver | 100 | % | 100 | % | ||||
| Average attendance rate at Board meetings | Average attendance rate at Committee meetings | ||||||||||
| 97 | % | 100 | % | ||||||||
* Chairman of the Board of Directors from January 1, 2023 to May 25, 2023.
** Chairman of the Board of Directors from May 25, 2023 onwards.
Directors who were absent from some meetings provided clear and substantiated explanations for their absence, which related mainly to personal matters or to unscheduled meetings called at short notice (especially where sudden developments on an ongoing project necessitated a Board meeting).
Declarations by Board members (including convictions and conflicts of interest)
As of December 31, 2023, no corporate officer has been the subject of any conviction or court order, or been associated with any bankruptcy or winding-up order. As of this day, there is no potential conflict of interest between any corporate officer and Sanofi.
As of December 31, 2023, the members of our Board of Directors collectively held (directly, or via the employee share ownership fund associated with the Group savings scheme) 24,270 of our shares, representing 0.0019% of our share capital.
Service agreements entered into with Board members
There are no existing service agreements between the Company and its subsidiaries on the one hand, and any Board member of corporate officer on the other, that stipulate any benefit whatsoever.
Executive Committee
The Executive Committee is chaired by the Chief Executive Officer.
Four new members joined the Executive Committee in 2023 and early 2024: Houman Ashrafian (Executive Vice President, Head of Research and Development); Emmanuel Frenehard (Executive Vice President, Chief Digital Officer); Madeleine Roach (Executive Vice President, Business Operations); and Brian Foard (Executive Vice President, Specialty Care).
As of February 22, 2024, the Executive Committee had 12 members, three of whom are women. In accordance with our Board Charter, the Board of Directors – in liaison with the Compensation Committee and the Appointments, Governance and CSR Committee, and on a proposal from the Chief Executive Officer – has established a policy on gender balance within Sanofi's executive bodies. A key objective of this policy is to support the creation of a talent pool of both women and men who can potentially join the Executive Committee in future.
106 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Paul Hudson
Chief Executive Officer
Date of birth: October 14, 1967.
Paul Hudson joined Sanofi as Chief Executive Officer on September 1, 2019.
Previously CEO of Novartis Pharmaceuticals (2016-2019), where he was a member of the Executive Committee, Paul has had an extensive international career in healthcare that spans the US, Japan and Europe.
Prior to Novartis, he worked for AstraZeneca, where he held several increasingly senior positions and most recently carried out the roles of President, AstraZeneca United States and Executive Vice President, North America.
He began his career in sales and marketing roles at GlaxoSmithKline UK and Sanofi-Synthélabo UK.
Paul holds a degree in economics from Manchester Metropolitan University in the UK and last year his alma mater awarded him an honorary Doctor of Business Administration for his achievements in industry. He also holds a diploma in marketing from the Chartered Institute of Marketing, also in the UK.
Paul Hudson is a citizen of the United Kingdom.
Houman Ashrafian
Executive Vice President, Head of Research and Development
Date of birth: February 4, 1975.
Houman Ashrafian joined Sanofi on September 11, 2023.
Houman joined Sanofi from SV Health Investors where he was Managing Partner of the global private equity and venture capital investment platform which has a special focus on biotechnology, healthcare growth equity, and medtech. He has a robust track record in building high value, successful companies in the healthcare space, that brought transformational medicines from discovery to market: he co-founded and chaired the biotechs Alchemab Therapeutics, Dualitas, Enara Bio, Mestag Therapeutics, Sitryx and Trex Bio. Previously, he was Vice President and head of the Clinical Science Group at UCB with a main focus on precision medicine strategies and early clinical activities across the R&D portfolio. He also co-founded Cardiac Report, a cardiac services company, Heart Metabolics, Catamaran Bio, as well as Weatherden, a boutique clinical consultancy.
Houman is an Honorary Consultant Cardiologist at the John Radcliffe Hospital in Oxford, and a Visiting Professor at the University of Oxford in the UK. He has received numerous prestigious awards and recognitions over the course of his career, including the Michael Davies Early Career Award from the British Cardiovascular Society and the Schuldham Prize.
Houman has a bachelor’s and master’s degree from the University of Cambridge (UK) and a BM BCh and DPhil from the University of Oxford (UK).
Houman Ashrafian is a citizen of the United Kingdom.
Natalie Bickford
Executive Vice President, Chief People Officer
Date of birth: July 16, 1970.
Natalie Bickford joined Sanofi on August 1, 2020. She has worked in HR and HR leadership for more than 20 years and brings a wealth of experience in consumer-facing industries to Sanofi.
Prior to joining Sanofi, Natalie was Group HR Director at Merlin Entertainments, the world’s second largest location-based entertainment business, where she was responsible for 30,000 employees across Europe, North America, and Asia Pacific. She also held senior HR positions at Sodexo, AstraZeneca and Kingfisher Plc.
Natalie has a solid track record of transforming organizations, with a strong focus on inclusion and diversity. She was awarded “HR Diversity Champion of the Year” at the European Diversity Awards in November 2019. Natalie is also Board member of the Kronos Workforce Institute, a reflection of her deep interest in understanding and shaping the future of work.
Natalie holds a degree in French and International Politics from the University of Warwick in the UK.
Natalie Bickford is a citizen of the United Kingdom.
SANOFI FORM 20-F 2023 | 107 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Olivier Charmeil
Executive Vice President, General Medicines
Date of birth: February 19, 1963.
From 1989 to 1994, Olivier Charmeil worked in the Mergers & Acquisitions department of Banque de l’Union Européenne. He joined Sanofi Pharma in 1994 as head of Business Development. Subsequently, he held various positions within Sanofi, including Chief Financial Officer (Asia) of Sanofi-Synthélabo in 1999 and Attaché to the Chairman, Jean-François Dehecq, in 2000, before being appointed as Vice President, Development within the Sanofi-Synthélabo International Operations Directorate, where he was responsible for China and support functions. In 2003, Olivier Charmeil was appointed Chairman and Chief Executive Officer of Sanofi-Synthélabo France, before taking the position of Senior Vice President, Business Management and Support within the Pharmaceutical Operations Directorate. In this role, he piloted the operational integration of Sanofi-Synthélabo and Aventis. He was appointed Senior Vice President Asia/Pacific, Pharmaceutical Operations in February 2006; Operations Japan reported to him from January 1, 2008, as did Asia/Pacific and Japan Vaccines from February 2009. On January 1, 2011, Olivier Charmeil was appointed Executive Vice President Vaccines, and joined our Executive Committee.
In May 2015, Olivier Charmeil and André Syrota were appointed as Co-Leaders of “Medicine of the Future”, an initiative developed by the French Minister for Economy, Industry and Digital Affairs, the French Minister for Social Affairs, Health and Women’s Rights and the French Minister for National and Higher Education and Research. They have been tasked with assembling a group of industrialists and academics, with the objective of imagining how French industry can accelerate the launch and export of innovative industrial products, with an emphasis on new biotechnologies.
From June 2016 to December 2018, Olivier Charmeil served as Executive Vice President of our General Medicines and Emerging Markets Global Business Unit.
He took up the position of Executive Vice President China & Emerging Markets in January 2019. In February 2020 he was appointed to lead the General Medicines GBU, created out of the former Primary Care and China & Emerging Markets GBUs. He also serves as sponsor for China. Also in 2020, Olivier became a Board Member of the European Federation of Pharmaceutical Industries and Associations (EFPIA).
Olivier is a graduate of HEC (École des Hautes Études Commerciales) and of the Institut d’Études Politiques in Paris.
Olivier Charmeil is a citizen of France.
Jean-Baptiste Chasseloup de Chatillon
Executive Vice President, Chief Financial Officer
Date of birth: March 19, 1965.
Jean-Baptiste Chasseloup de Chatillon joined Sanofi on October 1, 2018.
Until July 2018, Jean-Baptiste Chasseloup de Chatillon served as Chief Financial Officer and Executive Vice President of the PSA Group. In that capacity, he was also a member of the Managing Board and Executive Committee. He held various management positions within the PSA Group in finance (Treasurer in Spain, Chief Financial Officer in the United Kingdom) and in sales and marketing (Business units: Bank/Insurance, Spare parts, Used vehicles, Proprietary dealership network).
He was also Chairman of the Board of Banque PSA Finance (BPF) from 2012 to June 2016. He joined the Peugeot S.A. Managing Board in 2012.
He was appointed as Director and member of the Audit Committee of Sodexo (a French listed company) on December 14, 2021.
Jean-Baptiste holds a Masters from Paris Dauphine University and studied Finance in the United Kingdom at Lancaster University.
Jean-Baptiste Chasseloup de Chatillon is a citizen of France.
Brian Foard
Executive Vice President, Specialty Care
Date of birth: December 20, 1973.
As head of our Specialty Care GBU, Brian oversees an extensive portfolio of medicines in immunology, neuro-inflammation, rare diseases, and oncology. Brian and his colleagues are responsible for launching treatments in those fields, and for implementing the strategy to bring Sanofi’s scientific breakthroughs to patients.
Brian joined Sanofi in March 2017 as the Global Head of Dermatology and Respiratory, and held roles of increasing responsibility, including as Head of Global Immunology for Sanofi and then as US Country Lead and Head of Specialty Care for North America. He has over 20 years’ experience in the specialist biopharma industry, and began his career with Galderma where he spent more than 10 years in the US before relocating to Paris to lead global marketing and launch readiness. During his time at Galderma, Brian also served in roles including General Manager for Australia & New Zealand and Vice President & General Manager of the global prescription business unit.
Brian received a degree in business from East Carolina University and has completed an executive education course at Wharton.
Brian Foard is a citizen of the United States.
108 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Emmanuel Frenehard
Executive Vice President, Chief Digital Officer
Date of birth: October 18, 1972.
Emmanuel joined Sanofi in 2020 as Global Head of Digital, and was appointed to the Executive Committee on August 31, 2023.
Prior to being appointed Chief Digital Officer, he held the positions of Global Head, Digital GBU teams and Digital Products. He also led the Sanofi Digital Accelerator and a number of digital commerce initiatives.
Before joining Sanofi, Emmanuel spent 20 years leading large global organizations as well as three years in startups. He has built and launched multiple global digital products in support of existing and new business models. In particular, he managed iflix’s rollout across Southeast Asia and led the launch of DisneyLife, Disney’s direct-to-consumer digital subscription service, in the UK.
Emmanuel is a graduate of the European Business School (EBS) and holds a Master II in Business, Finance and Audit from the Institut Supérieur de Gestion (ISG).
Emmanuel Frenehard is a citizen of France.
Brendan O’Callaghan
Executive Vice President, Global Manufacturing & Supply
Date of birth: July 16, 1961.
Brendan O’Callaghan joined Sanofi on January 1, 2015. He joined the Executive Committee on October 1, 2021.
Brendan joined Sanofi in 2015 and was previously Global Head of Biologics and Industrial Affairs Head of the Specialty Care portfolio. He has played a key role in supporting our transformation to a fully integrated BioPharmaceutical company and advancing the digital transformation of our manufacturing network.
Prior to Sanofi, Brendan worked at Schering-Plough before moving to Merck/MSD as Head of Biologics and later Vice President of its Europe, Middle East and Africa Operations.
Brendan graduated in chemical engineering from the University College of Dublin, where he currently serves as an honorary adjunct Professor of Chemical and Biochemical Engineering.
Brendan O’Callaghan is a citizen of Ireland.
Julie Van Ongevalle
Executive Vice President, Consumer Healthcare
Date of birth: November 22, 1974.
Julie Van Ongevalle joined Sanofi on September 1, 2020.
With over 20 years of international experience, Julie Van Ongevalle has a deep knowledge of consumers and digital, as well as a proven track record in brand building, from identifying growth opportunities to building and implementing delivery strategies.
Prior to joining Sanofi, Julie worked at the Estée Lauder Companies, where she held roles of increasing responsibility across the company, starting in 2004. As Global Brand President of the Origins brand from 2016, she led a global organization of 4,000 people, growing the company’s market share across geographies. Prior to Origins, she spent eight years in the M.A.C. Cosmetics division, first as General Manager Benelux, then of the EMEA Region and finally North America.
Julie started her career as a marketing manager at GSK Consumer Healthcare and Clinique.
Julie graduated from the Institut Catholique des Hautes Études Commerciales (Belgium) with a Master of Science in Commercial and Financial Sciences.
Julie Van Ongevalle is a citizen of Belgium.
Roy Papatheodorou
Executive Vice President, General Counsel
Date of birth: May 15, 1978.
Roy Papatheodorou joined Sanofi on February 1, 2022.
Prior to joining Sanofi, Roy was General Counsel of Novartis Pharmaceuticals. He has a wealth of experience in leading global and diverse teams, having also headed the Legal Transactions team at Novartis and having previously been the General Counsel of the Actavis Group, one of the largest generics companies at the time.
He started his career at international law firm Linklaters, where he specialized in international M&A, corporate and private equity based in London, with time also spent in Russia and Brazil.
Roy holds a LLB in Law from King’s College London and a Legal Practice Course from BPP School of Law in London.
Roy Papatheodorou is a citizen of Cyprus and Italy.
SANOFI FORM 20-F 2023 | 109 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Madeleine Roach
Executive Vice President, Business Operations
Date of birth: May 23, 1984.
Madeleine Roach joined Sanofi in 2022 as Head of Internal Audit and Risk Management, before being appointed to the Executive Committee on October 1, 2023.
Prior to joining Sanofi, Madeleine served at AstraZeneca as Head of Group Finance Services, Asia-Pacific and Head of Global Business Services Site Lead in Malaysia, delivering a wide range of business services to stakeholders and further expanding the site with the addition of value-add services and digitalization capabilities, whilst attracting top talent through strong employer branding.
Madeleine also held positions of growing responsibilities in Finance and Global Business Services at AstraZeneca, after starting her career at PricewaterhouseCoopers and KPMG in Assurance and Advisory services, in Germany and the UK.
Madeleine holds a BA (Hons) in Economics and Politics from the School of Oriental and African Studies, University of London.
Madeleine Roach is a citizen of Germany.
Thomas Triomphe
Executive Vice President, Vaccines
Date of birth: August 6, 1974.
Thomas Triophe joined Vaccines in 2004 and has since advanced within the company in several roles of increasing responsibility in sales and marketing at country, regional and global levels. From 2015 to 2018, he was Head of the Asia-Pacific Region, based in Singapore. Before that, he served as Head of Vaccines Japan from 2012 to 2015. In 2010, he became Associate Vice President, Head of the Influenza-Pneumo Franchise after three years as Director for the same franchise, based in the United States. Earlier in his career, Thomas worked in banking and strategic consulting.
Thomas served as Vice President and Head of Franchise & Product Strategy for Vaccines from January 2018, in which position he implemented the strategy for our vaccine franchises, in close collaboration with Manufacturing & Supply and R&D.
He was appointed to his current position on June 15, 2020.
Thomas earned his MSc in industrial engineering from École des Ponts ParisTech and the IFP School, and he also holds an MBA from INSEAD.
Thomas Triomphe is a citizen of France.
B. Compensation
Compensation and other arrangements for corporate officers
Process for determining the compensation policy for corporate officers
The compensation policy for corporate officers is established by the Board of Directors, acting on the recommendation of the Compensation Committee. The Board of Directors applies the AFEP-MEDEF Code when determining the compensation and benefits awarded to our executive and non-executive corporate officers.
All members of the Compensation Committee are independent, and were chosen for their technical competencies and their good understanding of current standards, emerging trends and Sanofi’s practices.
To fulfill their remit, the Committee regularly invites Sanofi’s Chief People Officer and Head of Reward and Performance to attend their meetings, although the latter absent themselves when the Committee deliberates. Committee members also work with the Chairman and the Secretary of the Board, who have contacts with our principal institutional shareholders ahead of the Annual General Meeting.
In addition, the Chairman of the Committee:
•discusses the financial, accounting and tax impacts of the proposed compensation policy with the Chairman of the Audit Committee;
•plays an active role at meetings of the Appointments, Governance and CSR Committee and the Strategy Committee (to both of which he belongs), thereby gaining assurance that the proposed performance criteria are consistent and appropriate in light of Sanofi’s strategic ambitions.
The compensation policy is not subject to annual review, although some arrangements for implementing the policy – such as the performance criteria applicable to the Chief Executive Officer’s annual variable compensation, for example – are defined by the Board of Directors on an annual basis.
110 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
After consulting the Compensation Committee and as the case may be the other Board Committees, the Board of Directors may, under the second paragraph of item III of Article L. 22-10-8 of the French Commercial Code, temporarily derogate from the approved compensation policy for the Chief Executive Officer in exceptional circumstances and to the extent that the changes are aligned with the corporate interest and necessary to safeguard the continuity or viability of Sanofi. Derogations from the approved policy are possible in respect of the performance conditions applied to the Chief Executive Officer’s compensation, and may result in either an increase or a decrease in compensation. The circumstances in which it is possible to apply such a derogation are a change in the structure of the Sanofi group or major events affecting the markets. Such derogation may only be temporary and must be properly substantiated, and will remain subject to a binding vote at the next General Meeting of Sanofi shareholders.
Compensation policy for corporate officers
This section describes the compensation policy for corporate officers of Sanofi, as established pursuant to Article L. 22-10-8 of the French Commercial Code. That policy describes all the components of compensation awarded to corporate officers of Sanofi as consideration for holding office, and explains the process by which it is determined, divided, reviewed and implemented.
Our compensation policy for corporate officers has three distinct elements: (i) the compensation policy for directors; (ii) the compensation policy for the Chairman of the Board; and (iii) the compensation policy for the Chief Executive Officer.
Each of those policies is submitted for approval by our shareholders at the Annual General Meeting, in accordance with Article L. 22-10-8 II of the French Commercial Code. The compensation policy approved in any given year applies to any person holding corporate office in that year. When a corporate officer is appointed between two Annual General Meetings, their compensation is defined by applying the terms of the compensation policy approved by the most recent Annual General Meeting of shareholders.
General principles and objectives
Our compensation policy is based on the following general principles:
•the policy must be simple;
•the policy must prioritize long-term performance;
•the level of compensation must be competitive, so that we can attract and retain talent; and
•there must be a fair balance between the corporate interest, the challenges of delivering on our strategy, and the expectations of our stakeholders.
The Compensation Committee must ensure that trends in the compensation of corporate officers over the medium term are not uncorrelated with trends in the compensation of all our employees. In terms of annual variable compensation and equity-based compensation, the Compensation Committee aims to achieve convergence between the performance criteria applied to our Senior Leaders and those applied to the Chief Executive Officer.
Our equity-based compensation policy, which aims to align employee and shareholder interests and reinforce loyalty to Sanofi, is a critical tool for our worldwide attractiveness as an employer.
Grantees of equity-based compensation plans (including our Chief Executive Officer) can only be awarded performance shares. Awarding performance shares reduces the dilutive effect of equity-based compensation plans while maintaining the same level of motivation for grantees.
Acting on the recommendation of the Compensation Committee, the Board of Directors determines the performance conditions attached to equity-based compensation for all beneficiaries at Sanofi and its subsidiaries worldwide, thereby furthering the attainment of our objectives.
The Board of Directors makes any grant of performance shares contingent on multiple, exacting multi-year performance criteria in order to ensure that our equity-based compensation plans incentivize overall performance. Failure to achieve those criteria over the entire performance measurement period results in a reduction or loss of the initial grant.
In order to align equity-based compensation with our long-term performance, performance is measured over three financial years (the “vesting period”). Awards of performance shares are also contingent on continued employment in the Sanofi group during the vesting period, followed by stringent lock-up obligations in the case of the Chief Executive Officer (see below).
The terms of prior awards cannot be reset subsequently, for instance with less exacting performance conditions.
Compensation policy for directors
Directors hold office for a four-year term, as specified in our Articles of Association. They may be removed from office by a shareholders’ meeting, at any time and without restriction.
The maximum annual amount of overall compensation allocated to the directors is capped at €2,500,000 (the cap was raised to that level in 2023 to reflect the increasing number of Board and committee meetings in recent years and the growing proportion of Board members resident outside Europe). The arrangements for allocating the overall annual amount set by the Annual General Meeting between the directors are determined by the Board of Directors, acting on a recommendation from the Compensation Committee. Directors’ compensation comprises an annual fixed amount of €30,000, apportioned on a time basis for directors who assumed or left office during the year, and a variable amount, allocated by the Board according to actual attendance at Board and Committee meetings. As required by the AFEP-MEDEF Code, directors’ compensation is allocated predominantly on a variable basis.
SANOFI FORM 20-F 2023 | 111 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
The table below shows how the variable amount payable to directors for attendance at Board and committee meetings is determined.
The Board meeting of February 22, 2024, acting on a recommendation from the Compensation Committee, amended the allocation rules for that variable amount as follows, with effect from 2024 onwards:
•the amounts payable to (i) members of the Audit Committee resident outside France, (ii) members of the Appointments, Governance and CSR Committee resident outside Europe and (iii) the Chairman/Chairwoman of the Audit Committee, the Appointments, Governance and CSR Committee and the Compensation Committee were increased; and
•when a director residing outside France attends a Board meeting and one or more Committee meetings and/or strategic seminars during the same trip, he will receive a lower amount than the scale for attendance at certain Committee meetings and/or strategic seminars, except for Committee Chairmen/Chairwomen, whose usual compensation is unchanged.
| Compensation per meeting | ||||||||||||||
| Directors resident in France | Directors resident outside France but within Europe | Directors resident outside Europe | Chairman/Chairwoman | |||||||||||
| Board of Directors | €5,500 | €8,250 | €11,000 | N/A | ||||||||||
| Audit Committee | €8,250 | €11,000 | €13,750 | €13,750 | ||||||||||
| Compensation Committee | €5,500 | €8,250 | €11,000 | €11,000 | ||||||||||
| Appointments, Governance and CSR Committee | €5,500 | €8,250 | €11,000 | €11,000 | ||||||||||
| Strategy Committee | €5,500 | €8,250 | €11,000 | N/A | ||||||||||
| Scientific Committee | €5,500 | €8,250 | €11,000 | €11,000 | ||||||||||
The introduction of a separate compensation scale depending on whether or not the director is a European resident is intended to take into account the significantly longer travel time required to attend Board meetings in person.
Directors who take part via videoconference receive compensation equivalent to that paid to a director resident in France attending in person. Committee Chairs continue to receive the usual compensation in respect of the Committee they chair.
As an exception, in certain cases two meetings held on the same day give entitlement only to a single payment:
•if on the day of a Shareholders’ General Meeting, the Board of Directors meets both before and after the Meeting, only one payment is made for the two Board meetings; and
•if on the same day a director participates in a meeting of the Compensation Committee and a meeting of the Appointments, Governance and CSR Committee, only the higher of the two payments is made to cover both meetings.
Directors do not receive any exceptional compensation or equity-based compensation and have no entitlement to a top-up pension plan.
Neither the Chairman of the Board nor the Chief Executive Officer receives any compensation for serving as a director.
Compensation policy for the Chairman of the Board of Directors
The term of office of the Chairman of the Board is the same as that of the other directors (four years), and the Chairman’s term is aligned with his term of office as a director. He may be removed from office at any time by the Board of Directors.
The compensation policy for the Chairman of the Board of Directors is discussed by the Compensation Committee, which then makes a recommendation to the Board of Directors. The Chairman of the Board is not a member of the Committee, and does not attend meetings where his compensation is discussed.
The compensation of the Chairman of the Board of Directors (where the office of Chairman is separate from that of Chief Executive Officer, as is currently the case) consists solely of fixed compensation and benefits in kind and excludes any variable or exceptional compensation, any awards of stock options or performance shares, and any compensation for serving as a director.
The annual fixed compensation awarded to the Chairman of the Board of Directors is €880,000 gross; that amount was set at the Board meeting of February 22, 2023, and became applicable with effect from May 25, 2023, date on which the current Chairman took office.
This amount takes account of the specific remit of the Chairman of the Board of Directors as described in the Sanofi Board Charter, and of his membership of three Board Committees (the Strategy Committee, which he chairs, the Appointments, Governance and CSR Committee; and the Scientific Committee).
The compensation of the Chairman of the Board of Directors is not subject to annual review.
Where the office of Chairman is separate from that of Chief Executive Officer, the Chairman of the Board is not entitled to the Sanofi top-up defined-contribution pension plan.
Nor is he entitled to a termination benefit or a non-compete indemnity.
112 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Compensation policy for the Chief Executive Officer
General principles
Our Chief Executive Officer is not appointed for a fixed term of office. He may be removed from office on legitimate grounds at any time by the Board of Directors.
The compensation policy for the Chief Executive Officer is established by the Board of Directors, acting on the recommendation of the Compensation Committee. The compensation structure is not subject to annual review and is applicable for as long as it remains unchanged. The arrangements for implementing the policy may vary from year to year; a table showing the changes made to those arrangements in 2024 and 2023 is provided at the end of the present section.
The compensation of the Chief Executive Officer is determined with reference to compensation awarded to the chief executive officers of the following 12 leading global pharmaceutical companies(1): Amgen, AstraZeneca plc, Bayer AG, Bristol-Myers Squibb Inc., Eli Lilly and Company Inc., GlaxoSmithKline plc, Johnson & Johnson Inc., Merck Inc., Novartis AG, Novo Nordisk, Pfizer Inc., and Roche Holding Ltd. This panel comprises companies that are comparable to Sanofi, with no limitation as to geographical region given that Sanofi operates in a particularly competitive international environment. Consistency with market practice is fundamental in order to attract and retain the talents necessary to our success. In 2023, on the basis of information published as of the date of this annual report, the median fixed compensation of the chief executive officers of the aforementioned 12 leading global pharmaceutical companies was in the region of €1,619,000; the median of the annual variable compensation awarded was in the region of €2,523,000; and the median of the long-term compensation awarded (whether equity-based or in cash) represented around 861% of fixed compensation. Within this peer group, Paul Hudson’s overall compensation (fixed, variable and equity-based compensation) lies in the low range of the second quartile of the compensation paid by the panel companies. The practices of the main CAC 40 companies are also taken into account.
On taking up office
When the Chief Executive Officer is an outside appointment, the Board of Directors may decide, acting on a recommendation from the Compensation Committee, to compensate the appointee for some or all of the benefits he may have forfeited on leaving his previous employer. In such a case, the terms on which the Chief Executive Officer is hired aim to replicate the diversity of what was forfeited, with a comparable level of risk (variable portion, medium-term equity-based or cash compensation).
During the term of office
Compensation structure
Our policy aims at achieving and maintaining a balance in the compensation structure between fixed compensation, benefits in kind, short-term variable cash compensation, and medium-term variable equity-based compensation.
The compensation policy for the Chief Executive Officer is designed to motivate and reward performance by ensuring that a significant portion of compensation is contingent on the attainment of financial, operational and extra-financial criteria that reflect Sanofi’s objectives, and are aligned with the corporate interest and with the creation of shareholder value. Variable cash compensation and equity-based compensation are the two principal levers for action, and are intended to align the interests of the Chief Executive Officer with those of our shareholders and stakeholders.
During the meeting that follows the Board meeting held to close off the financial statements for the previous year, the Compensation Committee examines the levels of attainment of variable compensation for that year. In advance of that meeting, the Chief Executive Officer presents the Committee with a report containing narrative and quantitative information necessary to measure attainment of the objectives. The members of the Compensation Committee then discuss the information provided and report to the Board on those discussions, giving an evaluation of the Chief Executive Officer’s performance against each of the criteria (determining the level of attainment for quantitative objectives, and evaluating the level of attainment for qualitative objectives compared to the objectives set at the beginning of the year).
Annual fixed compensation
The annual fixed compensation of the Chief Executive Officer has been set at €1,400,000 gross since 2022. It had previously remained unchanged since 2019.
The amount of fixed compensation is not subject to annual review. It may however be changed, provided that such changes are not material:
•on the appointment of a new Chief Executive Officer, to reflect the new appointee’s competencies and/or then current market practice; and
•in exceptional circumstances, to take account of changes in (i) the role or responsibilities of the Chief Executive Officer, for example in terms of market conditions or the size of the Sanofi group or (ii) the performance level of Sanofi over a given period.
(1) Survey conducted on the basis of data supplied by Pay Governance and Baracay.
SANOFI FORM 20-F 2023 | 113 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Annual variable compensation
Annual variable compensation is in a range between 0% and 250% of fixed compensation, with a target of 150%. It is subject to a range of varied and exacting performance criteria, both quantitative and qualitative. The criteria are reviewed annually in light of the strategic objectives determined by Sanofi. The Board of Directors sets the criteria for each year at the start of that year on the recommendation of the Compensation Committee.
For 2024, the criteria are:
•60% based on financial indicators published by Sanofi: sales growth, free cash flow (FCF) and business earnings per share (business EPS), each accounting for 20%; and
•40% based on specific individual objectives: transformation (15%), R&D pipeline (15%), and corporate social responsibility (10%). The individual objectives set for variable remuneration for 2024 are described in “— Compensation and benefits of all kinds awardable to corporate officers in respect of 2024” below.
The Board of Directors has decided, acting on a proposal from the Compensation Committee, to streamline the annual variable compensation structure by refocusing it on three key financial indicators. The “business net income”, "business operating income margin" and "new asset growth" criteria have been replaced by the business EPS) criterion, chosen so as to align more closely with key industry-standard indicators in the pharmaceutical sector and with guidance communicated to the markets. The Board also decided to increase the weighting of financial criteria from 50% to 60%. This change takes into account actual market practices, and comments from investors who wished to see the weighting of financial objectives increased. Although for each of these financial objectives, the Board of Directors – acting on a proposal from the Compensation Committee – has set specific objectives, they cannot be disclosed for confidentiality reasons.
The percentage of variable compensation linked to the attainment of quantitative criteria may be scaled down regardless of actual performance, in order to give greater weight to the attainment of qualitative criteria. This flexibility can only operate to reduce the amount of variable compensation, and cannot compensate for underperformance on quantitative criteria.
Payment of annual variable compensation in a given year in respect of the previous year is contingent on a favorable shareholder vote at the Annual General Meeting.
Equity-based compensation
The Chief Executive Officer’s equity-based compensation, which can only be in the form of performance shares, may not exceed 250% of his target short-term compensation (fixed plus variable).
The Chief Executive Officer’s equity-based compensation is contingent upon attainment of exacting performance conditions, all of them quantitative, measured over a three-year-period. Such awards are contingent upon both:
•internal criteria based upon:
–business earnings per share (business EPS), free cash flow (FCF), and development of the R&D pipeline;
–Affordable Access and Planet Care – extra-financial criteria; and
•an external criterion based upon the change in total shareholder return (TSR) relative to a benchmark panel of 12 leading global pharmaceutical companies: Amgen, AstraZeneca plc, Bayer AG, Bristol-Myers Squibb Inc., Eli Lilly and Company Inc., GlaxoSmithKline plc, Johnson & Johnson Inc., Merck Inc., Novartis AG, Novo Nordisk, Pfizer Inc., and Roche Holding Ltd.
The Board of Directors, acting on a proposal from the Compensation Committee, has decided (i) to replace the "business net income" criterion by "business earnings per share (business EPS)", which is a central element of Sanofi's financial communication reflecting a significant part of the Company's performance, and (ii) to add an R&D-linked criterion, to demonstrate Sanofi’s commitment to building a robust pipeline of products in line with the Company's strategy.
Acting on a proposal from the Compensation Committee, the Board of Directors sought to maintain common criteria for annual variable compensation and equity-based compensation, in order to ensure that short-term performance does not come at the expense of long-term performance.
Measurable, material extra-financial criteria aligned with Sanofi’s CSR strategy were introduced into equity-based compensation plans in 2023 following discussions with investors, who were supportive of the criteria selected.
The valuation of performance shares is calculated at the date of grant, weighted between (i) fair value determined using the Monte Carlo model and (ii) the market price of Sanofi shares at the date of grant, adjusted for dividends expected during the vesting period.
Each award to our Chief Executive Officer takes into account previous awards and his overall compensation. In any event, the maximum number of shares to be delivered may not be more than the number of performance shares initially awarded.
For details of the proposed award to the Chief Executive Officer in respect of 2024, refer to “— Compensation and benefits of all kinds awardable to corporate officers in respect of 2024” below.
Share ownership and lock-up obligation of the Chief Executive Officer
The Chief Executive Officer is bound by the same obligations regarding share ownership specified in our Articles of Association and Board Charter as our other corporate officers.
In addition, until he ceases to hold office the Chief Executive Officer is required to retain a quantity of Sanofi shares equivalent to 50% of any gain (net of taxes and social contributions) arising on the vesting of performance shares, calculated as of the date on which those shares vest. Those shares must be retained in registered form until he ceases to hold office.
In compliance with the AFEP-MEDEF Code and our Board Charter, the Chief Executive Officer must undertake to refrain from entering into speculative or hedging transactions.
114 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Multi-year variable compensation
The Chief Executive Officer does not receive multi-year variable compensation.
Compensation for serving as a director
Executive officers of Sanofi do not receive any compensation for serving as directors. Consequently, the Chief Executive Officer does not receive compensation in his capacity as a director or as a member of the Strategy Committee.
Exceptional compensation
No exceptional compensation can be awarded to the Chief Executive Officer.
On leaving office
The Chief Executive Officer is entitled to a top-up defined-contribution pension plan, a termination benefit, and a non-compete indemnity.
Such arrangements are part of the overall compensation package generally awarded to executive officers; in line with the recommendations of the AFEP-MEDEF code, there are very strict rules about how they are implemented. The termination benefit and non-compete indemnity are intended to compensate for the fact that the Chief Executive Officer may be dismissed at any time.
Each of those benefits is taken into account by the Board of Directors when fixing the overall compensation of the Chief Executive Officer.
Pension arrangements
The Chief Executive Officer is entitled to benefits under the top-up defined-contribution pension plan introduced within Sanofi on January 1, 2020. This is a collective plan falling within the scope of Article 82 of the French General Tax Code. It is also offered to members of our Executive Committee and to all senior executives whose position is classified within the Sanofi grade scale as “Executive Level 1 or 2”. The Chief Executive Officer’s entitlement under this plan may be withdrawn by a decision of the Board of Directors, but not retroactively.
Under the terms of the plan, the Chief Executive Officer receives an annual contribution the amount of which (subject to attainment of a performance condition) may be up to 25% of his reference compensation (annual fixed and variable cash-based compensation only; all other compensation is excluded). The rights accruing under the plan are those that are generated by the capitalization contract taken out with the insurer, and vest even if the Chief Executive Officer does not remain with Sanofi until retirement. The Chief Executive Officer may elect for the rights to be transferable as a survivor’s pension.
The performance condition is as follows:
•if the level of attainment for variable compensation is equal to or greater than the target (i.e. 150% of fixed compensation), 100% of the contribution is paid;
•if the level of attainment for variable compensation is less than 100% of fixed compensation, no contribution is paid; and
•between those two limits, the contribution is calculated on a pro rata basis.
Because this performance condition is linked to the attainment of the performance criteria for annual variable compensation (which itself is determined with reference to the strategic objectives of Sanofi), it ensures that no pension contributions could be made in the event that the Chief Executive Officer fails to deliver.
The plan is wholly funded by Sanofi, which pays the full amount of the gross contributions. Because it is treated as equivalent to compensation, the contribution is subject to payroll taxes and employer’s social security charges, and to income tax in the hands of the Chief Executive Officer; all of the above are charged on the basis of the bands, rates and other conditions applicable to compensation, and paid and declared on his pay slips for the contribution period.
Subject to (i) formal confirmation by the Board of Directors that the performance condition for the previous year has been met and (ii) approval of the Chief Executive Officer’s compensation package for that year by the Annual General Meeting of our shareholders, the annual gross contribution is paid as follows:
•50% as a gross insurance premium to the fund manager; and
•50% to the Chief Executive Officer, to indemnify him for the social security and tax charges for which he will become immediately liable.
In accordance with Article 39,5 bis of the French General Tax Code, deferred compensation as defined in section 4 of Article L. 22-10-9,4 of the French Commercial Code can be offset against corporate profits as a taxable expense up to a limit set at three times the annual social security ceiling per beneficiary.
The pension entitlement is not cumulative with (i) any termination benefit paid in the event of forced departure or (ii) any non-compete indemnity.
SANOFI FORM 20-F 2023 | 115 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Termination arrangements
The termination benefit only becomes payable if the departure of the Chief Executive Officer is forced, i.e. in the event of removal from office or resignation linked to a change in strategy or control of the Company. Compensation for non-renewal of the term of office is irrelevant in the case of the Chief Executive Officer, because this office is held for an indefinite term.
In addition, no termination benefit is payable and the arrangement is deemed to have been rescinded in the following circumstances:
•removal from office for gross or serious misconduct (faute grave ou lourde);
•if the Chief Executive Officer elects to leave Sanofi to take up another position;
•if the Chief Executive Officer is assigned to another position within Sanofi; or
•if the Chief Executive Officer takes his pension.
Payment of the termination benefit is contingent upon fulfillment of a performance condition, which is deemed to have been met if the attainment rate for the individual variable compensation objectives exceeded 90% of the target; that condition is assessed over the three financial years preceding the Chief Executive Officer leaving office.
The amount of the termination benefit is capped at 24 months of the Chief Executive Officer’s most recent total compensation on the basis of (i) the fixed compensation effective on the date of leaving office and (ii) the last variable compensation received prior to that date subject to fulfilment of the performance condition.
The amount of the termination benefit is reduced by any amount received as consideration for the non-compete undertaking, such that the aggregate amount of those two benefits may never exceed two years of total fixed and variable compensation.
Non-compete undertaking
In the event of his departure from Sanofi, the Chief Executive Officer undertakes, during the 12-month period following his departure, not to join a competitor of Sanofi as an employee or corporate officer, or to provide services to or cooperate with such a competitor.
In return for this undertaking, he receives an indemnity corresponding to one year’s total compensation, based on his fixed compensation effective on the day he leaves office and on the last individual variable compensation he received prior to that date. This indemnity is payable in 12 monthly instalments.
However, the Board of Directors reserves the right to release the Chief Executive Officer from that undertaking for some or all of that 12-month period. In such cases, the non-compete indemnity would not be due for the period of time waived by the Company.
Consequences of the Chief Executive Officer’s departure for equity-based compensation
If the Chief Executive Officer leaves Sanofi for reasons other than resignation or removal from office for gross or serious misconduct (in which case any award of equity-based compensation is forfeited in full), the overall allocation percentage is prorated to reflect the amount of time the Chief Executive Officer remained with Sanofi during the vesting period.
If at any time prior to the expiration of the vesting period of his performance shares the Chief Executive Officer joins a competitor of Sanofi as an employee or corporate officer, or provides services to or cooperates with such a competitor, he irrevocably loses those performance shares regardless of any full or partial discharge by the Board of Directors of the non-compete undertaking relating to his office as Chief Executive Officer.
Since 2021, if the Chief Executive Officer retires at the statutory retirement age prior to the expiration of the vesting period of his performance shares, the overall allocation rate will be apportioned on a pro rata basis to reflect the amount of time for which the Chief Executive Officer remained in the employment of Sanofi during the vesting period.
116 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Summary of benefits awarded to the Chief Executive Officer on leaving office
The table below presents a summary of the benefits (as described above) that could be claimed by the Chief Executive Officer on leaving office, depending on the terms of his departure. The information provided in this summary is without prejudice to any decisions that may be made by the Board of Directors.
| Voluntary departure/Removal from office for gross or serious misconduct | Forced departure | Retirement | |||||||||
Termination benefit(a) | / | 24 months of fixed compensation as of the date of leaving office + 24 months of most recent individual variable compensation received(d) – Amounts received as non-compete indemnity | / | ||||||||
Non-compete indemnity(b) | 12 months of fixed compensation as of the date of leaving office + 12 months of most recent individual variable compensation received prior to leaving office | 12 months of fixed compensation as of date of leaving office + 12 months of most recent individual variable compensation received prior to leaving office(e) | / | ||||||||
Top-up pension(c) | / | / | Annual contribution of up to 25% of reference compensation | ||||||||
| Performance share plans not yet vested | Forfeited in full | Rights retained pro rata to period of employment within Sanofi(f) | Rights retained pro rata to period of employment within Sanofii(f) | ||||||||
(a) The amount of the termination benefit is reduced by any indemnity received as consideration for the non-compete undertaking, such that the aggregate amount of those two benefits may never exceed two years of total fixed and variable compensation.
(b) The Board of Directors may decide to release the Chief Executive Officer from the non-compete undertaking for some or all of the 12-month period. In that case, the non-compete indemnity would not be due, or would be scaled down proportionately.
(c) Defined-contribution pension plan, within the scope of Article 82 of the French General Tax Code. Subject to fulfillment of the performance condition, assessed annually.
(d) Subject to fulfillment of the performance condition assessed over the three financial years preceding departure from office, as described above.
(e) Subject to the Board of Directors enforcing the non-compete undertaking, the amount of the termination benefit is reduced by any indemnity received as consideration for the non-compete undertaking, such that the aggregate amount of those two benefits may never exceed two years of total fixed and variable compensation.
(f) In this case, the Chief Executive Officer remains subject to the terms of the plans, including the performance conditions and the non-compete clause.
Policy to recover erroneously-awarded compensation (“clawback”)
In 2023, the NASDAQ listing rules were amended to include Rule 5608, in application of Section 10D-1 of the Securities Exchange Act of 1934 which requires listed companies to implement a clawback policy.
On October 26, 2023, our Board of Directors adopted a clawback policy under which Sanofi must, within a reasonable time-frame, recover the portion of the Chief Executive Officer’s variable compensation (cash-based or equity-based) that is wholly or partly contingent on the attainment of financial performance criteria and was paid to him (according to the definition contained in the NASDAQ listing rules) based on financial information that has been determined to be erroneous and has required accounting restatement to correct an error in previously-published financial statements. The policy applies to compensation paid on or after October 2, 2023.
The clawback policy also applies to members of our Executive Committee and to our Head of Consolidation (equivalent to the Chief Accounting Officer within the meaning of the NASDAQ listing rules).
Summary of changes made to the compensation policy for the Chief Executive Officer
The table below summarizes adjustments made to how the compensation policy for the Chief Executive Officer is implemented. Some of them been thoroughly discussed with our shareholders.
2024 | 2023 | ||||
•Annual variable compensation: –To reflect shareholder expectations, the weighting of financial objectives has been increased from 50% to 60% (removal of criteria related to business net income, business operating income margin and new asset growth, addition of a criterion based on business earnings per share (business EPS)). •Equity-based compensation: –The criterion related to business net income has been replaced by business earnings per share (business EPS). –To demonstrate Sanofi's commitment to delivering on the strategic roadmap, a criterion linked to the R&D pipeline has been included in the Chief Executive Officer's equity-based compensation plan. •Clawback Policy: –Pursuant to the new NASDAQ listing rules as amended in 2023, on October 26, 2023, our Board of Directors adopted a clause allowing the clawback, in full or in part, of compensation paid to the Chief Executive Officer wholly or partly contingent on the attainment of financial criteria based on erroneous financial information. | •Annual variable compensation: –To reflect shareholder expectations, Sanofi is from now on disclosing the content of the qualitative criteria. •Variable equity-based compensation: –To link equity-based compensation (long-term compensation) to delivery of Sanofi's CSR strategy, measurable and material CSR criteria were introduced into performance share plans awarded during or after 2023. •Clawback Policy: –Pursuant to Section 10D-1 of the Exchange Act, SEC regulations and NASDAQ listing rules, the Board of Directors were to adopt a clause allowing for the recovery of some or all of the components of the Chief Executive Officer's compensation that are wholly or partially contingent on the attainment of financial performance criteria based on erroneous financial information. | ||||
SANOFI FORM 20-F 2023 | 117 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Arrangements in favor of executive officers in office as of December 31, 2023 (table No. 11 of the AFEP-MEDEF Code)
| Executive officer | Contract of employment | Top-up pension plan | Indemnities or benefits payable or potentially payable on cessation of office | Indemnities payable under non-compete clause | ||||||||||
| Chairman of the Board | No | No | No | No | ||||||||||
| Chief Executive Officer | No | Yes | Yes | Yes | ||||||||||
Compensation and benefits of all kinds awardable to corporate officers in respect of 2024
The section below describes the components of the compensation and benefits of all kinds awardable to corporate officers in respect of the 2024 financial year, pursuant to the compensation policies described in “— Compensation policy for corporate officers.”
Compensation and benefits of all kinds awardable to directors in respect of 2024
The amounts to be awarded to directors in respect of 2024 will be determined in accordance with the principles described above in “— Compensation policy for corporate officers — Compensation policy for directors.”
Compensation and benefits of all kinds awardable in respect of 2024 to the Chairman of the Board of Directors
The components of compensation awardable to the Chairman of the Board of Directors are described above in “— Compensation policy for corporate officers — Compensation policy for the Chairman of the Board of Directors.”
Acting on a recommendation from the Compensation Committee, the Board of Directors meeting of February 24, 2024 decided to maintain the amount of compensation payable to the Chairman of the Board of Directors at €880,000 gross with effect from May 25, 2023, the date on which Frédéric Oudéa took office.
The Chairman of the Board of Directors does not receive any variable compensation, stock options or performance shares, in accordance with AMF recommendations. Nor does he receive any compensation (i) for serving as a director or (ii) from any company included in Sanofi’s scope of consolidation within the meaning of Article L. 233-16 of the French Commercial Code.
Benefits in kind for 2024 comprise a company car with a driver.
Compensation and benefits of all kinds awardable in respect of 2024 to Paul Hudson, Chief Executive Officer
Fixed and variable compensation
Acting on a recommendation from the Compensation Committee, the Board of Directors meeting of February 22, 2024 determined the components of Paul Hudson’s compensation for the 2024 financial year.
Paul Hudson’s annual compensation comprises (i) annual fixed gross compensation of €1,400,000 (see the explanations provided under “— Compensation policy for corporate officers — Compensation policy for the Chief Executive Officer” above) and (ii) annual variable compensation in a range from 0% to 250% of his annual fixed compensation, with a target of 150%, and subject to both quantitative and qualitative criteria.
Acting on a recommendation from the Compensation Committee, and to take account of actual market practices and comments from investors who wished to see the weighting of financial objectives increased, the Board of Directors has decided to amend the structure of annual variable remuneration. Consequently, with effect from the 2024 financial year, the objectives are based 60% (versus 50% previously) on financial indicators (sales growth, free cash flow (FCF) and business earnings per share (business EPS)), each accounting for 20%.
Floors have been set for each financial criterion, below which no variable compensation is payable for that criterion.
Objectives based on financial indicators | ||||||||||||||
| 2024 | 2023 | |||||||||||||
Sales growth | 20 | % | Sales growth | 10 | % | |||||||||
Free Cash Flow (FCF) | 20 | % | Business net income | 10 | % | |||||||||
| Business earnings per share (business EPS) | 20 | % | Business operating income margin | 10 | % | |||||||||
Free Cash Flow (FCF) | 10 | % | ||||||||||||
Growth in new assets | 10 | % | ||||||||||||
TOTAL | 60 | % | 50 | % | ||||||||||
118 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
The Board of Directors has also streamlined the individual objectives for 2024. Individual objectives for 2024 and 2023 are shown below:
2024 individual objectives | 2023 individual objectives * | ||||||||||
Business transformation (Reallocation of pipeline resources, Centralization, hub strategy, Smart spending), Asset Portfolio, Digital Transformation) | 15.0% | Business transformation (CHC, Vaccines, General Medicines, Manufacturing & Supply, Digital & Information Systems, Specialty Care) | 15.0% | ||||||||
Development pipeline M1 (Lead selection), M2 (Candidate selection), First in Human, Pivotal Studies, Submissions, Approvals | 15.0% | People and Culture (Diversity, Culture, Succession Pipeline, Simplification) | 7.5% | ||||||||
CSR People & Culture, Environment, Governance (reinforcement of the strategic dialogue with the Board of Directors and functioning of the new Executive Committee) | 10.0% | Development pipeline M1 (Lead selection), M2 (Candidate selection), First in Human, Pivotal Studies, Submissions, Approvals | 12.5% | ||||||||
CSR –Enhancement and progress on CSR program: CO2 emissions, Affordable Access, Development of Sanofi Global Health Unit (GHU) –Image & Reputation: ongoing rollout of new corporate branding) –Compliance/ Ethics & Business Integrity: launch of new Code of Conduct | 15.0% | ||||||||||
(*) For details of individual objectives for 2023 refer to "— Compensation and benefits of all kinds paid during 2023 or awarded in respect of 2023 to Paul Hudson, Chief Executive Officer" below.
Equity-based compensation
Acting on a recommendation from the Compensation Committee and within the limits set out in the Chief Executive Officer's compensation policy, the Board of Directors meeting of February 22, 2024 proposes awarding 82,500 performance shares to Paul Hudson in respect of 2024. In accordance with the AFEP-MEDEF Code, the entire award will be subject to criteria that are both internal and external:
•internal criteria based on business earnings per share (business EPS) 35%, free cash flow 25%, R&D pipeline 10%, and CSR criteria 10%;
•external criterion (accounting for 20%) based on the change in TSR as compared with that of a panel of 12 leading global pharmaceutical companies: Amgen, AstraZeneca plc, Bayer AG, Bristol-Myers Squibb Inc., Eli Lilly and Company Inc., GlaxoSmithKline plc, Johnson & Johnson Inc., Merck Inc., Novartis AG, Novo Nordisk, Pfizer Inc., and Roche Holding Ltd.
For the plan applicable to Executive Committee members, the TSR criterion is measured in relative terms (variation from the previous ranking). That variation (the “Sanofi TSR Rank Improvement”) is determined by comparing the Endpoint Sanofi TSR Rank (established over a three-year measurement period) to the Baseline Sanofi TSR Rank (established over a one-year measurement period). TSR-linked awards would be 50% if the ranking improved by one; 100% if it improved by two; and 150% if it improved by three. For the Chief Executive Officer, any TSR-linked payment will remain contingent on Sanofi achieving an Endpoint Rank greater than or equal to the median of the TSR panel.
The CSR criteria, both of which are quantitative and which count for 10% of the award, are:
1.Affordable Access: providing essential medicines to non-communicable disease patients through Sanofi Global Health; and
2.Planet Care: Carbon Footprint Reduction, scopes 1 & 2 (reduction in CO2 emissions vs 2019).
Details of the performance objectives applicable to the Chief Executive Officer's equity-based compensation plan for 2024, including the mechanisms used to determine the attainment level for each criterion, will be published on our corporate website, in the "Compensation" section of the "Governance" pages, in advance of the Annual General Meeting.
Summary of performance objectives applicable to equity-based compensation plans | |||||||||||||||||
| 2024 | 2023 | ||||||||||||||||
| Business earnings per share (business EPS) | Internal financial criterion | 35 | % | Business net income (BNI) | 45 | % | |||||||||||
| Free Cash Flow (FCF) | Internal financial criterion | 25 | % | Free Cash Flow (FCF) | 25 | % | |||||||||||
R&D pipeline | Internal financial criterion | 10 | % | ||||||||||||||
CSR criteria | Internal extra-financial criteria | 10 | % | CSR criteria | 10 | % | |||||||||||
| TSR | External extra-financial criterion | 20 | % | TSR | 20 | % | |||||||||||
| TOTAL | 100 | % | 100 | % | |||||||||||||
In accordance with the AFEP-MEDEF Code, Paul Hudson is bound by rules on insider trading that impose blackout periods, as contained in our Board Charter.
In accordance with the AFEP-MEDEF Code and our Board Charter, Paul Hudson has undertaken not to engage in speculative or hedging transactions, and as far as the company is aware, no hedging instruments have been contracted.
SANOFI FORM 20-F 2023 | 119 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Compensation and benefits of all kinds paid during 2023 or awarded in respect of 2023 to corporate officers
The section below constitutes the report on compensation of corporate officers required by Article L. 225-37 of the French Commercial Code. The arrangements described therein will be submitted for approval by our shareholders at the Annual General Meeting called to approve the financial statements for the year ended December 31, 2023 pursuant to Article L. 22-10-34 of the French Commercial Code.
Compensation elements and benefits of all kinds paid during 2023 or awarded in respect of 2023 to directors
The compensation policy for directors (as described above in the section entitled “— Compensation policy for directors”) defines the fixed amount of compensation, and the principles for allocating the variable portion between directors, up to the limit of the overall amount approved by the Annual General Meeting.
Directors’ compensation includes an annual fixed payment, apportioned on a time basis for directors who assumed or left office during the year; and a variable amount, allocated by the Board according to actual attendance at Board and Committee meetings. As required by the AFEP-MEDEF Code, directors’ compensation is allocated predominantly on a variable basis.
For 2023, directors’ compensation was determined in accordance with the compensation policy for directors as described above in the section entitled “— Compensation policy for directors.”
Compensation allocated to directors for serving as directors (table No. 3 of the AFEP-MEDEF Code)
The table below shows amounts paid in respect of 2023 and 2022 to each member of our Board of Directors, including those whose term of office ended during those years.
Directors’ compensation for 2022, the amount of which was approved at the Board meeting of February 22, 2023, was partially paid in July 2022, with an additional payment made in 2023.
Directors’ compensation for 2023, the amount of which was approved at the Board meeting of February 22, 2024, was partially paid in July 2023, with an additional payment to be made in 2024.
(€) | Compensation in respect of 2023 | Compensation in respect of 2022 | |||||||||||||||||||||
Name | Fixed portion | Variable portion | Total amount (variable + fixed portion) | Fixed portion | Variable portion | Total gross compensation | Total gross compensation apportioned on a pro rata basis(*) | ||||||||||||||||
Christophe Babule | 30,000 | 104,500 | 134,500 | 30,000 | 129,250 | 159,250 | 134,912 | ||||||||||||||||
Rachel Duan(a) | 30,000 | 115,500 | 145,500 | 30,000 | 115,500 | 145,500 | 123,263 | ||||||||||||||||
Carole Ferrand(h) | 30,000 | 110,000 | 140,000 | 20,000 | 82,500 | 102,500 | 86,835 | ||||||||||||||||
Lise Kingo(b) | 30,000 | 118,250 | 148,250 | 30,000 | 140,250 | 170,250 | 144,231 | ||||||||||||||||
| Patrick Kron | 30,000 | 145,750 | 175,750 | 30,000 | 134,750 | 164,750 | 139,571 | ||||||||||||||||
Wolfgang Laux(c)(d) | 30,000 | 77,000 | 107,000 | 30,000 | 88,000 | 118,000 | 99,966 | ||||||||||||||||
Barbara Lavernos | 30,000 | 104,500 | 134,500 | 30,000 | 99,000 | 129,000 | 109,285 | ||||||||||||||||
| Fabienne Lecorvaisier | 30,000 | 126,500 | 156,500 | 30,000 | 143,000 | 173,000 | 146,560 | ||||||||||||||||
Melanie Lee(e) | N/A | N/A | N/A | 10,000 | 46,750 | 56,750 | 56,750 | ||||||||||||||||
Carole Piwnica(e) | N/A | N/A | N/A | 10,000 | 35,750 | 45,750 | 45,750 | ||||||||||||||||
| Gilles Schnepp | 30,000 | 145,750 | 175,750 | 30,000 | 154,000 | 184,000 | 155,879 | ||||||||||||||||
Diane Souza(a) | 30,000 | 187,000 | 217,000 | 30,000 | 206,250 | 236,250 | 200,144 | ||||||||||||||||
Thomas Südhof(a) | 30,000 | 192,500 | 222,500 | 30,000 | 203,500 | 233,500 | 197,814 | ||||||||||||||||
Yann Tran(d)fi)(g) | 30,000 | 60,500 | 90,500 | 30,000 | 77,000 | 107,000 | 90,647 | ||||||||||||||||
Emile Voest(b)(h) | 30,000 | 148,500 | 178,500 | 20,000 | 101,750 | 121,750 | 103,143 | ||||||||||||||||
Antoine Yver(a)(h) | 30,000 | 187,000 | 217,000 | 20,000 | 137,500 | 157,500 | 133,429 | ||||||||||||||||
Frédéric Oudéa(i) | 12,016 | 33,000 | 45,016 | 10,000 | 27,500 | 37,500 | 31,769 | ||||||||||||||||
| Total | 432,016 | 1,856,250 | 2,288,266 | 420,000 | 1,922,250 | 2,342,250 | 1,999,948 | ||||||||||||||||
(*) Due to the high number of Board and committee meetings, the theoretical amount of compensation payable to directors exceeded the maximum amount set by the Annual General Meeting of our shareholders. Consequently, the amount payable to each director was scaled down on a pro rata basis, as explained above.
The amounts reported are gross amounts before taxes.
(a) Director resident outside Europe.
(b) Director resident outside France but within Europe.
(c) Director appointed by the European Works Council.
(d) Director representing employees.
(e) Director who resigned from office on May 2, 2022.
(f) Compensation due to Yann Tran is paid directly to Fédération Chimie Énergie CFDT.
(g) Director appointed by the CFDT, the leading trade union organization with Sanofi in France.
(h) Director appointed by the General Meeting of May 3, 2022.
(i) Non-voting Board member appointed by the Board of Directors on September 2, 2022 until his appointment as Chairman of the Board on May 25, 2023. In accordance with the Articles of Association, the compensation of the non-voting Board member is deducted from the annual amount allocated by the General Meeting.
120 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Each of the two directors representing employees has a contract of employment with a Sanofi subsidiary, under which they receive compensation unrelated to their office as director. Consequently, that remuneration is not disclosed.
Variable compensation allocated to directors in respect of 2023 represented 81% of their total compensation.
Compensation and benefits of all kinds paid during 2023 or awarded in respect of 2023 to Serge Weinberg, Chairman of the Board of Directors from January 1, 2023 to May 25, 2023
Serge Weinberg held the office of Chairman of the Board of Directors from May 17, 2010 to May 25, 2023. He never had a contract of employment with Sanofi.
Serge Weinberg was a member of the Appointments, Governance and CSR Committee, the Scientific Committee and the Strategy Committee.
The remit of the Chairman of the Board is specified in the Board Charter, which is reproduced in its entirety in Exhibit 1.2. to this annual report.
During 2023, the activities of Serge Weinberg as Chairman of the Board of Directors included:
•chairing meetings held from January 1, 2023 through May 25, 2023 (six meetings of the Board of Directors, three meetings of the Strategy Committee); attending meetings of Committees of which he was a member (two meetings of the Appointments, Governance and CSR Committee, one meeting of the Scientific Committee); and participating in Committee meetings to which he was invited (Audit Committee and Compensation Committee);
•close monitoring of the proper implementation of the decisions taken by the Board;
•discussions with Frédéric Oudéa, appointed Chairman of the Board of Directors at the close of the Annual General Meeting held on May 25, 2023, to (i) explain to him how the Board operates and answer his questions, (ii) in connection with the evaluation of the Board’s operating procedures, and (iii) on matters relating to the projects presented to the Board;
•meetings with directors in connection with (i) the evaluation of the Board’s operating procedures and (ii) matters relating to the projects presented to the Board;
•regular meetings with the members of the Executive Committee;
•meetings with Sanofi employees and visits to subsidiaries of Sanofi;
•meetings with biotechs and medtechs;
•organizing the strategy seminar held in April 2023; and
•representing Sanofi at events or official meetings (in France and abroad) with representatives of the public authorities and other stakeholders, in line with his remit as defined by the Board Charter.
The Chairman also has a role in explaining positions taken by the Board within its sphere of competence, especially in terms of strategy, governance and executive compensation. In furtherance of this role, Serge Weinberg drew on his experience of corporate communication in:
• answering letters from investors and shareholders; and
•holding meetings with certain shareholders and proxy advisors.
Those tasks were carried out in coordination with the Chief Executive Officer.
Compensation paid in respect of the 2023 financial year (from January 1, 2023 to May 25, 2023)
On February 22, 2022, acting on a recommendation from the Compensation Committee, the Board of Directors determined the components of Serge Weinberg’s compensation for the 2023 financial year. For that financial year, Serge Weinberg’s annual fixed compensation was €800,000 gross, unchanged from the 2022 financial year. Over the period from January 1 to May 25, 2023, Serge Weinberg's compensation amounted to €324,964 gross.
In line with our compensation policy for the Chairman of the Board, as approved by our shareholders at the Annual General Meeting of May 25, 2023, he did not receive any variable compensation, and was not awarded any stock options or performance shares. He received no compensation for serving as a director, and no compensation from any company included in Sanofi’s scope of consolidation within the meaning of Article L. 233-16 of the French Commercial Code.
The amount reported in 2023 for benefits in kind (€3,225) relates to a company car with a driver.
Serge Weinberg was not covered by the Sanofi defined-contribution pension plan.
Compensation, options and shares awarded to Serge Weinberg (table No. 1 of the AFEP-MEDEF Code)
| (€) | 2023 | 2022 | ||||||
| Compensation awarded for the year (details provided in the following table) | 324,964 | 807,740 | ||||||
| Valuation of stock options awarded during the year | N/A | N/A | ||||||
| Valuation of performance shares awarded during the year | N/A | N/A | ||||||
| Valuation of other long-term compensation plans | N/A | N/A | ||||||
| Total | 324,964 | 807,740 | ||||||
SANOFI FORM 20-F 2023 | 121 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Compensation awarded to Serge Weinberg (table No. 2 of the AFEP-MEDEF Code)
| 2023 | 2022 | |||||||||||||
| (€) | Amounts due | Amounts paid | Amounts due | Amounts paid | ||||||||||
Fixed compensation(a) | 321,739 (b) | 321,739 (b) | 800,000 | 800,000 | ||||||||||
| Annual variable compensation | N/A | N/A | N/A | N/A | ||||||||||
| Exceptional compensation | N/A | N/A | N/A | N/A | ||||||||||
| Compensation for serving as a director | N/A | N/A | N/A | N/A | ||||||||||
| Benefits in kind | 3,225 | 3,225 | 7,740 | 7,740 | ||||||||||
| Total | 324,964 (b) | 324,964 (b) | 807,740 | 807,740 | ||||||||||
The amounts reported are gross amounts before taxes.
(a) Fixed compensation due in respect of a given year is paid during that year.
(b) Compensation apportioned on a pro rata time basis for the period from January 1, 2023 through May 25, 2023.
Compensation and benefits of all kinds paid during 2023 or awarded in respect of 2023 to Frédéric Oudéa, Chairman of the Board of Directors from May 25, 2023 onwards
Frédéric Oudéa was appointed Chairman of the Board of Directors on May 25, 2023. He does not have a contract of employment with Sanofi.
As Chairman of the Board, Frédéric Oudéa is a member of the Appointments, Governance and CSR Committee and the Scientific Committee, and Chair of the Strategy Committee.
The remit of the Chairman of the Board is specified in the Board Charter, which is reproduced in its entirety in Exhibit 1.2. to this annual report.
During 2023, the activities of Frédéric Oudéa as Chairman of the Board of Directors included:
•chairing meetings of the Board of Directors held from May 25, 2023 through December 31, 2023 (five meetings); attending meetings of Committees of which he is a member (three meetings of the Appointments, Governance and CSR Committee, five meetings of the Strategy Committee, and five meetings of the Scientific Committee); and participating in Committee meetings to which he was invited (Audit Committee and Compensation Committee);
•close monitoring of the proper implementation of the decisions taken by the Board;
•meetings with directors, including (i) in connection with the evaluation of the Board’s operating procedures, (ii) on matters relating to the projects presented to the Board, and (iii) on corporate governance matters;
•regular meetings with the members of the Executive Committee;
•meetings with Sanofi employees and visits to subsidiaries of Sanofi;
•meetings with biotechs and medtechs;
•organizing the strategy seminar held in October 2023; and
•representing Sanofi at events or official meetings (in France and abroad) with representatives of the public authorities and other stakeholders, in line with his remit as defined by the Board Charter.
The Chairman also has a role in explaining positions taken by the Board within its sphere of competence, especially in terms of strategy, governance and executive compensation. In furtherance of this role, Frédéric Oudéa drew on his experience of corporate communications in:
•answering letters from investors and shareholders;
•holding meetings with certain shareholders.
Those tasks were carried out in coordination with the Chief Executive Officer.
Compensation paid in respect of the 2023 financial year (from May 25, 2023 onwards)
On February 22, 2023, acting on a recommendation from the Compensation Committee, the Board of Directors set the annual compensation of the new Chairman of the Board of Directors at €880,000 gross. Over the period from May 25, 2023 to December 31, 2023, Frédéric Oudéa's compensation amounted to €528,505 gross.
In line with our compensation policy for the Chairman of the Board, Frédéric Oudéa did not receive any variable compensation, and was not awarded any stock options or performance shares. He received no compensation for serving as a director, and no compensation from any company included in Sanofi’s scope of consolidation within the meaning of Article L. 233-16 of the French Commercial Code.
Benefits in kind for the period from May 25, 2023 to December 31, 2023 were €2,418, and relate to a company car with a driver.
Frédéric Oudéa is not covered by the Sanofi defined-contribution pension plan.
122 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Compensation, options and shares awarded to Frédéric Oudéa (table No. 1 of the AFEP-MEDEF Code)
(€) | 2023 | 2022 | ||||||
| Compensation awarded for the year (details provided in the following table) | 528,505 | N/A (b) | ||||||
| Valuation of stock options awarded during the year | N/A | N/A | ||||||
| Valuation of performance shares awarded during the year | N/A | N/A | ||||||
| Valuation of other long-term compensation plans | N/A | N/A | ||||||
| Total | 528,505 | N/A (b) | ||||||
Compensation awarded to Frédéric Oudéa (table No. 2 of the AFEP-MEDEF Code)
| 2023 | 2022 | |||||||||||||
(€) | Amounts due | Amounts paid | Amounts due | Amounts paid | ||||||||||
Fixed compensation(a) | 526,087 | 526,087 | N/A | N/A | ||||||||||
| Annual variable compensation | N/A | N/A | N/A | N/A | ||||||||||
| Exceptional compensation | N/A | N/A | N/A | N/A | ||||||||||
| Compensation for serving as a director | N/A | N/A | N/A | N/A | ||||||||||
| Benefits in kind | 2,418 | 2,418 | N/A | N/A | ||||||||||
| Total | 528,505 | 528,505 | N/A | N/A | ||||||||||
The amounts reported are gross amounts before taxes.
(a) Fixed compensation due in respect of a given year is paid during that year.
(b) Compensation awarded to Frédéric Oudéa for service as a non-voting Board member, an office he held from September 2, 2022 to May 25, 2023 (the date on which he was appointed Chairman of the Board of Directors), is disclosed in the section entitled "Compensation elements and benefits of all kinds paid during 2023 or awarded in respect of 2023 to directors" above.
Compensation and benefits of all kinds paid during 2023 or awarded in respect of 2023 to Paul Hudson, Chief Executive Officer
Paul Hudson has served as Chief Executive Officer of Sanofi since September 1, 2019, and holds office for an indeterminate period.
Paul Hudson does not have a contract of employment with Sanofi, and receives no compensation from any company included in Sanofi’s scope of consolidation within the meaning of Article L. 233-16 of the French Commercial Code.
Compensation awarded to Paul Hudson (table No. 1 of the AFEP-MEDEF Code)
| (€) | 2023 | 2022 | ||||||
| Compensation awarded for the year (details provided in the following table) | 3,792,797 | 3,750,797 | ||||||
Valuation of performance shares awarded during the year(a) | 6,779,025 | 6,967,950 | ||||||
| Total | 10,571,822 | 10,718,747 | ||||||
(a) Weighting between (i) fair value determined using the Monte Carlo model and (ii) market price of Sanofi shares at the date of grant, adjusted for dividends expected during the vesting period.
The parameters used to calculate the valuations are market parameters available in the financial press.
SANOFI FORM 20-F 2023 | 123 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Fixed and variable compensation awarded to Paul Hudson (table No. 2 of the AFEP-MEDEF Code)
| 2023 | 2022 | |||||||||||||||||||||||||
| (€) | Amounts due | Amounts paid | Amounts due | Amounts paid | ||||||||||||||||||||||
Fixed compensation(a) | 1,400,000 | 1,400,000 | 1,400,000 | (a) | 1,400,000 | |||||||||||||||||||||
Annual variable compensation(b) | 2,379,300 | 2,337,300 | 2,337,300 | 2,308,800 | ||||||||||||||||||||||
Cash bonus (sign-on bonus) | N/A | N/A | N/A | 2,013,534 | (c) | |||||||||||||||||||||
| Exceptional compensation | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Compensation for serving as a director | N/A | N/A | N/A | N/A | ||||||||||||||||||||||
| Benefits in kind | 13,497 | 13,497 | 13,497 | 13,497 | ||||||||||||||||||||||
| Total | 3,792,797 | 3,750,797 | 3,750,797 | 5,735,831 | ||||||||||||||||||||||
The amounts reported are gross amounts before taxes.
(a) Fixed compensation due in respect of a given year is paid during that year.
(b) Variable compensation in respect of a given year is determined at the start of the following year and paid after the Annual General Meeting in that year, subject to shareholder approval.
(c) Cash bonus in respect of the 2021 financial year (Second Tranche of the Phantom Stock Units plan), vesting of which was subject to performance conditions (see separate section below). The Board meeting of February 22, 2022 formally noted the attainment level of the performance conditions, and the overall allocation rate. Paul Hudson was awarded 21,775 Phantom Stock Units in respect of 2021. The amount disclosed in this table represents the final valuation of the 21,775 Phantom Stock Units in respect of 2021 determined as of March 31, 2022 (the vesting date of the Second Tranche).
Fixed and variable compensation
On February 22, 2024, acting on a recommendation from the Compensation Committee, the Board of Directors determined the components of Paul Hudson’s compensation for the 2023 financial year.
The Chief Executive Officer’s annual compensation for 2023 comprises (i) annual fixed gross compensation of €1,400,000; and (ii) in line with our compensation policy for the Chief Executive Officer as approved by our shareholders at the Annual General Meeting of May 25, 2023, annual variable compensation in a range from 0% to 250% of his annual fixed compensation, with a target of 150%, and subject to both quantitative and qualitative criteria.
The objectives applicable to annual variable compensation in respect of 2023 were:
•50% based on financial indicators (sales growth, BNI, FCF, BOI margin and growth of new assets, each accounting for one-fifth); and
•50% based on specific individual objectives. For 2023, the individual objectives set by the Board were:
–business transformation (15%) – quantitative and qualitative objective,
–development pipeline (12.5%) – quantitative objective,
–people and culture (7.5%) – quantitative and qualitative objective, and
–CSR (15%) – quantitative and qualitative objective.
At the start of 2023, the Board of Directors established a precise matrix for determining each of the individual objectives. To reflect shareholder expectations, Sanofi discloses the content of the qualitative criteria, accompanied by narrative for each sub-criterion explaining the level of attainment reached. Those criteria are always assessed by reference to the performances of the leading global pharmaceutical companies.
124 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Acting on a recommendation from the Compensation Committee, the Board of Directors meeting of February 22, 2024 reviewed the attainment level of each criterion and sub-criterion. The Board’s conclusions are summarized in the table below.
| Criterion | Type | Weight | Target/Maximum (as % of fixed compensation) | Attainment level | Comments | Payout (as % of fixed compensation) | 2022 reference | |||||||||||||||||||
| Financial objectives | ||||||||||||||||||||||||||
Sales growth | Quantitative | 10% | 15%/25% | 112.90% | Confidential target, Performance above budget | 16.93 | % | 17.14 | % | |||||||||||||||||
Business net income(a) | Quantitative | 10% | 15%/25% | 112.43% | Confidential target, Performance above budget | 16.86 | % | 19.87 | % | |||||||||||||||||
Free cash flow | Quantitative | 10% | 15%/25% | 105.61% | Confidential target, Performance above budget | 15.84 | % | 17.77 | % | |||||||||||||||||
Business operating income margin | Quantitative | 10% | 15%/25% | 104.00% | Confidential target, Performance above budget | 15.60 | % | 15.30 | % | |||||||||||||||||
| Growth in new key assets | Quantitative | 10% | 15%/25% | 157.79% | DUPIXENT and vaccines performance significantly over budget | 23.66 | % | 16.35 | % | |||||||||||||||||
SANOFI FORM 20-F 2023 | 125 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Criterion | Type | Weight | Target/Maximum (as % of fixed compensation) | Attainment level | Comments | Payout (as % of fixed compensation) | 2022 reference | |||||||||||||||||||
| Individual objectives | ||||||||||||||||||||||||||
| Business Transformation | Quantitative / Qualitative | 15% | 22.5% / 37.5% | 101.83% | Vaccines: •mRNA: On track for First Visit of First Subject for a new lipid •R&D: 2 successful POCC achieved, 2 First-in-Human objectives achieved out of a target of 3 •Nirsevimab: US license for BEYFORTUS granted on time and file submission in Japan completed on time. Obtained unanimous Positive ACIP recommendation & VFC inclusion in August 2023 | 22.91 | % | 22.61 | % | |||||||||||||||||
General Medicines: •Accelerated core assets growth almost at budget impacted by price challenges •Continued portfolio simplification, exceeding the 2023 divestment and product family reduction targets •SOLIQUA launch and acceleration of TOUJEO in China | ||||||||||||||||||||||||||
Speciality Care: •DUPIXENT sales: performance above target •Launch of ALTUVIIIO in the US : sales above consensus •Amlitelimab data presented at the European Academy of Dermatology and Venerology (EADV) 2023 congress | ||||||||||||||||||||||||||
CHC: •Acceleration on digital and e-commerce sales •Carve-out scope finalized •Progress made on CIALIS and TAMIFLU switches | ||||||||||||||||||||||||||
Manufacturing and Supply: •Significant acceleration in 2023 for M&S Transformation, with key performance outcomes improved across Safety, Quality, Supply and Cost, and improved industrial performance delivered vs. 2022 •Excellent performance of service level for Specialty Care | ||||||||||||||||||||||||||
Digital: •Contribution to BOI above target due to value creation •Acceleration of commercial transformation with a Digital-first, AI-first approach to Health Care Providers and Sales Reps: on target, adjustments made to include priority products in the US launch •Digital and data-driven mindset development program for senior executives: exceeded target, with 93% of this population having completed the program by Feb 2024 | ||||||||||||||||||||||||||
People & Culture | Quantitative/ Qualitative | 7.5% | 11.25%/18.75% | 105.00% | •Reduction of voluntary turnover of women in senior roles •The succession pipeline for Key Value Driving Roles has been strengthened •Progress on Sanofi culture shift (engagement score increased) •Individual Development Plans in place for senior high potentials: exceeds target •Delivery of simplification projects above the original goal | 11.81 | % | 11.03 | % | |||||||||||||||||
126 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
| Criterion | Type | Weight | Target/Maximum (as % of fixed compensation) | Attainment level | Comments | Payout (as % of fixed compensation) | 2022 reference | |||||||||||||||||||
CSR | Further reinforce and expand on the CSR agenda | Quantitative/ Qualitative | 15% | 22.5%/37.5% | 105.00% | •Year on year Scope 1 and 2 CO2 emissions reduced by 12% •Global Access Plans developed for products in Vaccines, Specialty Care and General Medicines •5 countries enrolled in the A Million Conversations program (quantitative) •260K patients with non-communicable diseases (NCDs) reached by the Global Health Unit (GHU) Impact fund, ahead of target •Several investments made under the GHU | 23.63 | % | 24.75 | % | ||||||||||||||||
Image and Reputation | •Built and grew strong corporate brand equity, establishing solid brand governance, built strong connections of Sanofians with the brand, purpose, and ambition within 18 months post-launch •Partnership with Paris 2024 Olympic Games | |||||||||||||||||||||||||
Compliance / Ethics & Business Integrity | •Fully digital and modernized digital Code of Conduct with supporting training rolled out to all employees •Deployed a unified Sanofi thoughtful decision-making framework for all employees with practical experimentation and progress measured | |||||||||||||||||||||||||
Development pipeline | Quantitative | 12.5% | 18.75%/31.25% | 120.82% | •R&D has achieved above execution focused KPI : 15 entries into M1, 15 development candidates into M2, 9 assets have entered the clinical phase (FIH), 12 submissions (including 3 accelerated), 4 Phase 3 studies initiated •Total of 11 approvals (including 2 accelerated approvals of DUPIXENT in asthma and PN in China) vs 14 in 2022 and one NME (ALTUVIIIO in hemophilia) •Reinforcement of the pipeline through Business Development or Acquisitions: 16 pharma and 4 vaccines partnerships signed. Acquisition and full integration of Provention Bio (Pharma) •R&D and PLai.gra have made significant progress in delivering AI powered decision intelligence | 22.65 | % | 22.13 | % | |||||||||||||||||
| Total | 100% | 150%/250% | 113.30% | 169.89 | % | 166.95 | % | |||||||||||||||||||
(a) For a definition, see “Item 5. Operating and Financial Review and Prospects – A. Operating results — 1.5. Business net income” in this annual report.
Acting on a recommendation from the Compensation Committee, the Board of Directors meeting of February 22, 2024 set Paul Hudson’s variable compensation for 2023 at €2,379,300 gross, equivalent to 169,9% of his fixed compensation.
Payment of Paul Hudson’s variable compensation in respect of the 2023 financial year is contingent on approval of his compensation package by the shareholders in an Ordinary General Meeting, on the terms stipulated in Article L. 22-10-34 II of the French Commercial Code.
SANOFI FORM 20-F 2023 | 127 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Equity-based compensation
Using the authorizations granted by our shareholders via the twenty-fourth resolution at the Annual General Meeting of April 30, 2021, and acting on the recommendations of the Compensation Committee, the Board of Directors meeting of May 25, 2023 decided to award Paul Hudson 82,500 performance shares in respect of 2023. The valuation of that award as of May 25, 2023, determined in accordance with IFRS and incorporating a market-related condition, was €6,779,025, equivalent to 4.84 times his fixed compensation.
The entire amount of the award is contingent upon the achievement of performance objectives based on (i) internal criteria based upon BNI, FCF and CSR, and (ii) an external criterion based on improvement in TSR relative to that of a benchmark panel of 12 leading global pharmaceutical companies (plus Sanofi): Amgen, AstraZeneca plc, Bayer AG, Bristol-Myers Squibb Inc., Eli Lilly and Company Inc., GlaxoSmithKline plc, Johnson & Johnson Inc., Merck Inc., Novartis AG, Novo Nordisk, Pfizer Inc., and Roche Holding Ltd.
To align equity-based compensation on our medium-term performance, a three-year period (2023-2025) is used to measure performance.
The above criteria were selected because they align medium-term equity-based compensation on the strategy adopted by Sanofi.
The arrangements relating to these awards are as follows:
•the performance criterion based on BNI accounts for 45% of the award. That criterion corresponds to the ratio, at constant exchange rates, of actual BNI to budgeted BNI. It represents the average actual-to-budget ratio attained over the entire period. Budgeted BNI is derived from the budget as approved by the Board of Directors at the beginning of each financial year. The BNI objective may not be lower than the bottom end of the full-year guidance range publicly announced by Sanofi at the beginning of each year. If the attainment level is less than 95%, the corresponding performance shares are forfeited.
BNI actual-to-budget attainment level (B) | BNI allocation rate | ||||
If B < 95% | 0% | ||||
If B = 95% | 50% | ||||
If B is > 95% but < 98% | (50 + [(B - 95) x 16])% | ||||
If B is ≥ 98% but ≤ 105% | B% | ||||
If B is > 105% but < 110% | (105 + [(B - 105) x 3])% | ||||
If B is ≥ 110% | 120% | ||||
•the FCF criterion accounts for 25% of the award. This criterion was selected because it is aligned with Sanofi’s current strategic objectives, and is transparent both within and outside the company.
The FCF criterion represents the average actual-to-budget FCF ratio attained over the entire period. The award is based on a target FCF, below which some or all of the performance shares are forfeited.
FCF actual-to-budget attainment level (F) | FCF allocation rate | ||||
If F is ≤ 70% | 0% | ||||
If F is > 70% but < 80% | [(F - 70) x 5]% | ||||
If F = 80% | 50% | ||||
If F is > 80% but < 100% | (50 + [(F – 80) x 2.5])% | ||||
If F = 100% | 100% | ||||
If F is > 100% but < 120% | F% | ||||
If F is ≥ 120% | 120% | ||||
•the criterion based on TSR Rank Improvement accounts for 20% of the award. It corresponds to the change in rank of Sanofi’s TSR when compared to the TSR of peer companies included in a panel. The TSR corresponds to the trading price of Sanofi shares increased by the dividends per share during the measurement periods, without reinvestment. Sanofi TSR Rank Improvement is determined by comparing the Endpoint Sanofi TSR rank to the Baseline Sanofi TSR rank.
–The Baseline Sanofi TSR is equal to the following formula: (average prices of 2022 – average prices of 2021 + dividends per share 2022)/average prices of 2021.
–The Endpoint Sanofi TSR is equal to the following formula: (average prices of 2025 – average prices of 2022 + dividends per share 2023 to 2024)/average prices of 2022.
128 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Our TSR is compared with the benchmark panel of 12 companies listed above, so as to determine the ranking of Sanofi within the panel. The number of performance shares vesting depends upon the improvement in our TSR ranking, as follows:
| Sanofi's improvement in the rankings | TSR allocation rate | ||||
| +3 or more | 150 | % | |||
| +2 | 100 | % | |||
| +1 | 50 | % | |||
| No improvement | — | % | |||
Even if there is an improvement in Sanofi’s TSR ranking based on the principles set out above, no TSR allocation can be made if Sanofi’s ranking is below median TSR, defined as the performance of the company ranked seventh in the panel;
•the CSR-based criterion accounts for 10% of the award. This performance condition has been added to our equity-based compensation plans with effect from 2023, and equates to the attainment over a three-year period of annual objectives plus a "stretch" objective, linked to the following pillars of Sanofi’s CSR strategy:
1.Affordable Access: providing essential medicines to non-communicable disease patients through Sanofi Global Health,
2.Planet Care: Carbon Footprint Reduction, scopes 1 & 2 (% reduction in CO2 emissions vs 2019).
Attainment of each annual CSR objective will earn one performance point; a maximum of three points, plus one extra point linked to the "stretch" objective, can be earned for each CSR pillar. For each criterion, attainment of the objectives for 2025 will earn three points even if the annual objectives were not attained.
At the end of the period, the Board of Directors will determine the CSR Allocation Rate, corresponding to the number of points earned, as shown, below:
| CSR points earned | CSR Allocation Rate | ||||
Less than 3 points | 0% | ||||
| 3 points | 50% | ||||
| 4 points | 67% | ||||
| 5 points | 83 % | ||||
| 6 points | 100% | ||||
| 7 points | 110% | ||||
| 8 points | 120% | ||||
Other terms and conditions
Paul Hudson is under an obligation to retain, until he ceases to hold office, a quantity of Sanofi shares equivalent to 50% of any gain (net of taxes and social contributions) arising on the vesting of his performance shares, calculated as of the date on which those shares vest.
In compliance with the AFEP-MEDEF Code and our Board Charter, Paul Hudson has undertaken to refrain from entering into speculative or hedging transactions, and so far as Sanofi is aware no hedging instruments have been contracted.
Historical allocation rates
In the interests of transparency, we disclose below attainment levels and allocation rates for the most recent performance-linked equity-based compensation plans awarded to our Chief Executive Officer (bearing in mind that only the April 28, 2020 and the April 30, 2021 Plans apply to Paul Hudson):
| Attainment level | Allocation rate | |||||||||||||
| BNI | FCF | TSR | ||||||||||||
| April 30, 2019 plans | 2019-2021: 101.99% | 2019-2021: 127.67% | 2019-2021: 50% | 2019-2021: 97.00% i.e. 213,400 stock options and 48,500 performance shares | ||||||||||
| April 28, 2020 plans | 2020-2022: 103.27% | 2020-2022: 117.67% | 2020-2022: 0% | 2020-2022: 86.94% i.e. 65,205 performance shares | ||||||||||
April 30, 2021 plans | 2021-2023: 103.58% | 2021-2023: 110.31% | 2021-2023: 51.77% | 2021-2023: 95.23% i.e. 71,423 performance shares | ||||||||||
SANOFI FORM 20-F 2023 | 129 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Performance shares awarded to Paul Hudson in 2023 (table No. 6 of the AFEP-MEDEF Code)
| Source | Plan date | Valuation of performance shares (€) | Number of performance shares awarded during the period | Vesting date | Availability date(a) | Performance conditions | ||||||||||||||
| Sanofi | 05/25/2023 | 6,779,025 | 82,500 | 05/25/2026 | 05/25/2026 | Yes | ||||||||||||||
(a) Under the terms of our Board Charter, Paul Hudson is required to retain a quantity of shares corresponding to 50% of the capital gain arising on the vesting of the shares, net of the associated taxes and social contributions.
Each performance share awarded on May 25, 2023, was valued at €82.17, valuing the total benefit at €6,779,025.
The General Meeting of April 30, 2021 restricted the number of performance shares that can be awarded to executive officers to 5% of the overall limit (set at 1.5% of the share capital). The number of shares awarded to Paul Hudson in 2023 represents 0.44% of the total limit approved by that Meeting and 0.006% of our share capital at the date of grant.
Performance shares awarded to Paul Hudson which became available in 2023 (table No. 7 of the AFEP-MEDEF Code)
Paul Hudson was awarded 75,000 performance shares on April 28, 2020. The Board of Directors meeting of February 22, 2023 noted the level of achievement of the performance conditions applicable to this plan (86.94%), and Paul Hudson was definitively allotted 65,205 shares on May 2, 2023.
| Source | Plan date | Number of performance shares awarded (after vesting) during the period | ||||||
| Sanofi | April 28, 2020 | 65,205 | ||||||
Because awards of stock options to our Chief Executive Officer are not permitted under our compensation policy, tables No. 4 and No. 5 of the AFEP-MEDEF Code are not applicable.
Pension rights
Paul Hudson is entitled to benefits under the top-up defined-contribution pension plan introduced within Sanofi on January 1, 2020. Under the terms of the plan, the Chief Executive Officer receives (subject to attainment of a performance condition) an annual contribution of up to 25% of his reference compensation (annual fixed and variable compensation).
The performance condition for the vesting of pension rights is linked to the attainment of the performance criteria for 2023 variable compensation. The Board of Directors, at its meeting of February 22, 2024, ascertained whether that performance condition had been met, noting that the attainment level for the variable portion of Paul Hudson’s compensation for the 2023 financial year was 2,379,300%, i.e. 169.9% of his fixed compensation.
The annual gross contribution is paid as follows:
•50% as a gross insurance premium to the fund manager – the amount due to the fund manager with respect to 2023 is €472,412.50; and
•50% to Paul Hudson, to indemnify him for the social security and tax charges for which he will become immediately liable. The amount due to Paul Hudson with respect to 2023 was set by the Board of Directors at its meeting of February 22, 2024 at €472,412.50.
Payment of those amounts is contingent on approval of the Chief Executive Officer’s compensation package by the shareholders in an Ordinary General Meeting, on the terms stipulated in Article L. 22-10-34 II of the French Commercial Code.
Social welfare and health insurance
Paul Hudson is subject to, benefits from and contributes to the same health cover, and death and disability plans as are applicable to other employees of Sanofi based in France. He also benefits from an unemployment insurance scheme.
Benefits in kind
The benefits in kind received by Paul Hudson in 2023 were valued at €13,497, and correspond to a company car with a driver.
130 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Compensation and benefits for other Executive Committee members
Compensation
The compensation of Executive Committee members other than the Chief Executive Officer is reviewed by the Compensation Committee, taking into consideration the practices of leading global pharmaceutical companies.
In addition to fixed compensation, they receive variable compensation. Their target variable compensation depends on their position, and can represent up to 100% of their fixed compensation. The target amount of individual variable compensation is determined in line with market practice. It rewards the joint contribution of all Executive Committee members to Sanofi’s performance.
For 2023, the variable component consisted of two elements:
•attainment of quantitative objectives (accounting for 50%) which are measured at consolidated level: sales growth 30%, ratio of BOI to net sales (“BOI margin”) 35%, research and development outcomes 20%, and FCF 15%; and
•attainment of quantitative and qualitative objectives both individually (30%) and collectively (20%) within the Executive Committee (together accounting for 50%).
The indicators used are intended to measure Sanofi’s annual performance objectives; individual objectives; and the attainment of people objectives, individual career development plans, transformation of the corporate culture to align with the “Play to Win” strategy, and reducing Sanofi's carbon footprint.
In addition, Executive Committee members may be awarded performance shares.
For 2023, the total gross compensation paid and accrued in respect of members of the Executive Committee (excluding the Chief Executive Officer) was €21 million, including €8 million in fixed compensation.
A total of 190,648 performance shares were awarded in 2023 to members of the Executive Committee (excluding the award to the Chief Executive Officer). No stock options were awarded to members of the Executive Committee or the Chief Executive Officer in 2023.
In compliance with the AFEP-MEDEF Code, these entire awards are contingent upon three internal criteria, based on business net income (BNI)(1), free cash flow (FCF)(2), and Corporate Social Responsibility (CSR) indicators; and on an external criterion, based on TSR. Those criteria were selected because they align equity-based compensation with the strategy adopted by Sanofi. The Board believes that the performance conditions applied are good indicators of shareholder value creation in terms of the quality of investment decisions and the commitment to deliver exacting financial results in a difficult economic environment.
The arrangements relating to these awards are as follows:
•the BNI performance criterion accounts for 45% of the award. This criterion corresponds to the ratio, at constant exchange rates, of actual BNI to budgeted BNI. It represents the average actual-to-budget ratio attained over the entire period. Budgeted BNI is derived from the budget as approved by the Board of Directors at the beginning of each financial year. The BNI objective may not be lower than the bottom end of the full-year guidance range publicly announced by Sanofi at the beginning of each year. If the ratio is less than 95%, the corresponding performance shares are forfeited.
BNI actual-to-budget attainment level (B) | BNI allocation rate | ||||
If B is < 95% | 0 | % | |||
If B = 95% | 50% | ||||
If B is > 95% but < 98% | (50 + [(B –95) x 16])% | ||||
If B is ≥ 98% but ≤ 105% | B% | ||||
If B is > 105% but < 110% | (105 + [(B –105) x 3])% | ||||
If B is ≥ 110% | 120% | ||||
•the FCF criterion accounts for 25% of the award. It represents the average actual-to-budget ratio of FCF attained over the entire period. The award is based on a target FCF, below which some or all performance shares are forfeited.
FCF actual-to-budget attainment level (F) | FCF allocation rate | ||||
If F is ≤ 70% | 0 | % | |||
If F is > 70% but < 80% | [(F – 70) x 5]% | ||||
If F = 80% | 50 | % | |||
If F is > 80% but < 100% | (50 + [(F – 80) x 2.5])% | ||||
If F = 100% | 100% | ||||
If F is > 100% but < 120% | F% | ||||
If F is > 120% | 120% | ||||
(1) Non-IFRS financial measure. For a definition, refer to Item 5.A.1.5.3 Business net income (non-IFRS financial measure), in this Annual Report
(2) Non-IFRS financial measure. For a definition, refer to Item 5.B.1 B.1. Consolidated statement of cash flows, in this Annual Report
SANOFI FORM 20-F 2023 | 131 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
•the criterion based on Total Shareholder Return (“TSR”) Rank Improvement accounts for 20% of the award.
The TSR Rank Improvement corresponds to the change in Sanofi’s TSR rank relative to the TSR of a panel of Sanofi plus 12 peer companies (Amgen, AstraZeneca plc, Bayer AG, Bristol-Myers Squibb Inc., Eli Lilly and Company Inc., GlaxoSmithKline plc, Johnson & Johnson Inc., Merck Inc., Novartis AG, Novo Nordisk, Pfizer Inc., and Roche Holding Ltd).
The TSR Rank Improvement corresponds to the change in Sanofi’s TSR rank relative to the TSR of a panel of Sanofi plus 12 peer companies (Amgen, AstraZeneca plc, Bayer AG, Bristol-Myers Squibb Inc., Eli Lilly and Company Inc., GlaxoSmithKline plc, Johnson & Johnson Inc., Merck Inc., Novartis AG, Novo Nordisk, Pfizer Inc., and Roche Holding Ltd).
TSR corresponds to the market performance of Sanofi shares uplifted by dividends per share during the measurement periods, without reinvestment.
For the plan applicable to Executive Committee members, the TSR Rank Improvement is determined by comparing the Endpoint Sanofi TSR rank (measured over a three-year period) with the Baseline Sanofi TSR rank (measured over a one-year period). The TSR payment would amount to 50% for an improvement of one place in the rankings, 100% for two places in the rankings, and 150% for three places in the rankings;
•the criterion based on CSR accounts for 10% of the award. This performance criterion is linked to attainment of (i) annual objectives over a three-year period and (ii) a "stretch" objective, linked to the following pillars:
1.Affordable Access: providing essential medicines to non-communicable disease patients through Sanofi Global Health,
2.Planet Care - Carbon Footprint Reduction, scopes 1 & 2 (% CO2 emissions reduction vs 2019).
Attainment of each annual CSR objective will generate one performance point; a maximum of three points (plus one bonus point for the "stretch" objective) may be obtained for each pillar. For each criterion, attainment of the 2025 objectives will generate three points, even if the annual objectives are not attained;
•the number of performance shares vesting depends on the overall allocation rate, which for each period is the weighted average of the BNI allocation rate (45%), the FCF allocation rate (25%), the TSR allocation rate for the period (20%), and the CSR allocation rate;
•a multiplier is applied that will uplift the number of performance shares vesting by 10% if (i) the maximum TSR allocation rate is attained and (ii) Sanofi ranks greater than or equal to the median for the TSR benchmark panel at the endpoint;
•in order to align equity-based compensation with medium-term performance, performance is measured over three financial years;
•vesting is subject to a non-compete clause;
•the entire award is forfeited in the event of resignation, or dismissal for gross or serious misconduct;
•in the event of (i) individual dismissal other than for gross or serious misconduct, (ii) retirement before the age of 60, (iii) the beneficiary’s employer ceasing to be part of the Sanofi group or (iv) termination of employment contract under the terms of a collective separation plan initiated by the employer in accordance with locally applicable legislation or other measures approved by local authorities, the overall allocation percentage is apportioned on a pro rata time basis to reflect the amount of time the person remained with the Sanofi group during the vesting period;
•if any of the following events occur, full rights to the award are retained: (i) retirement on or after reaching the statutory retirement age, or early retirement under a statutory or contractual early retirement plan implemented by the relevant Sanofi entity and duly approved by the Chief Executive Officer of Sanofi; (ii) disability classified in the second or third categories stipulated in Article L. 314-4 of the French Social Security Code; or (iii) death of the beneficiary.
Pension arrangements
The total amount accrued as of December 31, 2023 in respect of corporate pension plans for persons who have held an executive position during the year 2023 was €10 million. That amount includes an expense of €1 million recognized in profit or loss during 2023.
Pay ratio between compensation of executive officers and average/median compensation of Sanofi employees – changes in compensation of executive officers and employees relative to the performance of Sanofi
This information is disclosed in accordance with Article L. 22-10-9 6° of the French Commercial Code, further to the enactment of the “Pacte” law.
Sanofi has referred to the guidance on compensation multiples issued by AFEP (version issued February 2021) in establishing the calculation methods used for the ratios presented.
Explanations of calculation methods and of year-on-year changes in the executive pay ratio:
•the scope includes Sanofi SA (the parent company) and all of its direct and indirect subsidiaries located in France, and hence covers more than 80% of total payroll of permanent employees in France. No separate ratios are published for Sanofi SA (the parent company), as the low headcount at Sanofi SA means that such ratios would not be representative of our total headcount in France;
•the employee compensation used in the calculation is the full time equivalent (FTE) compensation of permanent employees with at least two financial years of uninterrupted employment;
•compensation includes fixed compensation awarded during the reference year, and variable compensation related to the previous year and paid during the reference year. All compensation amounts are gross amounts;
•in order to maintain consistency, we have excluded from the numerator (i) compensation items not included in the denominator and (ii) non-recurring compensation items. This applies in particular to accommodation expenses related to the relocation to France of the Chief Executive Officer (Paul Hudson) in 2020, and to expenses related to unemployment insurance;
132 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
•long term variable compensation: performance shares and stock options awarded during each reference year are valued at the date of grant in accordance with international financial reporting standards. The valuation of performance shares that include the Total Shareholder Return (TSR) performance condition incorporates market conditions where applicable. Awards are subject to a continuing employment condition (three years minimum) and to performance conditions. Consequently, the valuation at the date of grant is not necessarily indicative of the value of stock options and performance shares at the end of the vesting period, especially if the performance conditions are not met;
•since Olivier Brandicourt (our previous Chief Executive Officer) received the same number of stock options and performance shares each year from 2016 to 2019, fluctuations in the Sanofi share price had a significant impact on the pay ratio during this period;
•2018 and 2019 figures have been restated for comparative purposes, to (i) exclude Sanofi’s equity-accounted share of Regeneron’s net profits (see note D.2. to our consolidated financial statements, included at Item 18. of this annual report) and (ii) include the effects of IFRS 16;
•regular benchmarking reviews are conducted to ensure that the level of compensation awarded to our employees and CEO is competitive and consistent with pharmaceutical industry levels.
Comparison of compensation of Sanofi executive officers with employee compensation* (parent company and all direct and indirect subsidiaries located in France), and year-on-year change in compensation of corporate officers and employees with reference to the company’s performance
Chief Executive Officer(a) | 2019 vs 2018 | 2020 vs 2019 | 2021 vs 2020 | 2022 vs 2021 | 2023 vs 2022 | ||||||||||||
Change in compensation (%) | 13.7 | % | 9.2 | % | -1.0 | % | 20.5 | % | -1.5 | % | |||||||
Ratio versus average employee compensation | 106.59 | 110.64 | 111.44 | 124.55 | 124.49 | ||||||||||||
Year-on-year change in ratio (%) | 13.6 | % | 3.8 | % | 0.7 | % | 11.8 | % | -0.1 | % | |||||||
Ratio to median employee compensation | 135.36 | 142.78 | 142.11 | 159.17 | 159.97 | ||||||||||||
Year-on-year change in ratio (%) | 12.5 | % | 5.5 | % | -0.5 | % | 12.0 | % | 0.5 | % | |||||||
Chairman of the Board(b) | 2019 vs 2018 | 2020 vs 2019 | 2021 vs 2020 | 2022 vs 2021 | 2023 vs 2022 | ||||||||||||
Change in compensation (%) | — | % | 14.1 | % | — | % | — | % | 5.7 | % | |||||||
Ratio versus average employee compensation | 9.21 | 9.98 | 10.15 | 9.41 | 10.09 | ||||||||||||
Year-on-year change in ratio (%) | -0.1 | % | 8.4 | % | 1.7 | % | -7.3 | % | 7.2 | % | |||||||
Ratio versus median employee compensation | 11.69 | 12.87 | 12.94 | 12.03 | 12.97 | ||||||||||||
Year-on-year change in ratio (%) | -1.1 | % | 10.1 | % | 0.5 | % | -7.1 | % | 7.8 | % | |||||||
| Employees | 2019 vs 2018 | 2020 vs 2019 | 2021 vs 2020 | 2022 vs 2021 | 2023 vs 2022 | ||||||||||||
Change in compensation (%) | 0.1 | % | 5.2 | % | -1.7 | % | 7.8 | % | -1.4 | % | |||||||
| Company Performance | |||||||||||||||||
Financial criterion | BNI | BNI | BNI | BNI | BNI | ||||||||||||
Year-on-year change (%) | 10.0 | % | 4.2 | % | 11.8 | % | 25.9 | % | -1.8 | % | |||||||
* Table based on the model table recommended in the AFEP guidance on compensation multiples (February 2021).
(a) 2019: Olivier Brandicourt left office on August 31. Paul Hudson was appointed as CEO on September 1, 2019.
2020: The 2020 CEO compensation includes Paul Hudson’s 2020 fixed compensation (€1.3 million), his 2019 variable compensation as paid in 2020 and annualized (€1.95 million), and 75,000 performance shares awarded in 2020.
(b) Frédéric Oudéa with effect from May 25, 2023, the date on which Serge Weinberg’s term of office expired.
Based on full-time equivalent permanent employees of all Sanofi legal entities worldwide with at least two years of uninterrupted employment, the ratios for 2023 were as follows:
•CEO:
–ratio versus average compensation: 125.6, and
–ratio versus median compensation: 182.9;
•Chairman of the Board of Directors:
–ratio versus average compensation: 10.2, and
–ratio versus median compensation: 14.8.
These ratios were calculated on the basis of annualized basic compensation, variable compensation in respect of the previous year, and performance shares awarded during 2023, applying 2023 average exchange rates.
SANOFI FORM 20-F 2023 | 133 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
C. Board Practices
Application of the AFEP-MEDEF Code
The corporate governance code applied by Sanofi is the December 2022 version of the AFEP-MEDEF Code (the “AFEP-MEDEF Code,” which is available at https://hcge.fr/le-code-afep-medef/.
Our Board Charter requires at least one-half of our directors to be independent; contains a section on the ethical rules applicable to our directors; sets out the remit and operating procedures of the Board; defines the roles and powers of our Chairman and our Chief Executive Officer; and describes the composition, remit and operating procedures of the Board committees, in accordance with the recommendations of the AFEP-MEDEF Code. Collectively, our Articles of Association and our Board Charter establish the framework within which Sanofi implements its principles of corporate governance
Our Board practices comply with the AFEP-MEDEF Code recommendations, with certain exceptions, and with the report of the Autorité de marchés financiers on Audit Committees, issued on July 22, 2010.
Board diversity matrix
The table below provides certain information regarding the diversity of our board of directors as of the date of this annual report.
| Board Diversity Matrix | ||||||||||||||||||||||||||
| Country of Principal Executive Offices: | France | |||||||||||||||||||||||||
| Foreign Private Issuer | Yes | |||||||||||||||||||||||||
| Disclosure Prohibited under Home Country Law | Yes | |||||||||||||||||||||||||
As of February 23, 2024 | As of July 21, 2023 | |||||||||||||||||||||||||
| Total Number of Directors | 16 | 16 | ||||||||||||||||||||||||
| Gender Identity | Female | Male | Non-Binary | Did Not Disclose Gender | Female | Male | Non-Binary | Did Not Disclose Gender | ||||||||||||||||||
| Directors | 6 | 10 | 0 | 0 | 6 | 10 | 0 | 0 | ||||||||||||||||||
| Demographic Background | ||||||||||||||||||||||||||
| Underrepresented Individual in Home Country Jurisdiction | ||||||||||||||||||||||||||
| LGBTQ+ | ||||||||||||||||||||||||||
| Did Not Disclose Demographic Background | ||||||||||||||||||||||||||
Under Nasdaq Rule 5606, Nasdaq-listed companies are required to annually disclose, to the extent permitted by applicable law, information on each director’s voluntary self-identified characteristics. This information must be provided in either the issuer’s annual report or on the Company’s website and all companies must disclose the current year and immediately prior year diversity statistics. Sanofi has collected and disclosed (on an anonymous basis) the gender identity of its directors but does not collect or disclose the demographic (race or ethnicity and LGBTQ+ status) background of its directors, because Sanofi does not believe the collection and disclosure is permitted by French law
Activities of the Board of Directors in 2023
During 2023, the Board of Directors met 11 times (including strategy seminars), with an overall attendance rate among Board members of 97%. Individual attendance rates of serving directors varied between 64% and 100%.
The following persons attended meetings of the Board of Directors:
•the directors;
•the Secretary to the Board;
•frequently: members of the Executive Committee; and
•occasionally: the statutory auditors, managers of our global support functions, and other company employees.
The agenda for each meeting of the Board is prepared by the Secretary after consultation with the Chairman, taking account of the agendas for the meetings of the specialist Committees and the suggestions of the directors.
Approximately one week prior to each meeting of the Board of Directors, the directors each receive a file containing the agenda, the minutes of the previous meeting, and documentation relating to the agenda.
The minutes of each meeting are expressly approved at the next meeting of the Board of Directors.
In compliance with our Board Charter, certain issues are examined in advance by the various Committees according to their areas of competence, to enable them to make a recommendation; those issues are then submitted for a decision by the Board of Directors.
134 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Since 2016, acting on a recommendation from the Appointments, Governance and CSR Committee, each year the Board has held at least two executive sessions, i.e. meetings held without the Chief Executive Officer present. If the Chairman of the Board so decides, such sessions may also be held without the directors representing employees (or any other Sanofi employee) being present. The primary purpose of such sessions is to evaluate the way the Board and its Committees operate, discuss the performance of the Chief Executive Officer, and to debate succession planning. Two executive sessions lasting an hour and a half were held in 2023, in February and July.
In 2023, the main activities of the Board of Directors related to the following issues:
FINANCIAL STATEMENTS AND FINANCIAL MANAGEMENT | |||||
l | Review of the individual company and consolidated financial statements for the 2022 financial year and for the first half of 2023, review of the consolidated financial statements for the first three quarters of 2023, and review of draft press releases and presentations to analysts relating to the publication of those financial statements. | ||||
l | Review of forward-looking management documents. | ||||
l | Projected 2023 accounting close, presentation of 2024 budget and 2024-2026 financial forecasts. | ||||
l | Proposed dividend for the 2022 financial year. | ||||
l | Renewal of share repurchase program. | ||||
l | Formally recording the share capital, and amending the Articles of Association accordingly. | ||||
l | Delegation to the Chief Executive Officer of the power to issue bonds. | ||||
OPERATIONS, STRATEGY AND RISK MANAGEMENT | |||||
l | Play to Win strategy: delivery on the strategy, proposed separation of Consumer Healthcare business. | ||||
l | Review of minutes of Strategy Committee and Scientific Committee meetings. | ||||
l | Update on risks, and review of risk management activity report and 2023 risk profile analysis. | ||||
l | Review of acquisition projects. | ||||
« | Update on ZANTAC. | ||||
« | Update on France. | ||||
APPOINTMENTS AND GOVERNANCE | |||||
l | Composition of the Board and its committees: •appointment of Frédéric Oudéa as Chairman of the Board of Directors; •composition of Board committees. | ||||
l | Review of director independence. | ||||
l | Review of management report, corporate governance report, and statutory auditors' reports. | ||||
l | Adoption of draft resolutions, the Board report on the resolutions, and special reports on awards of stock options and performance shares. | ||||
l | Annual evaluation of the work of the Board and its Committees. | ||||
l | Review of previously-approved related-party agreements. | ||||
l | Update on the Action 2023 employee share ownership plan. | ||||
l | 2024 training plan for Board members. | ||||
COMPENSATION | |||||
l | Determination of the compensation of corporate officers: •review of fixed compensation and determination of variable compensation objectives for the Chief Executive Officer for 2023; •determination of the compensation of the Chairman of the Board of Directors for 2023. | ||||
l | Allocation of directors' compensation for 2022, and principles for the 2023 allocation. | ||||
l | Review of fixed and variable Executive Committee compensation for 2022 and 2023. | ||||
l | Adoption of performance share plans for 2023, sign-off on attainment of performance conditions for prior equity-based compensation plans. | ||||
« | Adoption of a clawback policy. | ||||
CORPORATE SOCIAL RESPONSIBILITY | |||||
l | Monitoring of progress on the CSR strategy. | ||||
l | Monitoring of objectives for gender balance in executive bodies, and more generally of Sanofi's diversity policy. | ||||
l | Monitoring of Sanofi's equal pay and equal opportunity policy. | ||||
« | Implementation of the European Corporate Sustainability Reporting Directive (CSRD). | ||||
l Annual items | « Non-recurring items | ||||||||||
SANOFI FORM 20-F 2023 | 135 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
In addition, two strategy seminars were held, in April and October 2023, in which all members of the Executive Committee took part. The seminar gave directors an opportunity to address issues including:
•monitoring delivery of the Play to Win strategy;
•pipeline review, especially in oncology;
•strategy and progress to date of the Sanofi Ventures investment fund;
•modernization of Manufacturing & Supply;
•changes in the regulatory environment;
•acquisition opportunities;
•R&D strategy;
•strategy for the General Medicines segment;
•proposed separation of Consumer Healthcare business;
•financial roadmap, including the new cost savings program.
Remit and Operation of Board Committees
Our Board of Directors is assisted in its deliberations and decisions by five specialist Committees (for a description of the remit of each Committee, refer to our Board Charter, provided as Exhibit 1.2 to this annual report). Chairs and members of these Committees are chosen by the Board from among its members, based on their experience.
The Committees are responsible for the preparation of certain items on the agenda of the Board of Directors. Decisions of the Committees are adopted by a simple majority with the Chair of the Committee having a casting vote. Minutes are prepared, and approved by the Committee members.
The Chair of each Committee reports to the Board on the work of that Committee, so that the Board is fully informed whenever it takes a decision.
Audit Committee
Composition of the Committee in 2023
Audit Committee | ||||||||
Composition as of January 1, 2023 | Composition as of December 31, 2023 | |||||||
Chair | Fabienne Lecorvaisier (independent director) | Fabienne Lecorvaisier (independent director) | ||||||
Members | Diane Souza (independent director) Christophe Babule(a) Carole Ferrand (independent director) | Diane Souza (independent director) Christophe Babule(a) Carole Ferrand (independent director) | ||||||
| Proportion of independent directors: 75% (3/4) | Proportion of independent directors: 75% (3/4) | |||||||
(a) This table only refers to independence as defined under the AFEP-MEDEF Code. However, Christophe Babule is independent for the purposes of the NASDAQ Listing Rules and Rule 10A-3 under the Exchange Act.
All members of the Audit Committee have financial or accounting expertise as a consequence of their training and professional experience, and all are deemed to be financial experts as defined by the Sarbanes-Oxley Act and by Article L. 823-19 of the French Commercial Code. See “Item 16A. Audit Committee Financial Expert”.
Remit of the Committee
The remit of the Committee is described in our Board Charter, provided as Exhibit 1.2 to this annual report.
Pursuant to Order no. 2023-1142 of December 6, 2023 transposing the European Corporate Sustainability Reporting Directive (CSRD) into French law, our Audit Committee has been given a remit to review the process for the preparation and certification of sustainability disclosures. Our Board Charter was amended by the Board of Directors on December 13, 2023 to reflect this change. In fulfilling this role, the Audit Committee works in conjunction with the Appointments, Governance and CSR Committee.
Operation of the Committee
In addition to the statutory auditors, the principal financial officers, the Senior Vice President Group Internal Audit and other members of the senior management team attend meetings of the Audit Committee.
The Committee members had an exemplary attendance record, with an overall attendance rate of 100%.
The statutory auditors attend all meetings of the Audit Committee; they presented their opinions on the annual and half-year financial statements at the Committee meetings of February 20 and July 26, 2023, respectively. The Committee meets regularly with the statutory auditors without management present.
The Chair of the Committee also meets regularly with certain members of management, in particular the heads of Internal Audit, Risk Management and Ethics/Compliance.
For information about Audit Committee oversight of internal control and risks relating to the processing of accounting and financial information, refer to “Item 15. Controls and Procedures.”
136 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Work of the Committee in 2023
The Audit Committee met six times in 2023, including meetings held in advance of the Board meeting tasked with finalizing the financial statements.
The work of the Committee in 2023 is summarized below:
FINANCIAL POSITION | ||||||||
l | Preliminary review of the individual company and consolidated financial statements for the 2022 financial year, review of the individual company and consolidated financial statements for the first half of 2023, review of the consolidated financial statements for the first three quarters of 2023, and review of draft press releases. | |||||||
l | Financial position of Sanofi, indebtedness and liquidity, off balance sheet commitments. | |||||||
« | Use of non-IFRS financial measures. | |||||||
INTERNAL AUDIT, INTERNAL CONTROL AND RISK MANAGEMENT | ||||||||
l | Review of the work of the Internal Control function and evaluation of that work for 2022 as certified by the statutory auditors pursuant to Section 404 of the Sarbanes-Oxley Act, and examination of the 2022 annual report. | |||||||
l | Principal risks (risk management and risk profiles) including CSR risks; Risk Committee report for 2023; tracking of whistleblowing and material compliance investigations; review of emerging risks, including geopolitical and macroeconomic risks; review of tax risks and deferred tax assets; review of material litigation. | |||||||
l | Conclusions of Sanofi senior management on internal control procedures and review of the 2022 Management Report, in particular the description of risk factors in the Universal Registration Document. | |||||||
l | Internal audit report and audit program for 2023. | |||||||
l | Reporting on guarantees and endorsements. | |||||||
l | IT obsolescence. | |||||||
« | Protection of information and prevention of internal threats. | |||||||
« | Ethics and data protection. | |||||||
« | Talent Management Review. | |||||||
STRATEGY AND COMPENSATION | ||||||||
l | Presentation of 2024 budget. | |||||||
l | Review of attainment of performance conditions for 2020 equity-based compensation plans. | |||||||
« | Proposed separation of Consumer Healthcare business - Tax aspects of legal reorganization of entities. | |||||||
COMPLIANCE, BUSINESS ETHICS AND CSR | ||||||||
l | Review of European Green Taxonomy indicators included in the Universal Registration Document. | |||||||
« | Implementation of the European Corporate Sustainability Reporting Directive (CSRD) and relating update of the work organisation with the Appointments, Governance and CSR Committee. | |||||||
RELATIONS WITH STATUTORY AUDITORS | ||||||||
l | Audit engagements and fees. | |||||||
l | Review and budget for non-audit services (audit-related services, tax, and other). | |||||||
« | Update on audit mandates: •recommendation to reappoint PwC at the 2023 AGM; •update on transitional arrangements relating to the end of the EY mandate in 2024, and appointment of a new statutory auditor. | |||||||
l Annual items | « Non-recurring items | ||||||||||
The Committee did not use external consultants in 2023.
Attendance rates in 2023
Committee members had an exemplary attendance rate of 100%.
SANOFI FORM 20-F 2023 | 137 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Appointments, Governance and CSR Committee
Composition of the Committee in 2023
Appointments, Governance and CSR Committee | ||||||||
Composition as of January 1, 2023 | Composition as of December 31, 2023 | |||||||
Chair | Gilles Schnepp (independent director) | Gilles Schnepp (independent director) | ||||||
Members | Serge Weinberg Patrick Kron (independent director) Lise Kingo (independent director) Barbara Lavernos | Frédéric Oudéa (independent director)(a) Patrick Kron (independent director) Lise Kingo (independent director) Barbara Lavernos | ||||||
Proportion of independent directors: 60% (3/5) | Proportion of independent directors: 80% (4/5) | |||||||
(a) Frédéric Oudéa, an independent director, was appointed as a member of the Appointments, Governance and CSR Committee by a Board decision of May 25, 2023.
The Chief Executive Officer is involved in the work of the Committee.
Remit of the Committee
The remit of the Committee is described in our Board Charter, provided as Exhibit 1.2 to this annual report.
The remit to review the process for the preparation and certification of sustainability disclosures has been given to our Audit Committee (see above). The Appointments, Governance and CSR Committee plays a role in this work through joint meetings.
Work of the Committee in 2023
The work of the Appointments, Governance and CSR Committee during 2023 covered the following issues:
APPOINTMENTS | |||||
l | Succession planning for the Chairman, Chief Executive Officer and Executive Committee. | ||||
l | Changes to the composition of the Board and its committees. | ||||
l | Review of expiring terms of office, and appointment of new Board members. | ||||
GOVERNANCE | |||||
l | Update on annual evaluation of the Board and its committees. | ||||
l | Review of director independence. | ||||
l | Review of management report and corporate governance report in the 2022 Universal Registration Document. | ||||
l | Governance roadshows with key Sanofi investors, and analysis of the policies of proxy advisors. | ||||
CSR | |||||
l | Updates on the four pillars of the CSR strategy: •Affordable Access to Healthcare; •Innovating for Unmet Medical Needs; •Planet Care; •Inclusive, Diverse Workplace and Communities. | ||||
l | New regulatory requirements and roadmap to implementation. | ||||
l | Review of the CSR chapter in the 2022 Universal Registration Document. | ||||
« | Update on Foundation S. | ||||
« | Update on the Corporate Sustainability Reporting Directive (CSRD). | ||||
l Annual items | « Non-recurring items | ||||||||||
The Committee did not use external consultants in 2023.
Attendance rates in 2023
The Committee met five times in 2023, with an attendance rate of 100%.
138 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Compensation Committee
Composition of the Committee in 2023
Compensation Committee | ||||||||
Composition as of January 1, 2023 | Composition as of December 31, 2023 | |||||||
Chair | Patrick Kron (independent director) | Patrick Kron (independent director) | ||||||
Members | Wolfgang Laux Diane Souza (independent director) Rachel Duan (independent director) | Wolfgang Laux Diane Souza (independent director) Rachel Duan (independent director) | ||||||
Proportion of independent directors: 75% (3/4) | Proportion of independent directors: 75% (3/4) | |||||||
Work of the Committee in 2023
The work of the Compensation Committee during 2023 covered the following issues:
COMPENSATION OF CORPORATE OFFICERS | |||||
l | Components of the compensation of corporate officers (Chief Executive Officer and Chairman of the Board). | ||||
l | Review of performance conditions applicable to the compensation of the Chief Executive Officer, in particular CSR criteria. | ||||
l | Allocation of directors’ compensation for 2022, and review of the compensation policy applicable to directors. | ||||
l | Review of the disclosures about compensation contained in the corporate governance section of the 2022 Universal Registration Document and the annual report, and of equal pay ratios. | ||||
l | Review of the draft "say on pay" resolutions to be submitted to the Annual General Meeting of May 25, 2023. | ||||
l | Governance roadshows with key Sanofi investors, and analysis of the policies of proxy advisors. | ||||
« | Review of the structure of the Chief Executive Officer's compensation, and objectives for 2024. | ||||
EQUITY-BASED COMPENSATION | |||||
l | Implementation of equity-based compensation plans awarded in prior years (sign-off on attainment of performance conditions for 2020 plans). | ||||
« | Introduction of CSR criteria into the new equity-based compensation plan for the Chief Executive Officer. | ||||
EMPLOYEE SHARE OWNERSHIP | |||||
l | Status report and analysis of 2023 employee share ownership plan. | ||||
l | Consideration of next employee share ownership plan, and implementation of Action 2024 plan. | ||||
EXECUTIVE COMMITTEE COMPENSATION | |||||
l | Monitoring of fixed and variable compensation of Executive Committee members in 2022 and 2023. | ||||
« | Adoption of clawback policy. | ||||
« | Terms for incoming and outgoing Executive Committee members. | ||||
l Annual items | « Non-recurring items | ||||||||||
When the Committee discusses the compensation policy for members of senior management who are not corporate officers, i.e. the members of the Executive Committee, the Committee invites the Chief Executive Officer to attend.
The Committee did not use external consultants in 2023.
Attendance rates in 2023
The Committee met three times in 2023, with an attendance rate of 100%.
SANOFI FORM 20-F 2023 | 139 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Strategy Committee
Composition of the Committee in 2023
Strategy Committee | ||||||||
Composition as of January 1, 2023 | Composition as of December 31, 2023 | |||||||
Chair | Serge Weinberg | Frédéric Oudéa (independent director) (a) | ||||||
Members | Paul Hudson Patrick Kron (independent director) Gilles Schnepp (independent director) | Paul Hudson Patrick Kron (independent director) Barbara Lavernos (b) Gilles Schnepp (independent director) | ||||||
Proportion of independent directors: 50% (2/4) | Proportion of independent directors: 60% (3/5) | |||||||
(a) Frédéric Oudéa was appointed as Chair of the Strategy Committee by a Board decision of May 25, 2023.
(b) Barbara Lavernos was appointed as a member of the Strategy Committee by a Board decision of February 22, 2023.
Work of the Committee in 2023
During 2023, the Committee's work included the following key issues:
l | Divestment and acquisition projects, and business development priorities. | ||||
l | Play to Win strategy and financial roadmap for 2024-2026. | ||||
l | Opportunities for alliances. | ||||
« | Proposed separation of Consumer Healthcare business. | ||||
« | Update on strategy in France. | ||||
« | Update on regulatory environment and competition. | ||||
l Annual items | « Non-recurring items | ||||||||||
The Committee did not use external consultants in 2023.
Attendance rates in 2023
The Committee met eight times in 2023, with an attendance rate of 100%.
Scientific Committee
Composition of the Committee in 2023
Scientific Committee | ||||||||
Composition as of January 1, 2023 | Composition as of December 31, 2023 | |||||||
Chair | Thomas Südhof (independent director) | Thomas Südhof (independent director) | ||||||
Members | Emile Voest (independent director) Serge Weinberg Antoine Yver (independent director) | Frédéric Oudéa (independent director) Emile Voest (independent director) Antoine Yver (independent director) | ||||||
Proportion of independent directors: 75% (3/4) | Proportion of independent directors: 100% (4/4) | |||||||
Work of the Committee in 2023
During 2023, the Committee’s work included the following key issues:
l | Review of product portfolio. | ||||
l | Review of acquisition and alliance projects. | ||||
l | Update on Immunology & Inflammation. | ||||
l | Update on Oncology. | ||||
l | Update on Chemistry, Manufacture & Controls (CMC). | ||||
l | Update on Neurology. | ||||
« | Update on Rare Diseases. | ||||
« | Use of artificial intelligence in R&D. | ||||
l Annual items | « Non-recurring items | ||||||||||
The Committee did not use external consultants in 2023.
Attendance rates in 2023
The Committee met seven times in 2023, with an attendance rate of 100%.
140 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
D. Employees
Number of Employees(a)
In 2023, Sanofi employed 86,088 people worldwide, 3,736 fewer than in 2022. The tables below give a breakdown of employees by geographical area and function as of December 31, 2023, 2022 and 2021.
Employees by Geographical Area(a)
| As of December 31, | ||||||||||||||||||||
| 2023 | % | 2022 | % | 2021 | % | |||||||||||||||
| Europe | 42,115 | 48.9 | % | 42,151 | 46.9 | % | 45,351 | 48.5 | % | |||||||||||
| United States | 13,418 | 15.6 | % | 13,761 | 15.3 | % | 12,886 | 13.8 | % | |||||||||||
| Rest of the World | 30,555 | 35.5 | % | 33,912 | 37.8 | % | 35,311 | 37.7 | % | |||||||||||
| Total | 86,088 | 100.0 | % | 89,824 | 100.0 | % | 93,548 | 100.0 | % | |||||||||||
Employees by Function(a)
| As of December 31, | |||||||||||
| 2023 | 2022 | 2021 | |||||||||
| Sales Force | 16,835 | 19,210 | 20,477 | ||||||||
| Research and Development | 11,660 | 11,943 | 11,756 | ||||||||
| Production | 34,313 | 35,953 | 39,268 | ||||||||
| Marketing and Support Functions | 23,280 | 22,718 | 22,047 | ||||||||
| Total | 86,088 | 89,824 | 93,548 | ||||||||
(a) Employees on garden leave and ExCom management level excluded from the data.
Industrial Relations
In all countries where we operate, we seek to strike a balance between our economic interests and those of our employees, which we regard as inseparable.
Our belief in a balanced workplace for our employees is based on the basic principles of our Social Charter, which outlines the rights and duties of all Sanofi employees. The Social Charter addresses our key ambitions vis-à-vis our workforce: equal opportunity for all people without discrimination, the right to health and safety, respect for privacy, the right to information and professional training, social protection for employees and their families, freedom of association and the right to collective bargaining, and respect for the principles contained in the Global Compact on labor relations and ILO conventions governing the physical and emotional well-being and safety of children.
Our labor relations are based on respect and dialogue. In this spirit, management and employee representatives meet regularly to exchange views, negotiate, sign agreements and ensure that agreements are being implemented.
Employee dialogue takes place in different ways from country to country, as dictated by specific local circumstances. Depending on the circumstances, employee dialogue relating to information, consultation and negotiation processes may take place at national, regional or company level. It may be organized on an interprofessional or sectorial basis, or both. Employee dialogue may be informal or implemented through a specific formal body, or a combination of both methods. Whatever the situation, Sanofi encourages employees to voice their opinions, help create a stimulating work environment and take part in decisions aiming to improve the way we work. These efforts reflect one of the principles of the Social Charter, whereby improving working conditions and the necessary adaptation to our business environment go hand-in-hand.
Profit-sharing Schemes, Employee Savings Schemes and Employee Share Ownership
Profit-sharing schemes
All employees of our French companies belong to voluntary and statutory profit-sharing schemes.
Voluntary schemes
Voluntary schemes (intéressement des salariés) are collective schemes that are optional for the employer and contingent upon performance. The aim is to give employees an interest in the growth of the business and improvements in its performance.
The amount distributed by our French companies during 2023 in respect of the voluntary scheme for the year ended December 31, 2023 represented 1.05% of total payroll.
SANOFI FORM 20-F 2023 | 141 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
In April 2020, we entered into a new fixed-term statutory profit-sharing agreement for the 2020, 2021 and 2022 financial years, which applies to all employees of our French companies. Under the agreement, Sanofi pays collective variable compensation determined on the basis of the more favorable of (i) growth in consolidated net sales (at constant exchange rates and on a constant structure basis) or (ii) BOI margin. For each of those criteria, a matrix determines what percentage of total payroll is to be allocated to the scheme. An additional sum may be distributed, based on a CSR-related performance condition reflecting progress in environmental matters (reduction in greenhouse gas emissions) and capped at 0.5% of total payroll.
This overall allocation is reduced by the amount required by law to be transferred to a special profit-sharing reserve. The balance is then distributed between the employees unless the transfer to the reserve equals or exceeds the maximum amount determined under the specified criteria, in which case no profit share is paid to the employees.
In June 2023, we entered into a new fixed-term statutory profit-sharing agreement for the 2023, 2024 and 2025 financial years, which applies to all employees of our French companies.
Statutory scheme
The statutory scheme (participation des salariés aux résultats de l’entreprise) is a French legal obligation for companies with more than 50 employees that made a profit in the previous financial year.
The amount distributed by our French companies during 2023 in respect of the statutory scheme for the year ended December 31, 2022 represented 9.95% of total payroll.
Distribution formula
In order to favor lower-paid employees, the voluntary and statutory profit-sharing agreements entered into since 2005 split the benefit between those entitled as follows:
•60% prorated on the basis of time spent in the Company’s employment in the year; and
•40% prorated on the basis of gross annual salary received during the year, subject to a lower limit equal to the social security ceiling and an upper limit of three times the social security ceiling.
Employee savings schemes and collective retirement savings plan
The employee savings arrangements operated by Sanofi are based on a collective savings scheme (Plan d’Épargne Groupe) and a collective retirement savings scheme (Plan d’Épargne pour la Retraite Collectif). Those schemes reinvest the sums derived from the statutory and voluntary profit-sharing schemes, plus voluntary contributions from employees.
In 2023, 90% of the employees who benefited from the profit-sharing schemes opted to invest in the collective savings scheme, and nearly 80% opted to invest in the collective retirement savings scheme.
Sanofi supplements the amount invested by employees in these schemes by making a top-up contribution.
In 2023, €143.5 million and €157.6 million were invested in the collective savings scheme and the collective retirement savings scheme respectively through the voluntary and statutory schemes for 2022, and through top-up contributions.
Employee share ownership
As of December 31, 2023, shares held under the collective savings scheme or in registered form by employees of Sanofi, employees of related companies and former employees amounted to 2.58% of our share capital.
For more information about our most recent employee share ownership plan, refer to “Item 10. Additional Information — Changes in Share Capital — Increases in Share Capital”.
E. Share Ownership
Senior Management
Members of the Executive Committee hold shares of our Company amounting in the aggregate to less than 1% of our share capital.
Existing Option Plans as of December 31, 2023
In 2019, the Board of Directors reviewed Sanofi’s compensation policy and decided that stock options would no longer be awarded from 2020 onwards. That decision was taken to standardize the terms of equity-based compensation awards within Sanofi, and in response to feedback from some shareholders and proxy advisors who had concerns about stock options given their dilutive effect and potential unintended consequences.
Share Purchase Option Plans
As of December 31, 2023 there were no stock purchase option plans outstanding.
142 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Share Subscription Option Plans
| Source | Date of shareholder authorization | Date of grant | Total number of options granted | to corporate officers(a) | to the 10 employees awarded the most options(b) | Start date of exercise period | Expiry date | Exercise price (€) | Number of shares subscribed as of 12/31/2023 | Number of options canceled as of 12/31/2023(c) | Number of options outstanding | ||||||||||||||||||||||||
| Sanofi | May 6, 2011 | March 5, 2013 | 548,725 | — | 261,000 | March 6, 2017 | March 5, 2023 | 72.19 | 439,660 | 109,065 | — | ||||||||||||||||||||||||
| Sanofi | May 6, 2011 | March 5, 2013 | 240,000 | 240,000 | — | March 6, 2017 | March 5, 2023 | 72.19 | 175,920 | 64,080 | — | ||||||||||||||||||||||||
| Sanofi | May 3, 2013 | March 5, 2014 | 769,250 | — | 364,500 | March 6, 2018 | March 5, 2024 | 73.48 | 575,270 | 102,625 | 91,355 | ||||||||||||||||||||||||
| Sanofi | May 3, 2013 | March 5, 2014 | 240,000 | 240,000 | — | March 6, 2018 | March 5, 2024 | 73.48 | 193,440 | 46,560 | — | ||||||||||||||||||||||||
| Sanofi | May 3, 2013 | June 24, 2015 | 12,500 | — | 12,500 | June 25, 2019 | June 24, 2025 | 89.38 | 2,250 | 8,500 | 1,750 | ||||||||||||||||||||||||
| Sanofi | May 3, 2013 | June 24, 2015 | 202,500 | — | 202,500 | June 25, 2019 | June 24, 2025 | 89.38 | 45,000 | — | 157,500 | ||||||||||||||||||||||||
| Sanofi | May 3, 2013 | June 24, 2015 | 220,000 | 220,000 | — | June 25, 2019 | June 24, 2025 | 89.38 | — | 41,536 | 178,464 | ||||||||||||||||||||||||
| Sanofi | May 4, 2016 | May 4, 2016 | 17,750 | — | 17,750 | May 5, 2020 | May 4, 2026 | 75.9 | 4,500 | 9,750 | 3,500 | ||||||||||||||||||||||||
| Sanofi | May 4, 2016 | May 4, 2016 | 165,000 | — | 165,000 | May 5, 2020 | May 4, 2026 | 75.9 | 82,500 | — | 82,500 | ||||||||||||||||||||||||
| Sanofi | May 4, 2016 | May 4, 2016 | 220,000 | 220,000 | — | May 5, 2020 | May 4, 2026 | 75.9 | — | 41,250 | 178,750 | ||||||||||||||||||||||||
| Sanofi | May 10, 2017 | May 10, 2017 | 158,040 | — | 157,140 | May 11, 2021 | May 10, 2027 | 88.97 | 34,184 | 44,276 | 79,580 | ||||||||||||||||||||||||
| Sanofi | May 10, 2017 | May 10, 2017 | 220,000 | 220,000 | — | May 11, 2021 | May 10, 2027 | 88.97 | — | 42,570 | 177,430 | ||||||||||||||||||||||||
| Sanofi | May 2, 2018 | May 2, 2018 | 220,000 | 220,000 | — | May 3, 2022 | May 3, 2028 | 65.84 | — | 51,216 | 168,784 | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | April 30, 2019 | 220,000 | 220,000 | — | May 1, 2023 | April 30, 2029 | 76.71 | — | 6,600 | 213,400 | ||||||||||||||||||||||||
(a) Comprises the Chief Executive Officer, and any Deputy Chief Executive Officers or members of the Management Board in office at the date of grant.
(b) In office at the date of grant.
(c) Includes 293,812 options cancelled due to partial non-fulfilment of performance conditions.
In 2023, 10,813 stock options were exercised by individuals who were Executive Committee members as of December 31, 2023. All of the plans involved post-date the creation of the Executive Committee (Sanofi plan of March 5, 2013, exercise price €72.19; and Sanofi plan of March 5, 2014, exercise price €73.48).
As of December 31, 2023, a total of 1,333,013 stock subscription options remained outstanding. As of the same date, 1,333,013 options were immediately exercisable.
Existing Performance Share Plans as of December 31, 2023
The Board of Directors awards shares to certain employees in order to give them a direct stake in our future and performances via trends in the share price, as a partial substitute for the granting of stock options.
Shares are awarded to employees by the Board of Directors on the basis of a list submitted to the Compensation Committee. The Board of Directors sets terms of the awards, including continuing employment conditions and performance conditions (measured over three financial years).
The employee plans have a three-year vesting period, with no lock-up period.
•At its meeting of May 25, 2023, the Board of Directors awarded a share performance plan, cascaded down into three sub-plans:
–a plan under which 466 beneficiaries classified as “Senior Executives” were awarded a total of 1,209,790 shares;
–a plan under which 7,874 beneficiaries not classified as “Senior Executives” were awarded a total of 2,425,047 shares;
–a plan under which 82,500 performance shares were awarded to the Chief Executive Officer.
Of the 8,341 beneficiaries, 49% were women.
•At its meeting of December 13, 2023, the Board of Directors awarded a share performance plan, cascaded down into two sub-plans:
–a plan under which seven beneficiaries classified as “Senior Executives” were awarded a total of 58,347 performance shares;
–a plan under which one beneficiary not classified as a “Senior Executive” was awarded a total of 944 performance shares.
Of those eight beneficiaries, 25% were women.
SANOFI FORM 20-F 2023 | 143 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
The entirety of those awards is contingent upon criteria based on business net income (BNI), free cash flow (FCF) and Corporate Social Responsibility (CSR); in the case of employees classified as “Senior Executives”, an additional criterion based on total shareholder return (TSR) is added, accounting for 20% of the total. Vesting is subject to a non-compete clause.
The number of shares awarded to the Chief Executive Officer in 2023 represents 0.4% of the total limit approved by our shareholders at the Annual General Meeting of April 30, 2021 (1.5% of our share capital) and 2.18% of the total amount awarded to all beneficiaries in 2023.
The 2023 awards represent a dilution of approximately 0.19% of our undiluted share capital as of December 31, 2023.
Not all of our employees were awarded performance shares, but a new voluntary profit-sharing agreement was signed in April 2020 which gives all of our employees an interest in Sanofi’s performance (for more details refer to “— Profit-Sharing Schemes, Employee Savings Schemes and Employee Share Ownership”, above).
Performance Share Plans
| Source | Date of shareholder authorization | Date of award | Total number of shares awarded | to corporate officers(a) | to the 10 employees awarded the most shares(b) | Start date of vesting period(c) | Vesting date | End of lock-up period | Number of shares vested as of 12/31/2023 | Number of rights canceled as of 12/31/2023(d) | Number of shares not yet vested | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | April 28, 2020 | 75,000 | 75,000 | — | April 28, 2020 | May 01, 2023 | May 02, 2023 | 65,205 | 9,795 | 0 | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | April 28, 2020 | 328,113 | — | 120,951 | April 28, 2020 | May 01, 2023 | May 02, 2023 | 245,789 | 82,324 | — | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | April 28, 2020 | 400,495 | — | 151,761 | April 28, 2020 | May 01, 2023 | May 02, 2023 | 212,753 | 187,742 | — | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | April 28, 2020 | 753,720 | — | 19,027 | April 28, 2020 | May 01, 2023 | May 02, 2023 | 717,595 | 36,125 | — | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | April 28, 2020 | 1,783,173 | — | 26,542 | April 28, 2020 | May 01, 2023 | May 02, 2023 | 1357661 | 425512 | — | ||||||||||||||||||||||||
| Sanofi | April 30, 2019 | October 28, 2020 | 73,027 | — | 73,027 | October 28, 2020 | October 29, 2023 | October 30, 2023 | 57,745 | 15,282 | — | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | April 30, 2021 | 1,614,023 | — | 19,407 | April 30, 2021 | May 01, 2024 | May 01, 2024 | 2,489 | 314,691 | 1,296,843 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | April 30, 2021 | 701,824 | — | 163,877 | April 30, 2021 | May 01, 2024 | May 01, 2024 | — | 202,582 | 499,242 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | April 30, 2021 | 595,878 | — | 10,918 | April 30, 2021 | May 01, 2024 | May 01, 2024 | 469 | 36,618 | 558,791 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | April 30, 2021 | 497,695 | — | 150,339 | April 30, 2021 | May 01, 2024 | May 01, 2024 | — | 43,973 | 453,722 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | April 30, 2021 | 75,000 | 75,000 | — | April 30, 2021 | May 01, 2024 | May 01, 2024 | — | — | 75,000 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | October 27, 2021 | 13,521 | — | 13,521 | October 27, 2021 | October 28, 2024 | October 28, 2024 | — | 3,706 | 9,815 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | May 03, 2022 | 2,000,627 | — | 25,882 | May 03, 2022 | May 03, 2025 | May 04, 2025 | 1,145 | 183,551 | 1,815,931 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | May 03, 2022 | 1,146,431 | — | 192,542 | May 03, 2022 | May 03, 2025 | May 04, 2025 | — | 170,559 | 975,872 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | May 03, 2022 | 82,500 | 82,500 | — | May 03, 2022 | May 03, 2025 | May 04, 2025 | — | — | 82,500 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | December 14, 2022 | 90,580 | — | 77,111 | December 14, 2022 | December 14, 2025 | December 15, 2025 | — | — | 90,580 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | December 14, 2022 | 10,335 | — | 10,335 | December 14, 2022 | December 14, 2025 | December 15, 2025 | — | — | 10,335 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | May 25, 2023 | 2,425,047 | — | 25,417 | May 25, 2023 | May 25, 2026 | May 25, 2026 | 620 | 71,695 | 2,352,732 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | May 25, 2023 | 1,209,790 | — | 192,417 | May 25, 2023 | May 25, 2026 | May 25, 2026 | — | 50,735 | 1,159,055 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | May 25, 2023 | 82,500 | 82,500 | — | May 25, 2023 | May 25, 2026 | May 25, 2026 | — | — | 82,500 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | December 13, 2023 | 58,347 | — | 58,347 | December 13, 2023 | December 14, 2026 | December 14, 2026 | — | — | 58,347 | ||||||||||||||||||||||||
| Sanofi | April 30, 2021 | December 13, 2023 | 944 | — | 944 | December 13, 2023 | December 14, 2026 | December 14, 2026 | — | — | 944 | ||||||||||||||||||||||||
(a) Comprises the Chief Executive Officer, and any Deputy Chief Executive Officers or members of the Management Board in office at the date of grant.
(b) In office at the date of grant.
(c) Subject to the conditions set.
(d) 87,416 rights were cancelled due to partial non-fulfilment of performance conditions.
As of December 31, 2023, 9,522,209 shares had not yet vested pending fulfilment of performance conditions.
144 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 6. Directors, senior management and employees | ||
Shares Owned by Members of the Board of Directors
As of December 31, 2023, members of our Board of Directors held in the aggregate 24,270 shares, or under 1% of the share capital and of the voting rights, excluding the beneficial ownership of 118,227,307 shares held by L’Oréal as of such date which may be attributed to Barbara Lavernos or Christophe Babule (who disclaim beneficial ownership of such shares).
Transactions in Shares by Members of the Board of Directors and Equivalent Persons in 2023
As far as Sanofi is aware, transactions in our securities carried out during 2023 by (i) Board members, (ii) executives with the power to make management decisions affecting our future development and corporate strategy and (iii) persons with close personal ties to such individuals (as per Article L. 621-18-2 of the French Monetary and Financial Code), were as follows:
•March 13, 2023: Barbara Lavernos, director, acquired 500 shares at a price of €88.50 per share;
•May 2, 2023: Frédéric Oudéa, non-voting member (at the date of the transaction), acquired 500 shares at a price of €97.67 per share;
•June 9, 2023: Wolfgang Laux subscribed 9.74 units in the FCPE at a price of €95.12 per unit;
•July 20, 2023: Wolfgang Laux subscribed 250 units in the FCPE at a price of €79.58 per unit.
F. Disclosure of action to recover erroneously awarded compensation
N/A
SANOFI FORM 20-F 2023 | 145 | ||||
| PART I | ||
ITEM 7. Major shareholders and related party transactions | ||
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
The table below shows the ownership of our shares as of January 31, 2024, indicating the beneficial owners of our shares. To the best of our knowledge and on the basis of the notifications received as disclosed below, except for L’Oréal and BlackRock, Inc., no other shareholder currently holds more than 5% of our share capital or voting rights.
Total number of issued shares | Number of actual voting rights (excluding treasury shares)(d) | Theoretical number of voting rights (including treasury shares)(e) | ||||||||||||||||||
| Number | % | Number | % | Number | % | |||||||||||||||
| L’Oréal | 118,227,307 | 9.35 | 236,454,614 | 16.81 | 236,454,614 | 16.61 | ||||||||||||||
BlackRock(a) | 86,592,005 | 6.85 | 86,592,005 | 6.15 | 86,592,005 | 6.08 | ||||||||||||||
Employees(b) | 32,435,981 | 2.56 | 63,771,330 | 4.53 | 63,771,330 | 4.48 | ||||||||||||||
| Public | 1,011,302,360 | 79.96 | 1,020,079,451 | 72.51 | 1,020,079,451 | 71.69 | ||||||||||||||
Treasury shares(c) | 16,245,648 | 1.28 | — | — | 16,245,648 | 1.14 | ||||||||||||||
| Total | 1,264,803,301 | 100 | 1,406,897,400 | 100 | 1,423,143,048 | 100 | ||||||||||||||
(a) Based on BlackRock’s declaration dated December 15, 2023.
(b) Shares held by the employees according to article L.225-102 of the French Commercial Code.
(c) Number of shares repurchased as of January 31, 2024 under the share repurchase program in force.
(d) Based on the total number of voting rights as of January 31, 2024.
(e) Based on the total number of voting rights as of January 31, 2024 as published in accordance with Article 223-11 and seq. of the General Regulations of the Autorité des marchés financiers (i.e. including treasury shares, the voting rights of which are suspended).
Our Articles of Association provide for double voting rights for shares held in registered form for at least two years. All of our shareholders may benefit from double voting rights if these conditions are met, and no shareholder benefits from specific voting rights. For more information relating to our shares, see “Item 10. Additional Information — B. Memorandum and Articles of Association.”
Neither L’Oréal nor BlackRock holds different voting rights from those of our other shareholders.
To the best of our knowledge, no other shareholder currently holds, directly or indirectly and acting alone or in concert, more than 5% of our share capital or voting rights. Furthermore, we believe that we are not directly or indirectly owned or controlled by another corporation or government, or by any other natural or legal persons. To our knowledge, there are no arrangements that may result in a change of control.
During the year ended December 31, 2023 we received one share ownership declaration informing us that a legal threshold had been passed, as required under Article L. 233-7 of the French Commercial Code. Dodge & Cox, acting on behalf of its clients and funds under its management, declared that on June 23, 2023 it had passed below the 5% threshold in terms of share capital, and holds, on behalf of its clients and funds, 4.99% of the share capital and 4.43% of the voting rights.
In addition to the statutory requirement to inform the Company and the Autorité des marchés financiers (AMF, the French Financial Markets Regulator) that they hold a number of shares (or of securities equivalent to shares or of voting rights pursuant to Article L. 233-9 of the French Commercial Code) representing more than one-twentieth (5%), one-tenth (10%), three-twentieths (15%), one-fifth (20%), one-quarter (25%), three-tenths (30%), one-third (1/3), one-half (50%), two-thirds (2/3), nine-tenths (90%) or nineteen-twentieths (95%) of the share capital or theoretical voting rights within four trading days after crossing any such ownership threshold (Article L. 233-7 of the French Commercial Code), any natural or legal person who directly or indirectly comes to hold a percentage of the share capital, voting rights or securities giving future access to the Company’s capital that is equal to or greater than 1% or any multiple of that percentage, is obliged to inform the Company thereof by registered mail, return receipt requested, indicating the number of securities held, within five trading days following the date on which each of the thresholds was crossed.
If such declaration is not made, the shares in excess of the fraction that should have been declared will be stripped of voting rights at shareholders’ meetings, if on the occasion of such meeting, the failure to declare has been formally noted and one or more shareholders collectively holding at least 5% of the Company’s share capital or voting rights so request at that meeting.
Any natural or legal person is also required to inform the Company, in the forms and within the time limits stipulated above for passing above a specified threshold, if their direct or indirect holding passes below any of the aforementioned thresholds.
Since January 1, 2024 Sanofi has only received share ownership declarations of statutory threshold.
146 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 7. Major shareholders and related party transactions | ||
As of December 31, 2023, individual shareholders (including employees of Sanofi and its subsidiaries, as well as retired employees holding shares via the Sanofi Group Employee Savings Plan) held approximately 7.9% of our share capital. Institutional shareholders (excluding L’Oréal) held approximately 77.7% of our share capital. Such shareholders are primarily from the United-States (34.7%), French (10.8%) and British (13.1%). German institutions held 4.2% of our share capital, Scandinavian institutions held 3.5%, Swiss institutions held 2.2%, Benelux institutions held 1.8%, and institutions from other European countries held 1.9%. Other international institutional investors (excluding those from Europe, North America and Asia) held approximately 1% of our share capital. In France, our home country, we have 10,492 identified shareholders of record. In the United States, our host country, we have 55 identified shareholders of record and 18,346 identified ADS holders of record.
(Source: analysis performed by NASDAQ as of December 31, 2023, and internal information.)
Shareholders’ Agreement
We are unaware of any shareholders’ agreement currently in force.
B. Related Party Transactions
See Note D.33. to our consolidated financial statements included at Item 18. of this annual report.
C. Interests of Experts and Counsel
N/A
SANOFI FORM 20-F 2023 | 147 | ||||
| PART I | ||
ITEM 8. Financial information | ||
Item 8. Financial Information
A. Consolidated Financial Statements and Other Financial Information
Our consolidated financial statements as of and for the years ended December 31, 2023, 2022 and 2021 are included in this annual report at “Item 18. Financial Statements.”
Dividends on Ordinary Shares
We paid annual dividends for the years ended December 31, 2019, 2020, 2021 and 2022 and our shareholders will be asked to approve the payment of an annual dividend of €3.76 per share for the 2023 fiscal year at our next annual shareholders’ meeting. If approved, this dividend will be paid on May 15, 2024.
We expect that we will continue to pay regular dividends based on our financial condition and results of operations. The proposed 2023 dividend equates to a distribution of 46.3% of our business net income. For information on the non-IFRS financial measure “business earnings per share” see “Item 5. Operating and Financial Review and Prospects — Business Net Income.”
The following table sets forth information with respect to the dividends paid by our Company in respect of the 2019, 2020, 2021 and 2022 fiscal years and the dividend that will be proposed for approval by our shareholders in respect of the 2023 fiscal year at our April 30, 2024 shareholders’ meeting.
| 2023 | (a) | 2022 | 2021 | (b) | 2020 | 2019 | |||||||||||||||||
Dividend per Share (€) | 3.76 | 3.56 | 3.33 | 3.2 | 3.15 | ||||||||||||||||||
(a) Proposal, subject to shareholder approval.
(b) And a dividend in kind of EUROAPI shares, at a ratio of one EUROAPI share per 23 Sanofi shares.
The declaration, amount and payment of any future dividends will be determined by majority vote of the holders of our shares at an ordinary general meeting, following the recommendation of our Board of Directors. Any declaration will depend on our results of operations, financial condition, cash requirements, future prospects and other factors deemed relevant by our shareholders. Accordingly, we cannot assure you that we will pay dividends in the future on a continuous and regular basis. Under French law, we are required to pay dividends approved by an ordinary general meeting of shareholders within nine months following the meeting at which they are approved.
Disclosure pursuant to Section 13(r) of the United States Exchange Act of 1934
Sanofi engages in limited business activities with Iran related to human health products – namely, sales of bulk and branded pharmaceuticals and vaccines. These activities, which are disclosed pursuant to Section 13(r) of the United States Exchange Act of 1934, as amended, are not financially material to Sanofi and contributed well under 1% of Sanofi’s consolidated net sales in 2023.
Sanofi’s US affiliates and non-US affiliates owned or controlled by Sanofi’s US affiliates either do not engage in Iran-related activities or act under licenses issued by the US Department of the Treasury’s Office of Foreign Assets Control (OFAC).
Sanofi and certain non-US Sanofi affiliates engage in limited business activities that neither are expressly authorized by OFAC nor require such authorization.
In 2016, Sanofi and the Iran Food and Drug Administration (IFDA), an entity affiliated with the Iranian Ministry of Health and Medical Education, signed a Memorandum of Cooperation (MOC) regarding: (i) potential future projects to reinforce current partnerships with reputable Iranian manufacturers (in particular, to enhance industrial quality standards); (ii) collaborating with the Ministry of Health and Medical Education on programs for the prevention and control of certain chronic and non-communicable diseases (in particular, diabetes); and (iii) potential future collaboration on epidemiological studies. In 2023, activities conducted under the MOC did not generate any revenue or net profits.
Certain non-US Sanofi affiliates engage in limited business with Iranian counterparties associated with the Iranian Ministry of Health, such as public hospitals or distributors. In 2023, those business activities generated approximately €27.1 million in gross revenue and contributed no more than €3.9 million in net profits.
Finally, a representative office in Tehran incurs incidental expenses from state-owned utilities.
Sanofi believes that it and its affiliates’ activities are compliant with applicable law, and in light of the nature of the activities concerned, Sanofi and its affiliates intend to continue their ongoing activities in Iran.
148 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 8. Financial information | ||
Information on Legal or Arbitration Proceedings
This Item 8. incorporates by reference the disclosures found in Note D.22. to the consolidated financial statements at Item 18. of this annual report; material updates thereto as of the date of this annual report are found below under the heading “— B. Significant Changes — Updates to Note D.22.”.
Sanofi and its subsidiaries are involved in litigation, arbitration and other legal proceedings. These proceedings typically are related to product liability claims, intellectual property rights (particularly claims against generic companies seeking to limit the patent protection of Sanofi products), competition law and trade practices, commercial claims, employment and wrongful discharge claims, tax assessment claims, waste disposal and pollution claims, and claims under warranties or indemnification arrangements relating to business divestitures. As a result, we may become subject to substantial liabilities that may not be covered by insurance and could affect our business and reputation. While we do not currently believe that any of these legal proceedings will have a material adverse effect on our financial position, litigation is inherently unpredictable. As a consequence, we may in the future incur judgments or enter into settlements of claims that could have a material adverse effect on results of operations, cash flows and/or our reputation.
Government Investigations and Related Litigation
From time to time, subsidiaries of Sanofi are subject to governmental investigations and information requests from regulatory authorities inquiring as to the practices of Sanofi with respect to the sales, marketing, and promotion of its products.
From 2017 through 2023, several government agencies issued Civil Investigative Demands (CIDs) or other discovery requests calling for the production of documents and information relating to Sanofi’s trade and pricing practices for its insulin products and/or LANTUS-related litigation. Sanofi US is cooperating with each of the previously reported investigations (including those conducted by the State Attorney General’s offices in Washington, California, Colorado and Vermont), and reached a resolution with the New York State Attorney General in April 2023. In addition, Sanofi US is cooperating with investigations initiated by the US Federal Trade Commission in June 2022; by the Texas State Attorney General’s office in August 2022; and by the Ohio Attorney General’s office in October 2022.
In September 2019, Sanofi US received a CID from the US Department of Justice concerning DUPIXENT, KEVZARA, PRALUENT and ZALTRAP. In June 2021, the government declined to intervene in the underlying complaint which was filed in November 2018. The government investigation into this matter is now closed. Relators, however, filed their First Amended Complaint in October 2021, which the Court dismissed with prejudice in August 2023.
In February 2020, Genzyme Corporation received a CID from the US Department of Justice. The CID requests documents and information relating to Genzyme Corporation’s payments made to vendors or developers of electronic health record technology. Genzyme Corporation is cooperating with this investigation.
In October 2022, Sanofi US received a CID from the Ohio State Attorney General’s office, seeking documents and information about the pricing, sale, and distribution of pharmaceuticals and pharmacy benefit manager services in the State of Ohio. Sanofi US is cooperating with this investigation.
In November 2023, Sanofi US received a CID from the US Department of Justice regarding an investigation into Sanofi’s pricing submissions for ADMELOG. Sanofi US is cooperating with this investigation.
Insulin-Related Litigation
In December 2016 and January 2017, two putative class actions were filed against Sanofi US and Sanofi GmbH in the US Federal Court in Massachusetts on behalf of direct purchasers of LANTUS alleging certain antitrust violations. Sanofi GmbH was later dismissed from the actions. In January 2018, the Court dismissed Plaintiffs’ consolidated amended complaint against Sanofi US. Plaintiffs appealed that order to the Court of Appeals for the First Circuit, which issued its decision on February 13, 2020 reversing and remanding to the district court. In January 2021, Sanofi-Aventis Puerto Rico, Inc. (Sanofi PR) was added as a defendant. In October 2022, plaintiffs informed Sanofi US and Sanofi PR that they would proceed via joinder rather than move for class certification. Consistent with the Court's joinder deadline, new plaintiffs moved to intervene on January 3, 2023.
There are a number of insulin-related litigation matters pending in the US federal and state courts. These include cases brought on behalf of putative classes of consumers, wholesale purchasers of insulin, and state and local governments. The cases, which have been filed against Sanofi US along with other insulin manufacturers and, in some cases, pharmacy benefit managers, challenge those entities’ insulin pricing practices (including Sanofi’s pricing practices for LANTUS, APIDRA, TOUJEO and/or SOLIQUA). The suits allege some combination of: violations of the Racketeer Influenced and Corrupt Organizations Act (“RICO Act”); violations of various state unfair/deceptive trade practices statutes; unjust enrichment; common-law fraud; and civil conspiracy. The status of these matters varies from initial motions to dismiss the complaints to active discovery. In August 2023, the vast majority of the insulin-related litigation was consolidated in an MDL (Multi-District Litigation - MDL) in federal court in New Jersey. On January 24, 2024, the District Court for the District of New Jersey issued a decision denying plaintiffs’ motion for class certification. Plaintiffs have appealed that decision.
Mylan vs Sanofi antitrust complaint
In May 2023, Mylan Pharmaceuticals Inc., Mylan Specialty LP and Mylan Inc. (Mylan) filed suit against Sanofi-Aventis US LLC, Sanofi SA, Aventis Pharma SA and Sanofi-Aventis Puerto Rico (Sanofi) in the Western District of Pennsylvania for alleged antitrust violations related to Mylan’s insulin product SEMGLEE. Sanofi has moved to dismiss the complaint.
SANOFI FORM 20-F 2023 | 149 | ||||
| PART I | ||
ITEM 8. Financial information | ||
B. Significant Changes
Updates to Note D.22.
TAXOTERE - Mississippi Attorney General Litigation in the United States
In February 2024, Sanofi reached a settlement to resolve this case, which now fully concludes the matter.
Other Changes
On January 23, 2024 Sanofi announced the entry into a merger agreement with Inhibrx, Inc. (Inhibrx), a publicly traded, clinical-stage biopharmaceutical company focused on developing a broad pipeline of novel biologic therapeutic candidates in oncology and orphan diseases (the Merger Agreement), pursuant to which Sanofi has agreed to acquire Inhibrx following the spin-off of Inhibrx's non-INBRX-101 assets and liabilities into a new publicly traded company ("New Inhibrx"). Under the terms of the Merger Agreement, Sanofi has agreed to: (i) provide Inhibrx's stockholders with consideration of $30 per share of Inhibrx common stock at the closing of the merger (approximately $1.7 billion), and to additionally issue one non-transferable contingent value right per share of Inhibrx common stock, which will entitle its holder to receive a deferred cash payment of $5, conditioned upon the achievement of a regulatory milestone (approximately $0.3 billion, if the regulatory milestone is achieved); (ii) pay off Inhibrx’s outstanding third-party debt (approximately $0.2 billion); and (iii) provide a capital contribution to New Inhibrx (up to $0.2 billion). At the closing of the merger, Sanofi will acquire 100% of the equity interests in Inhibrx, which will become a 100% wholly owned subsidiary of Sanofi. In addition, Inhibrx will retain a minority stake (approximately 8% equity interest) in New Inhibrx. INBRX-101 is a human recombinant protein that holds the promise of allowing Alpha-1 Antitrypsin Deficiency (AATD) patients to achieve normalization of serum AAT levels with less frequent (monthly vs. weekly) dosing. AATD is an inherited rare disease characterized by low levels of AAT protein, predominantly affecting the lung with progressive deterioration of the tissue. INBRX-101 may help to reduce inflammation and prevent further deterioration of lung function in affected individuals. INBRX-101 acquisition is expected to support Sanofi’s portfolio growth strategy and complements the Company’s 30-year heritage in rare diseases and track record in immunology and inflammation. The transaction is subject to various closing conditions, including the receipt of regulatory approvals and completion of the spin-off of New Inhibrx. Assuming satisfaction of those closing conditions, Sanofi currently anticipates that the transaction will close in the second quarter of 2024.
On February 1, 2024, Sanofi announced that François-Xavier Roger will be appointed Chief Financial Officer and a member of Sanofi’s Executive Committee effective April 1, 2024. He will be based in Paris and will succeed Jean-Baptiste Chasseloup de Chatillon who will step down from his role to become Head of Apprentis d’Auteuil.
150 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 9. The offer and listing | ||
Item 9. The Offer and Listing
A. Offer and Listing Details
We have one class of shares. Each American Depositary Share, or ADS, represents one-half of one share. The ADSs are evidenced by American Depositary Receipts, or ADRs, which are issued by JPMorgan Chase Bank, NA.
Our shares trade on Compartment A of the regulated market of Euronext Paris under the symbol "SAN," and our ADSs trade on the Nasdaq Global Select Market, or Nasdaq,under the symbol "SNY."
B. Plan of Distribution
N/A
C. Markets
Shares and ADSs
Our shares are listed on Euronext Paris under the symbol “SAN” and our ADSs are listed on the Nasdaq under the symbol “SNY.”
As of the date of this annual report, our shares are included in a large number of indexes, including the “CAC 40 Index,” the principal French index published by Euronext Paris. This index contains 40 stocks selected among the top 100 companies based on free-float capitalization and the most active stocks listed on the Euronext Paris market. The CAC 40 Index indicates trends in the French stock market as a whole and is one of the most widely followed stock price indices in France.
Our shares are included in European indexes, such as the EURO STOXX 50, STOXX Europe 600 index, FTSE Eurofirst 300, MSCI Europe, MSCI Pan Euro, Euronext 100, and STOXX Europe 600 Health Care. They are also included in American and international indexes, such as the NASDAQ Composite, NASDAQ Health Care, S&P Global 100, MSCI World, and MSCI World Pharmaceuticals, Biotechnology and Life Sciences.
Our shares are also part of the main extra-financial rating indices, taking into account environmental, social, and governance criteria (FTSE4Good, STOXX Global ESG Leaders, and EURO STOXX 50 Low Carbon).
Trading by Sanofi in our own Shares
Under French law, a company may not issue shares to itself, but it may purchase its own shares in the limited cases described at “Item 10. Additional Information — B. Memorandum and Articles of Association — Trading in Our Own Shares.”
D. Selling Shareholders
N/A
E. Dilution
N/A
F. Expenses of the Issue
N/A
SANOFI FORM 20-F 2023 | 151 | ||||
| PART I | ||
ITEM 10. Additional information | ||
Item 10. Additional Information
A. Share Capital
N/A
B. Memorandum and Articles of Association
General
Our Company is a société anonyme, a form of limited liability company, organized under the laws of France. The LEI number of the Company is 549300E9PC51EN656011.
In this section, we summarize material information concerning our share capital, together with material provisions of applicable French law and our Articles of Association (statuts), an English translation of which has been filed as an exhibit to this annual report. For a description of certain provisions of our Articles of Association relating to our Board of Directors and statutory auditors, see “Item 6. Directors, Senior Management and Employees." You may obtain copies of our Articles of Association in French from the greffe (Clerk) of the Registre du Commerce et des Sociétés de Paris (Registry of Commerce and Companies of Paris, France, registration number: 395 030 844). Please refer to that full document for additional details.
Our Articles of Association specify that our corporate affairs are governed by:
•applicable laws and regulations (in particular, Title II of the French Commercial Code); and
•the Articles of Association themselves.
Article 3 of our Articles of Association specifies that the Company’s corporate purpose, in France and abroad, is:
•acquiring interests and holdings, in any form whatsoever, in any company or enterprise, in existence or to be created, connected directly or indirectly with the health and fine chemistry sectors, human and animal therapeutics, nutrition and bio-industry:
–in the following areas:
◦purchase and sale of all raw materials and products necessary for these activities,
◦research, study and development of new products, techniques and processes,
◦manufacture and sale of all chemical, biological, dietary and hygienic products,
◦obtaining or acquiring all intellectual property rights related to results obtained and, in particular, filing all patents, trademarks and models, processes or inventions,
◦operating directly or indirectly, purchasing, and transferring – for free or for consideration – pledging or securing all intellectual property rights, particularly all patents, trademarks and models, processes or inventions,
◦obtaining, operating, holding and granting all licenses,
◦within the framework of a group-wide policy and subject to compliance with the relevant legislation, participating in treasury management transactions, whether as lead company or otherwise, in the form of centralized currency risk management or intra-group netting, or any other form permitted under the relevant laws and regulations,
–and, more generally:
◦all commercial, industrial, real or personal property, financial or other transactions, connected directly or indirectly, totally or partially, with the activities described above and with all similar or related activities and even with any other purposes likely to encourage or develop the Company’s activities.
Directors
Transactions in which directors are materially interested
Under French law, any agreement entered into (directly or through an intermediary) between our Company and any one of the members of the Board of Directors that is not entered into (i) in the ordinary course of our business and (ii) under normal conditions, is subject to the prior authorization of the disinterested members of the Board of Directors. The same provision applies to agreements between our Company and another company if one of the members of the Board of Directors is the owner, general partner, manager, director, general manager or member of the executive or supervisory board of the other company, as well as to agreements in which one of the members of the Board of Directors has an indirect interest.
The Board of Directors must also approve any undertaking taken by our Company for the benefit of our Chairman, Chief Executive Officer (directeur général) or his delegates (directeurs généraux délégués) pursuant to which such persons will or may be granted compensation, benefits or any other advantages as a result of the termination of or a change in their offices or following such termination or change, in accordance with Article L. 22-10-8 III of the French Commercial Code. Each such undertaking must be included in our compensation policy for corporate officers, which is submitted for approval by our shareholders at the Annual General Meeting in accordance with Article L. 22-10-8 II of the French Commercial Code. No such compensation or undertaking may be determined, awarded or paid unless in accordance with such compensation policy.
152 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 10. Additional information | ||
See “Item 6. Directors, Senior Management and Employees — B. Compensation” for a description of the process for establishing and authorizing such compensation policy.
Directors’ compensation
The aggregate amount of compensation of the Board of Directors is determined at the Shareholders’ Ordinary General Meeting. The Board of Directors then divides this aggregate amount among its members by a simple majority vote. In addition, the Board of Directors may grant exceptional compensation (rémunérations exceptionnelles) to individual directors on a case-by-case basis for special assignments following the procedures described above at “— Transactions in which directors are materially interested”. The Board of Directors may also authorize the reimbursement of travel and accommodation expenses, as well as other expenses incurred by Directors in the corporate interest. See also “Item 6. Directors, Senior Management and Employees.” Furthermore, under our Articles of Association, the Board of Directors may compensate any observers (censeurs) to the Board of Directors, which would reduce by the same amount the total annual compensation available for allocation to the Board of Directors.
Board of Directors’ authority to take out loans or borrow money on behalf of the Company
All loans or borrowings on behalf of the Company may be decided by the Board of Directors within the limits, if any, imposed by the Shareholders’ Extraordinary General Meeting. There are currently no limits imposed on the amounts of loans or borrowings that the Board of Directors may approve.
Directors’ age limits
For a description of the provisions of our Articles of Association relating to age limits applicable to our Directors, see “Item 6. Directors, Senior Management and Employees – A. Directors and Senior Management."
Directors’ share ownership requirements
Pursuant to our Articles of Association, each director appointed by a Shareholders’ Ordinary General Meeting must own at least 500 shares throughout their term of office. In addition, pursuant to the Board Charter, our Directors must within no more than two years from their appointment hold at least 1,000 Sanofi shares in their own name, which must be retained until they cease to hold office.
Shareholders’ meetings
General
In accordance with the provisions of the French Commercial Code, there are three types of shareholders’ meetings: ordinary, extraordinary and special.
Ordinary general meetings of shareholders are required for matters such as:
•electing, replacing and removing Directors;
•appointing independent auditors;
•approving the annual financial statements;
•declaring dividends or authorizing dividends to be paid in shares, provided the Articles of Association contain a provision to that effect; and
•approving share repurchase programs.
Extraordinary general meetings of shareholders are required for approval of matters such as amendments to our Articles of Association, including any amendment required in connection with extraordinary corporate actions. Extraordinary corporate actions include:
•changing our Company’s name or corporate purpose;
•increasing or decreasing our share capital;
•creating a new class of equity securities;
•authorizing the issuance of:
–shares giving access to our share capital or giving the right to receive debt instruments, or
–other securities giving access to our share capital;
•establishing any other rights to equity securities;
•selling or transferring substantially all of our assets; and
•the voluntary liquidation of our Company.
Special meetings of shareholders of a certain category of shares or shares with certain specific rights (such as shares with double voting rights) are required for any modification of the rights derived from that category of shares. The resolutions of the shareholders’ general meeting affecting these rights are effective only after approval by the relevant special meeting.
SANOFI FORM 20-F 2023 | 153 | ||||
| PART I | ||
ITEM 10. Additional information | ||
Annual ordinary meetings
The French Commercial Code requires the Board of Directors to convene an annual ordinary general shareholders’ meeting to approve the annual financial statements. This meeting must be held within six months of the end of each fiscal year.
The Board of Directors may also convene an ordinary or extraordinary general shareholders’ meeting upon proper notice at any time during the year. If the Board of Directors fails to convene a shareholders’ meeting, our independent auditors may call the meeting. In case of bankruptcy, the liquidator or court-appointed agent may also call a shareholders’ meeting in some instances. In addition, any of the following may request the court to appoint an agent for the purpose of calling a shareholders’ meeting:
•one or several shareholders holding at least 5% of our share capital;
•duly qualified associations of shareholders who have held their shares in registered form for at least two years and who together hold at least 1% of our voting rights;
•the works council in cases of urgency; or
•any interested party in cases of urgency.
Under our Articles of Association, the Board of Directors may take decisions by written consultation under the conditions permitted by law and as specified in the Board Charter (an English language version of which is reproduced in full as Exhibit 1.2 to this annual report), including the possibility to convene an ordinary or extraordinary general meeting.
Notice of shareholders’ meetings
All prior notice periods provided for below are minimum periods required by French law and cannot be shortened, except in case of a public tender offer for our shares.
We must announce general meetings at least thirty-five days in advance by means of a preliminary notice (avis de réunion), which is published in the Bulletin des Annonces Légales Obligatoires, or BALO. The preliminary notice must first be sent to the French Financial markets authority (Autorité des marchés financiers, the “AMF”), with an indication of the date on which it will be published in the BALO. It must be published on our website at least twenty-one days prior to the general meeting. The preliminary notice must contain, among other things, the agenda, a draft of the resolutions to be submitted to the shareholders for consideration at the general meeting and a detailed description of the voting procedures (proxy voting, electronic voting or voting by mail), the procedures permitting shareholders to submit additional resolutions or items to the agenda and to ask written questions to the Board of Directors. The AMF also recommends that, prior to or simultaneously with the publication of the preliminary notice, we publish a summary of the notice indicating the date, time and place of the meeting in a newspaper of national circulation in France and on our website.
At least fifteen days prior to the date set for a first convening, and at least ten days prior to any second convening, we must send a final notice (avis de convocation) containing the final agenda, the date, time and place of the meeting and other information related to the meeting. Such final notice must be sent by mail to all registered shareholders who have held shares in registered form for more than one month prior to the date of the final notice and by registered mail, if shareholders have asked for it and paid the corresponding charges. The final notice must also be published in a newspaper authorized to publish legal announcements in the local administrative department (département) in which our Company is registered as well as in the BALO, with prior notice having been given to the AMF for informational purposes. Even if there are no proposals for new resolutions or items to be submitted to the shareholders at the meeting, we must publish a final notice in a newspaper authorized to publish legal announcements in the local administrative department (département) in which our Company is registered as well as in the BALO.
Other issues
In general, shareholders can only take action at shareholders’ meetings on matters listed on the agenda. As an exception to this rule, shareholders may take action with respect to the appointment and dismissal of directors even if this action has not been included on the agenda.
Additional resolutions to be submitted for approval by the shareholders at the shareholders’ meeting may be proposed to the Board of Directors, for recommendation to the shareholders at any time from the publication of the preliminary notice in the BALO until twenty-five days prior to the general meeting and in any case no later than twenty days following the publication of the preliminary notice in the BALO by:
•one or several shareholders together holding a specified percentage of shares;
•a duly qualified association of shareholders who have held their shares in registered form for at least two years and who together hold at least 1% of our voting rights; or
•the works council.
Within the same period, the shareholders may also propose additional items (points) to be submitted and discussed during the shareholders’ meeting, without a shareholders’ vote. The shareholders must substantiate the reasons for their proposals of additional items.
The resolutions and the list of items added to the agenda of the shareholders’ meeting must be promptly published on our website.
The Board of Directors must submit the resolutions to a vote of the shareholders after having made a recommendation thereon. The Board of Directors may also comment on the items that are submitted to the shareholders’ meeting.
154 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 10. Additional information | ||
Following the date on which documents must be made available to the shareholders (including documents to be submitted to the shareholders’ meeting and resolutions proposed by the Board of Directors, which must be published on our website at least twenty-one days prior to the general meeting), shareholders may submit written questions to the Board of Directors relating to the agenda for the meeting until the fourth business day prior to the general meeting. The Board of Directors must respond to these questions during the meeting or may refer to a Q&A section located on our website in which the question submitted by a shareholder has already been answered.
Attendance at shareholders’ meetings; proxies and votes by mail
In general, all shareholders may participate in general meetings either in person or by proxy. Shareholders may vote in person, by proxy or by mail.
The right of shareholders to participate in general meetings is subject to the recording (inscription en compte) of their shares on the second business day, 12:00 a.m. (Paris time), preceding the general meeting:
•for holders of registered shares: in the registered shareholder account held by the Company or on its behalf by an agent appointed by it; and
•for holders of bearer shares: in the bearer shareholder account held by the accredited financial intermediary with whom such holders have deposited their shares; such financial intermediaries shall deliver to holders of bearer shares a shareholding certificate (attestation de participation) enabling them to participate in the general meeting.
Attendance in person
Any shareholder may attend ordinary general meetings and extraordinary general meetings and exercise its voting rights subject to the conditions specified in the French Commercial Code, the French Civil Code and our Articles of Association.
An attendance sheet and written minutes are established for each shareholders’ meeting; failure to do so could lead to cancellation of the decisions at the shareholders’ meeting.
Proxies and votes by mail
Proxies are sent to any shareholder upon a request received between the publication of the final notice of meeting and six days before the general meeting and must be made available on our website at least twenty-one days before the general meeting. In order to be counted, such proxies must be received at our registered office, or at any other address indicated on the notice of the meeting or by any electronic mail indicated on the notice of the meeting, prior to the date of the meeting (in practice, we request that shareholders return proxies at least three business days prior to the meeting; electronic proxies must be returned before 3 p.m. Paris time, on the day prior to the general meeting). A shareholder may grant proxies to any natural person or legal entity. The agent may be required to disclose certain information to the shareholder or to the public.
A proxy is only valid for one meeting (or by way of exception for two meetings, one being ordinary and the other extraordinary, held on the same day or within a single 15-day period); it remains valid in the event such meeting is convened multiple times for the same agenda, and may be revoked by written statement of the shareholder granting the proxy.
Alternatively, the shareholder may send us a blank proxy without nominating any representative. In this case, the chairman of the meeting will vote the blank proxies in favor of all resolutions proposed or approved by the Board of Directors and against all others.
With respect to votes by mail, we must send shareholders a voting form upon request or must make available a voting form on our website at least twenty-one days before the general meeting. The completed form must be returned to us at least three days prior to the date of the shareholders’ meeting. For holders of registered shares, in addition to traditional voting by mail, instructions may also be given via the Internet.
Quorum
The French Commercial Code requires that shareholders holding in the aggregate at least 20% of the shares entitled to vote must be present in person, or vote by mail or by proxy, in order to fulfill the quorum requirement for:
•an ordinary general meeting; and
•an extraordinary general meeting where the only resolutions pertain to either (a) a proposed increase in our share capital through incorporation of reserves, profits or share premium, or (b) the potential issuance of free share warrants in the event of a public tender offer for our shares (Article L. 233-32 of the French Commercial Code).
For any other extraordinary general meeting the quorum requirement is at least 25% of the shares entitled to vote, held by shareholders present in person, voting by mail or by proxy.
For a special meeting of holders of a certain category of shares, the quorum requirement is one third of the shares entitled to vote in that category, held by shareholders present in person, voting by mail or by proxy.
If a quorum is not present at a meeting, the meeting is adjourned. However, only questions that were on the agenda of the adjourned meeting may be discussed and voted upon once the meeting resumes.
When an adjourned meeting is resumed, there is no quorum requirement for meetings cited in the first paragraph of this “Quorum” section. In the case of any other reconvened extraordinary general meeting or special meeting, the quorum requirement is 20% of the shares entitled to vote (or voting shares belonging to the relevant category for special meetings of holders of shares of such specific category), held by shareholders present in person or voting by mail or by proxy. If a quorum is not met, the reconvened meeting may be adjourned for a maximum of two months with the same quorum requirement. No deliberation or action by the shareholders may take place without a quorum.
SANOFI FORM 20-F 2023 | 155 | ||||
| PART I | ||
ITEM 10. Additional information | ||
C. Material Contracts
In the ordinary course of our business, we enter into agreements for licensing or collaboration in the development and commercialization of products. Certain of the agreements which have led to successful commercialization to date are summarized in “Item 5. Operating and financial review and prospects — A.1.7 Financial presentation of alliances.”
D. Exchange Controls
French exchange control regulations currently do not limit the amount of payments that we may remit to non-residents of France. Laws and regulations concerning foreign exchange controls do require, however, that all payments or transfers of funds made by a French resident to a non-resident be handled by an accredited intermediary.
E. Taxation
General
The following generally summarizes the material French and US federal income tax consequences to US holders (as defined below) of purchasing, owning and disposing of our ADSs and ordinary shares (collectively the “Securities”). This discussion is intended only as a descriptive summary and does not purport to be a complete analysis or listing of all potential tax effects of the purchase, ownership or disposition of our Securities. All of the following is subject to change. Such changes could apply retroactively and could affect the consequences described below.
This summary does not constitute a legal opinion or tax advice. Holders are urged to consult their own tax advisers regarding the tax consequences of the purchase, ownership and disposition of Securities in light of their particular circumstances, including the effect of any US federal, state, local or other national tax laws.
A set of tax rules is applicable to French assets that are held by or in foreign trusts. These rules provide inter alia for the inclusion of trust assets in the settlor’s net assets for purpose of applying the French real estate wealth tax, for the application of French gift and death duties to French assets held in trust, for a specific tax on capital on the French assets of foreign trusts not already subject to the French real estate wealth tax and for a number of French tax reporting and disclosure obligations. The following discussion does not address the French tax consequences applicable to Securities held in trusts. If Securities are held in trust, the grantor, trustee and beneficiary are urged to consult their own tax adviser regarding the specific tax consequences of acquiring, owning and disposing of Securities.
The description of the French and US federal income tax consequences set forth below is based on the laws (including, for US federal income tax purposes, the Internal Revenue Code of 1986, as amended (the “Code”), final, temporary and proposed US Treasury Regulations promulgated thereunder and administrative and judicial interpretations thereof) in force as of the date of this annual report, the Convention Between the Government of the United States of America and the Government of the French Republic for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and Capital of August 31, 1994 (the “Treaty”), which entered into force on December 30, 1995 (as amended by any subsequent protocols, including the protocol of January 13, 2009), and the tax regulations issued by the French tax authorities within the Bulletin Officiel des Finances Publiques-Impôts (the “Regulations”) in force as of the date of this report. US holders are advised to consult their own tax advisers regarding their eligibility for Treaty benefits, especially with regard to the “Limitations on Benefits” provision, in light of their own particular circumstances.
No advance ruling has been obtained with respect to the tax consequences of the acquisition, ownership or disposition of the Securities from either the French or US tax authorities. Thus, there can no assurances that either or both of such authorities will not take a position concerning said tax consequences different from that set out herein or that such a position would not be sustained by a court.
For the purposes of this discussion, a US holder is a beneficial owner of Securities that is (i) an individual who is a US citizen or resident for US federal income tax purposes, (ii) a US domestic corporation created or organized in or under the laws of the United States or any state thereof, including the District of Columbia, or (iii) certain estates or trusts that are subject to US tax jurisdiction. A non-US holder is a person other than a US holder.
If a partnership holds Securities, the tax treatment of a partner generally will depend upon the status of the partner and the activities of the partnership. If a US holder is an estate or trust or partner in a partnership that holds Securities, the holder is urged to consult its own tax adviser regarding the specific tax consequences of acquiring, owning and disposing of Securities.
This discussion is intended only as a general summary and does not purport to be a complete analysis or listing of all potential tax effects of the acquisition, ownership or disposition of the Securities to any particular investor, and does not discuss tax considerations that arise from rules of general application or that are generally assumed to be known by investors. The discussion applies only to investors that hold our Securities as capital assets that have the US dollar as their functional currency, that are entitled to Treaty benefits under the “Limitation on Benefits” provision contained in the Treaty, and whose ownership of the Securities is not effectively connected to a permanent establishment or a fixed base in France. Certain holders (including, but not limited to, US expatriates, partnerships or other entities classified as partnerships for US federal income tax purposes, banks, insurance companies, regulated investment companies, tax-exempt organizations, financial institutions, persons subject to the alternative minimum tax, persons who acquired the Securities pursuant to the exercise of employee stock options or otherwise as compensation, persons that own (directly, indirectly or by attribution) 5% or more of our voting stock or 5% or more of our outstanding share capital, dealers in securities or currencies, persons that elect to mark their securities to market for US federal income tax purposes, persons that acquire ADSs in “pre-release” transactions (i.e. prior to deposit of the relevant ordinary shares,
156 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 10. Additional information | ||
although our depositary has indicated that such transactions have been halted) and persons holding Securities as a position in a synthetic security, straddle or conversion transaction) may be subject to special rules not discussed below. Holders of Securities are advised to consult their own tax advisers with regard to the application of French tax law and US federal tax law to their particular situations, as well as any tax consequences arising under the laws of any state, local or other foreign jurisdiction.
French taxes
Estate and gift taxes and transfer taxes
In general, a transfer of Securities by gift or by reason of death of a US holder that would otherwise be subject to French gift or inheritance tax, respectively, will not be subject to such French tax by reason of the Convention between the Government of the United States of America and the Government of the French Republic for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Estates, Inheritances and Gifts, dated November 24, 1978, unless the donor or the transferor is domiciled in France at the time of making the gift or at the time of his or her death, or the Securities were used in, or held for use in, the conduct of a business through a permanent establishment or a fixed base in France.
Pursuant to Article 235 ter ZD of the French General Tax Code, purchases of Securities are subject to a 0.3% French tax on financial transactions (the “FTFF”) provided that Sanofi’s market capitalization exceeds €1 billion as of December 1 of the year preceding the taxation year. A list of companies whose market capitalization exceeds €1 billion as of December 1 of the year preceding the taxation year used to be published annually by the French Ministry of Economy. It is now published by the French tax authorities, and could be amended at any time. Pursuant to Regulations BOI-ANNX-000467-20/12/2023 issued on December 20, 2023, purchases of Sanofi’s Securities in 2024 should be subject to the FTFF as the market capitalization of Sanofi exceeded €1 billion as of December 1, 2023. In accordance with Article 726-II-d of the French General Tax Code, purchases which are subject to the FTFF should however not be subject to transfer taxes (droits d’enregistrement) in France.
Wealth tax
The French wealth tax (impôt de solidarité sur la fortune) has been replaced with a French real estate wealth tax (impôt sur la fortune immobilière) with effect from January 1, 2018. French real estate wealth tax applies only to individuals and does not generally apply to the Securities if the holder is a US resident, as defined pursuant to the provisions of the Treaty, provided that the individual does not own directly or indirectly a shareholding exceeding 10% of the financial rights and voting rights.
US taxes
Ownership of the securities
Deposits and withdrawals by a US holder of ordinary shares in exchange for ADSs, will not be taxable events for US federal income tax purposes. For US tax purposes, holders of ADSs will be treated as owners of the ordinary shares represented by such ADSs. Accordingly, the discussion that follows regarding the US federal income tax consequences of acquiring, owning and disposing of ordinary shares is equally applicable to ADSs.
Information reporting and backup withholding tax
Distributions made to holders and proceeds paid from the sale, exchange, redemption or disposal of Securities may be subject to information reporting to the Internal Revenue Service. Such payments may be subject to backup withholding taxes unless the holder (i) is a corporation or other exempt recipient or (ii) provides a taxpayer identification number and certifies that no loss of exemption from backup withholding has occurred. Holders that are not US persons generally are not subject to information reporting or backup withholding. However, such a holder may be required to provide a certification of its non-US status in connection with payments received within the United States or through a US-related financial intermediary to establish that it is an exempt recipient. Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a holder’s US federal income tax liability. A holder may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the Internal Revenue Service and furnishing any required information.
Foreign asset reporting
In addition, a US holder that is an individual or certain entities may be subject to reporting obligations with respect to ordinary shares and ADSs if the aggregate value of these and certain other “specified foreign financial assets” exceeds $50,000 on the last day of the tax year or more than $75,000 at any time during the tax year. If required, this disclosure is made by filing Form 8938 with the US Internal Revenue Service. Significant penalties can apply if holders are required to make this disclosure and fail to do so. In addition, a US holder should consider the possible obligation to file online a FinCEN Form 114 – Foreign Bank and Financial Accounts Report as a result of holding ordinary shares or ADSs. Holders are encouraged to consult their US tax advisors with respect to these and other reporting requirements that may apply to their acquisition of ordinary shares and ADSs.
State and local taxes
In addition to US federal income tax, US holders of Securities may be subject to US state and local taxes with respect to such Securities. Holders of Securities are advised to consult their own tax advisers with regard to the application of US state and local income tax law to their particular situation.
SANOFI FORM 20-F 2023 | 157 | ||||
| PART I | ||
ITEM 10. Additional information | ||
ADSs-Ordinary Shares
French taxes
Taxation of dividends
Under French law, dividends paid by a French corporation, such as Sanofi, to non-residents of France are generally subject to French withholding tax at a rate of (i) 25% for payments benefiting legal persons who are not French tax residents (and 15% for distributions made to not-for-profit organizations with a head office in a Member State of the European Economic Area which would be subject to the tax regime set forth under Article 206 paragraph 2 of the French General Tax Code if its head office were located in France and which meet the criteria set forth in the Regulations BOI-RPPM-RCM-30-30-10-70-24/12/2019, No. 130), and (ii) 12.8% for payments benefiting individuals who are not French tax residents. Dividends paid by a French corporation, such as Sanofi, towards non-cooperative States or territories, as defined in Article 238-0 A of the French General Tax Code, will generally be subject to French withholding tax at a rate of 75%, irrespective of the tax residence of the beneficiary of the dividends if the dividends are received in such States or territories; however, eligible US holders entitled to Treaty benefits under the “Limitation on Benefits” provision contained in the Treaty who are US residents, as defined pursuant to the provisions of the Treaty and who receive dividends in non-cooperative States or territories, will not be subject to this 75% withholding tax rate.
Under the Treaty, the rate of French withholding tax on dividends paid to an eligible US holder who is a US resident as defined pursuant to the provisions of the Treaty and whose ownership of the ordinary shares or ADSs is not effectively connected with a permanent establishment or fixed base that such US holder has in France, is reduced to 15%, or to 5% if such US holder is a corporation and owns directly or indirectly at least 10% of the share capital of the issuing company; such US holder may claim a refund from the French tax authorities of the amount withheld in excess of the Treaty rates of 15% or 5%, if any. For US holders that are not individuals but are US residents, as defined pursuant to the provisions of the Treaty, the requirements for eligibility for Treaty benefits, including the reduced 5% or 15% withholding tax rates contained in the “Limitation on Benefits” provision of the Treaty, are complicated, and certain technical changes were made to these requirements by the protocol of January 13, 2009. US holders are advised to consult their own tax advisers regarding their eligibility for Treaty benefits in light of their own particular circumstances.
Dividends paid to an eligible US holder may immediately be subject to the reduced rates of 5% or 15% provided that such holder establishes before the date of payment that it is a US resident under the Treaty by completing and providing the depositary with a treaty form (Form 5000). Dividends paid to a US holder that has not filed the Form 5000 before the dividend payment date will be subject to French withholding tax at the rate of 25% and then reduced at a later date to 5% or 15%, provided that such holder duly completes and provides the French tax authorities with the treaty forms Form 5000 and Form 5001 before December 31 of the second calendar year following the year during which the dividend is paid. Pension funds and certain other tax-exempt entities are subject to the same general filing requirements as other US holders except that they may have to supply additional documentation evidencing their entitlement to these benefits.
The depositary agrees to use reasonable efforts to follow the procedures established, or that may be established, by the French tax authorities (i) to enable eligible US holders to qualify for the reduced withholding tax rate provided by the Treaty, if available at the time the dividends are paid, or (ii) to recover any excess French withholding taxes initially withheld or deducted with respect to dividends and other distributions to which such US holders may be eligible from the French tax authorities and (iii) to recover any other available tax credits. In particular, associated forms (including Form 5000 and Form 5001, together with their instructions), will be made available by the depositary to all US holders registered with the depositary, and are also generally available from the US Internal Revenue Service.
The withholding tax refund, if any, ordinarily is paid within 12 months of filing the applicable French Treasury Form, but not before January 15 of the year following the calendar year in which the related dividend is paid.
Tax on sale or other disposition
In general, under the Treaty, a US holder who is a US resident for purposes of the Treaty will not be subject to French tax on any capital gain from the redemption (other than redemption proceeds characterized as dividends under French domestic law), sale or exchange of ordinary shares or ADSs unless the ordinary shares or the ADSs form part of the business property of a permanent establishment or fixed base that the US holder has in France. Special rules apply to holders who are residents of more than one country.
US Taxes
Taxation of dividends
For US federal income tax purposes, the gross amount of any distribution paid to US holders (that is, the net distribution received plus any tax withheld therefrom) will be treated as ordinary dividend income to the extent paid or deemed paid out of the current or accumulated earnings and profits of Sanofi (as determined under US federal income tax principles). Dividends paid by Sanofi will not be eligible for the dividends-received deduction generally allowed to corporate US holders.
Subject to certain exceptions for short-term and hedged positions, the US dollar amount of dividends received by an individual US holder with respect to the ADSs or our ordinary shares is currently subject to taxation at a maximum rate of 20% if the dividends are “qualified dividends”. Dividends paid on the ordinary shares or ADSs will be treated as qualified dividends if (i) the issuer is eligible for the benefits of a comprehensive income tax treaty with the United States that the Internal Revenue Service has approved for the purposes of the qualified dividend rules and (ii) the issuer was not, in the year prior to the year in which the dividend was paid, and is not, in the year in which the dividend is paid, a passive foreign investment company (PFIC). The Treaty
158 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 10. Additional information | ||
has been approved for the purposes of the qualified dividend rules. Based on our financial statements and relevant market and shareholder data, we believe Sanofi was not a PFIC for US federal income tax purposes with respect to its 2023 taxable year. In addition, based on its current expectations regarding the value and nature of its assets, the sources and nature of its income, and relevant market and shareholder data, we do not anticipate that Sanofi will become a PFIC for its 2024 taxable year. Holders of ordinary shares and ADSs should consult their own tax advisers regarding the availability of the reduced dividend tax rate in light of their own particular circumstances.
If you are a US holder, dividend income received by you with respect to ADSs or ordinary shares generally will be treated as foreign source income for foreign tax credit purposes. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. Distributions out of earnings and profits with respect to the ADSs or ordinary shares generally will be treated as “passive category” income (or, in the case of certain US holders, “general category” income). Subject to certain limitations, French income tax withheld in connection with any distribution with respect to the ADSs or ordinary shares may be claimed as a credit against the US federal income tax liability of a US holder if such US holder elects for that year to credit all foreign income taxes. Alternatively, such French withholding tax may be taken as a deduction against taxable income. Foreign tax credits will not be allowed for withholding taxes imposed in respect of certain short-term or hedged positions in Securities and may not be allowed in respect of certain arrangements in which a US holder’s expected economic profit is insubstantial. Further, recently issued Treasury regulations that apply to taxes paid or accrued in taxable years beginning on or after December 28, 2021 impose additional requirements for foreign taxes to be eligible for a foreign tax credit, and there can be no assurance that those requirements will be satisfied. The IRS has indicated that taxpayers may defer the application of many of the additional requirements until further notice. The US federal income tax rules governing the availability and computation of foreign tax credits are complex. US holders should consult their own tax advisers concerning the implications of these rules in light of their particular circumstances.
To the extent that an amount received by a US holder exceeds the allocable share of our current and accumulated earnings and profits, such excess will be applied first to reduce such US holder’s tax basis in its ordinary shares or ADSs and then, to the extent it exceeds the US holder’s tax basis, it will constitute capital gain from a deemed sale or exchange of such ordinary shares or ADSs (see “— Tax on Sale or Other Disposition”, below).
The amount of any distribution paid in euros will be equal to the US dollar value of the euro amount distributed, calculated by reference to the exchange rate in effect on the date the dividend is received by a US holder of ordinary shares (or by the depositary, in the case of ADSs) regardless of whether the payment is in fact converted into US dollars on such date. US holders should consult their own tax advisers regarding the treatment of foreign currency gain or loss, if any, on any euros received by a US holder that are converted into US dollars on a date subsequent to receipt.
Distributions to holders of additional ordinary shares (or ADSs) with respect to their ordinary shares (or ADSs) that are made as part of a pro rata distribution to all ordinary shareholders generally will not be subject to US federal income tax. However, if a US holder has the option to receive a distribution in shares (or ADSs) or to receive cash in lieu of such shares (or ADSs), the distribution of shares (or ADSs) will be taxable as if the holder had received an amount equal to the fair market value of the distributed shares (or ADSs), and such holder’s tax basis in the distributed shares (or ADSs) will be equal to such amount.
Tax on sale or other disposition
In general, for US federal income tax purposes, a US holder that sells, exchanges or otherwise disposes of its ordinary shares or ADSs will recognize capital gain or loss in an amount equal to the US dollar value of the difference between the amount realized for the ordinary shares or ADSs and the US holder’s adjusted tax basis (determined in US dollars and under US federal income tax rules) in the ordinary shares or ADSs. Such gain or loss generally will be US-source gain or loss, and will be treated as long-term capital gain or loss if the US holder’s holding period in the ordinary shares or ADSs exceeds one year at the time of disposition. If the US holder is an individual, any capital gain generally will be subject to US federal income tax at preferential rates (currently a maximum of 20%) if specified minimum holding periods are met. The deductibility of capital losses is subject to significant limitations.
Medicare tax
Certain US holders who are individuals, estates or trusts are required to pay a Medicare tax of 3.8% (in addition to taxes they would otherwise be subject to) on their “net investment income” which would include, among other things, dividends and capital gains from the ordinary shares and ADSs.
SANOFI FORM 20-F 2023 | 159 | ||||
| PART I | ||
ITEM 10. Additional information | ||
F. Dividends and Paying Agents
N/A
G. Statement by Experts
N/A
H. Documents on Display
We are subject to the information requirements of the US Securities Exchange Act of 1934, as amended, or Exchange Act, and, in accordance therewith, we are required to file reports, including this annual report, and other information with the US Securities and Exchange Commission, or Commission, by electronic means.
You may review a copy of our filings with the Commission, as well as other information furnished to the Commission, including exhibits and schedules filed with it, at the Commission’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information. In addition, the Commission maintains an Internet site at http://www.sec.gov that contains reports and other information regarding issuers that file electronically with the Commission (these documents are not incorporated by reference in this annual report).
I. Subsidiary Information
N/A.
J. Annual Report to Security Holders
To the extent we furnish an annual report to security holders, we will promptly submit an English version of this annual report to US security holders under the cover of Form 6-K.
160 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 11. Quantitative and qualitative disclosures about market risk | ||
Item 11. Quantitative and Qualitative Disclosures about Market Risk(1)
General policy
Liquidity risk, foreign exchange risk and interest rate risk, as well as related counterparty risks, are managed centrally by our dedicated treasury team within the Group Finance Department. Where it is not possible to manage those risks centrally – in particular due to regulatory restrictions (such as foreign exchange controls) or local tax restrictions – credit facilities and/or currency lines, guaranteed whenever necessary by the parent company, are contracted by our subsidiaries locally with banks, under the supervision of the central treasury team.
Our financing and investment strategies, and our interest rate and currency hedging strategies, are reviewed monthly by the Group Finance Department.
Our policy prohibits the use of derivatives for speculative purposes.
Counterparty risk
Our financing and investing transactions, and our currency and interest rate hedges, are contracted with leading counterparties. We set limits for investment and derivative transactions with individual financial institutions, depending on the rating of each institution. Compliance with these limits, which are based on the notional amounts of the investments and the fair value of the hedging instruments, is monitored on a daily basis.
The table below shows our total exposure as of December 31, 2023 by rating and in terms of our percentage exposure to the dominant counterparty.
| (€ million) | Cash and cash equivalents (excluding mutual funds) | (a) | Notional amounts of currency hedges | (b) | Fair value of currency hedges | Notional amounts of interest rate hedges | (b) | Fair value of interest rate hedges | General corporate purpose credit facilities | ||||||||||||||||||||
| AA | 318 | 1,050 | (6) | 45 | — | 500 | |||||||||||||||||||||||
| AA- | 663 | 7,277 | 11 | 1,165 | (47) | 1,000 | |||||||||||||||||||||||
| A+ | 728 | 12,403 | 43 | 1,145 | (65) | 3,500 | |||||||||||||||||||||||
| A | 246 | 5,676 | 19 | 673 | (27) | 2,500 | |||||||||||||||||||||||
| A- | 20 | 1,283 | 8 | 377 | (25) | 500 | |||||||||||||||||||||||
| BBB+ | — | — | — | — | — | — | |||||||||||||||||||||||
| Unallocated | 324 | — | — | — | — | — | |||||||||||||||||||||||
| Total | 2,299 | 27,689 | 75 | 3,405 | (165) | 8,000 | |||||||||||||||||||||||
%/rating of dominant counterparty | 26.2%/AA- | 14.9%/A+ | 19.3%/AA- | 6%/A | |||||||||||||||||||||||||
(a) Cash equivalents include mutual fund investments of €5,321 million.
(b) The notional amounts are translated into euros at the relevant closing exchange rate as of December 31, 2023.
As of December 31, 2023, we held investments in euro and US dollar denominated money-market mutual funds. Those instruments have low volatility, low sensitivity to interest rate risk, and a very low probability of loss of principal. The depositary banks of the mutual funds, and of Sanofi itself, have a long-term rating of at least A. Realization of counterparty risk could impact our liquidity in certain circumstances.
(1) The disclosures in this section supplement those provided in Note B.8.7. to the consolidated financial statements as regards the disclosure requirements of IFRS 7, and are covered by the independent registered public accounting firms' opinion on the consolidated financial statements.
SANOFI FORM 20-F 2023 | 161 | ||||
| PART I | ||
ITEM 11. Quantitative and qualitative disclosures about market risk | ||
Foreign exchange risk
A. Operating foreign exchange risk
A substantial portion of our net sales is generated in countries where the euro, which is our reporting currency, is not the functional currency. In 2023, for example, 43.0% of our net sales were generated in the United States; 24.1% in Europe; and 32.9% in the Rest of the World region (see the definition in “Item 5. Operating and Financial Review and Prospects — A/ Operating results), including countries that are, or may in the future become, subject to exchange controls, of which 6.8% was generated in China and 3.7% in Japan. Although we also incur expenses in those countries, the impact of those expenses is not enough wholly to offset the impact of exchange rates on our net sales. Consequently, our operating income may be materially affected by fluctuations in exchange rates between the euro and other currencies. Sanofi operates a foreign exchange risk hedging policy to reduce the exposure of operating income to exchange rate movements. That policy involves regular assessments of Sanofi’s worldwide foreign currency exposure, based on foreign currency transactions carried out by the parent company and its subsidiaries. Those transactions mainly comprise sales, purchases, research costs, co-marketing and co-promotion expenses, and royalties. To reduce the exposure of those transactions to exchange rate movements, Sanofi contracts hedges using liquid derivative instruments, mainly forward currency purchases and sales, and also foreign exchange swaps.
The table below shows operating currency hedging instruments in place as of December 31, 2023, with the notional amount translated into euros at the relevant closing exchange rate (see Note D.20. to the consolidated financial statements for the accounting classification of those instruments as of December 31, 2023).
Operating foreign exchange derivatives as of December 31, 2023
| (€ million) | Notional amount | Fair value | ||||||
| Forward currency sales | 6,112 | 30 | ||||||
| of which US dollar | 2,981 | 35 | ||||||
| of which Chinese yuan renminbi | 788 | 7 | ||||||
| of which Singapore dollar | 419 | (1) | ||||||
of which Japanese yen | 339 | (6) | ||||||
| of which Korean won | 192 | (4) | ||||||
| Forward currency purchases | 4,246 | (8) | ||||||
| of which US dollar | 2,022 | (12) | ||||||
| of which Singapore dollar | 876 | — | ||||||
| of which Chinese yuan renminbi | 364 | (1) | ||||||
| of which Korean won | 137 | 2 | ||||||
of which Japanese yen | 123 | 1 | ||||||
| Total | 10,358 | 22 | ||||||
The above positions mainly hedge future material foreign-currency cash flows arising after the end of the reporting period in relation to transactions carried out during the year ended December 31, 2023 and recognized in the balance sheet at that date. Gains and losses on hedging instruments (forward contracts) are calculated and recognized in parallel with the recognition of gains and losses on the hedged items. Due to this hedging relationship, the commercial foreign exchange profit or loss on these items (hedging instruments and hedged transactions) was immaterial in 2023.
B. Financial foreign exchange risk
The cash pooling arrangements for foreign subsidiaries outside the euro zone, and some of Sanofi’s financing activities, expose certain Sanofi entities to financial foreign exchange risk (i.e. the risk of changes in the value of borrowings and loans denominated in a currency other than the functional currency of the borrower or lender). That foreign exchange exposure is hedged using derivative instruments (foreign exchange swaps, forward contracts or currency swaps) that alter the currency split of Sanofi’s net debt once those instruments are taken into account.
The table below shows financial currency hedging instruments in place as of December 31, 2023, with the notional amounts translated into euros at the relevant closing exchange rate (see also Note D.20. to the consolidated financial statements for the accounting classification of these instruments as of December 31, 2023).
162 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
ITEM 11. Quantitative and qualitative disclosures about market risk | ||
Financial foreign exchange derivatives as of December 31, 2023
| (€ million) | Notional amount | Fair value | Expiry | |||||||||||
| Forward currency sales | 10,279 | 111 | ||||||||||||
| of which US dollar | 6,628 | (a) | 101 | 2024 | ||||||||||
| of which Singapore dollar | 1,556 | 6 | 2024 | |||||||||||
| of which Chinese yuan renminbi | 513 | 4 | 2024 | |||||||||||
| Forward currency purchases | 7,055 | (58) | ||||||||||||
| of which US dollar | 3,073 | (b) (c) | (52) | 2024 | ||||||||||
| of which Singapore dollar | 2,696 | (10) | 2024 | |||||||||||
| of which Japanese yen | 341 | 3 | 2024 | |||||||||||
| Total | 17,334 | 53 | ||||||||||||
(a) Includes forward sales with a notional amount of $3,615 million expiring in 2024, designated as a hedge of Sanofi’s net investment in Bioverativ. As of December 31, 2023, the fair value of these forward contracts represented an asset of €54 million; the opposite entry was recognized in “Other comprehensive income," with the impact on financial income and expense being immaterial.
(b) Includes forward purchases with a notional amount of $1,000 million expiring in 2024, designated as a fair value hedge of the exposure of $1,000 million of bond issues to fluctuations in the EUR/USD spot rate. As of December 31, 2023, the fair value of the contracts represented a liability of €31 million, the opposite entry for €2.7 million of which was credited to "Other comprehensive income" under the cost of hedging accounting treatment.
(c) Includes forward purchases with a notional amount of $1,044 million expiring in 2024, designated as a fair value hedge of the exposure of $1,044 million of commercial paper. As of December 31, 2023, the fair value of these forward contracts swaps represented a liability of €3 million, the opposite entry for €0.7 million of which was credited to "Other comprehensive income" under the cost of hedging accounting treatment.
These hedging instruments generate a net financial gain or loss arising from the interest rate differential between the hedged currency and the euro, given that the foreign exchange gain or loss on the foreign-currency borrowing and loans is offset by the change in the intrinsic value of the hedging instruments. The interest rate differential is recognized within cost of net debt (see Note D.29. to our consolidated financial statements). We may also hedge some future foreign-currency investment or divestment cash flows.
C. Other foreign exchange risks
A significant proportion of our net assets is denominated in US dollars (see Note D.35. to the consolidated financial statements). As a result, any fluctuation in the exchange rate of the US dollar against the euro automatically impacts the amount of our equity as expressed in euros.
In addition, we use the euro as our reporting currency. Consequently, if one or more European Union Member States were to abandon the euro as a currency, the resulting economic upheavals – in particular, fluctuations in exchange rates – could have a significant impact on the terms under which we can obtain financing and on our financial results, the extent and consequences of which are not currently foreseeable.
Liquidity risk
We operate a centralized treasury platform whereby all surplus cash and financing needs of our subsidiaries are invested with or funded by the parent company (where permitted by local legislation). The central treasury department manages our current and projected financing, and ensures that Sanofi is able to meet its financial commitments by maintaining sufficient cash and confirmed credit facilities for the size of our operations and the maturity of our debt (see Notes D.17.1.c. and D.17.1.g. to the consolidated financial statements).
We diversify our short-term investments with leading counterparties using money-market products with instant access or with a maturity of less than three months.
As of December 31, 2023, cash and cash equivalents amounted to €8,710 million, and short-term investments predominantly comprised:
•collective investments in euro and US dollar denominated money-market mutual funds. All such funds can be traded on a daily basis and the amount invested in each fund may not exceed 10% of the aggregate amount invested in such funds; and
•amounts invested directly with banks and non-financial institutions in the form of instant access deposits, term deposits, and Negotiable European Commercial Paper with a maturity of no more than three months.
As of December 31, 2023 we also had €8 billion of undrawn general corporate purpose confirmed credit facilities, half of which expires in December 2027 and half in March 2029. Those credit facilities are not subject to financial covenant ratios.
Our policy is to diversify our sources of funding through public or private issuances of debt securities, in the United States (shelf registration statement) and Europe (Euro Medium Term Note program). In addition, our A-1+/P-1 short-term rating gives us access to commercial paper programs in the United States, and to Negotiable European Commercial Paper programs in France. The average maturity of our total debt was 4.45 years as of December 31, 2023, compared with 4.71 years as of December 31, 2022.
Average drawdowns under the Negotiable European commercial paper program during 2023 were €0.2 billion (with a maximum of €0.8 billion); the average maturity of those drawdowns was three months. As of December 31, 2023, drawdowns under the program amounted to €40 million.
SANOFI FORM 20-F 2023 | 163 | ||||
| PART I | ||
ITEM 11. Quantitative and qualitative disclosures about market risk | ||
Average drawdowns under the US Commercial Paper program during 2023 were €3.4 billion (with a maximum of €6.8 billion); the average maturity of those drawdowns was four months. As of December 31, 2023, drawdowns under the program amounted to €0.9 billion.
In the event of a liquidity crisis, we could be exposed to difficulties in calling up our available cash, a scarcity of sources of funding including the above-mentioned programs, and/or a deterioration in their terms. This situation could damage our capacity to refinance our debt or to issue new debt on reasonable terms.
Interest rate risk
Sanofi issues debt in two currencies, the euro and the US dollar, and also invests its cash and cash equivalents in those currencies. Sanofi also operates cash pooling arrangements to manage the surplus cash and short-term liquidity needs of foreign subsidiaries located outside the euro zone.
To optimize the cost of debt or reduce the volatility of debt and manage its exposure to financial foreign exchange risk, Sanofi uses derivative instruments (interest rate swaps, currency swaps, foreign exchange swaps and forward contracts) that alter the fixed/floating rate split and the currency split of its net debt.
The projected full-year sensitivity to interest rate fluctuations of our debt, net of cash and cash equivalents for 2024 is as follows:
| Change in short-term interest rates | Impact on pre-tax net income (€ million) | Impact on pre-tax income/(expense) recognized directly in equity (€ million) | ||||||
| +100 bp | 48 | — | ||||||
| +25 bp | 12 | — | ||||||
| -25 bp | (12) | — | ||||||
| -100 bp | (48) | — | ||||||
Stock market risk
It is our policy not to trade on the stock market for speculative purposes.
164 | SANOFI FORM 20-F 2023 | ||||
| PART I | ||
| ITEM 12. Description of Securities other than Equity Securities | ||
Item 12. Description of Securities other than Equity Securities
12.A. Debt securities
Not applicable.
12.B. Warrants and rights
Not applicable.
12.C. Other securities
Not applicable.
12.D. American depositary shares
General
JPMorgan Chase Bank, NA (“JPMorgan”), as depositary, issues Sanofi ADSs in certificated form (evidenced by an ADR) or book-entry form. Each ADR is a certificate evidencing a specific number of Sanofi ADSs. Each Sanofi ADS represents one-half of one Sanofi ordinary share (or the right to receive one-half of one Sanofi ordinary share) deposited with the Paris, France office of BNP Paribas, as custodian. Each Sanofi ADS also represents an interest in any other securities, cash or other property that may be held by the depositary under the Second Amended and Restated Deposit Agreement between Sanofi and JPMorgan dated February 13, 2015, as amended by Amendment No. 1 dated July 23, 2020 (“Amendment No. 1”), Amendment No. 2 dated December 18, 2023 ("Amendment No. 2"), and as may be further amended from time to time (together, the “deposit agreement”). The depositary’s principal executive office is located at 383 Madison Avenue, 11th Floor, New York, New York 10179.
For additional information on our ADSs, please refer to Exhibit 2.2 “Description of Securities” of this Annual Report.
Fees and expenses
Fees payable by ADS holders
Pursuant to the deposit agreement, holders of our ADSs may have to pay to JPMorgan, either directly or indirectly, fees, charges and expenses up to the amounts set forth in the table below.
| Associated Fee | Depositary Action | ||||
| $5.00 or less per 100 ADSs (or portion thereof) | The deposit of shares and/or the execution and delivery of ADRs (pursuant to distribution in shares or distribution of rights to subscribe for additional shares, or distribution of any rights of any other nature), and/or the reduction of ADSs and surrender of ADRs for the purposes of withdrawal, including the termination of the deposit agreement. | ||||
| $0.05 or less per ADS (or portion thereof) | Any distribution made pursuant to the deposit agreement, including, among other things: •any cash distribution made, or for any elective cash/stock dividend offered; and •the direct or indirect distribution of securities (other than ADSs or rights to purchase additional ADSs) or the net cash proceeds from the public or private sale of any such securities. | ||||
$0.05 or less per ADS per calendar year (or portion thereof) | Services performed in administering the ADRs (which fee may be charged on a periodic basis during each calendar year). | ||||
An amount for the reimbursement of such fees, charges and expenses as are incurred by JPMorgan and/or any of its agents (including, without limitation BNP Paribas, as custodian and expenses incurred on behalf of owners in connection with compliance with foreign exchange control regulations or any law or regulation relating to foreign investment) | Compliance with foreign exchange control regulations or any law or regulation relating to foreign investment, servicing of shares or other deposited securities, sale of securities, delivery of deposited securities or otherwise. | ||||
| Expenses incurred by JPMorgan | Foreign currency conversion into dollars. | ||||
The Depositary may sell (by public or private sale) sufficient securities and property received in respect of Share distributions, rights and other distributions prior to a deposit to pay any charge owing.
SANOFI FORM 20-F 2023 | 165 | ||||
| PART I | ||
| ITEM 12. Description of Securities other than Equity Securities | ||
In addition to the fees outlined above, each holder will be responsible for any taxes or other governmental charges payable on his or her Sanofi ADSs or on the deposited securities underlying his or her Sanofi ADSs. The depositary may refuse to transfer a holder’s Sanofi ADSs or allow a holder to withdraw the deposited securities underlying his or her Sanofi ADSs until such taxes or other charges are paid. It may apply payments owed to a holder or sell deposited securities underlying a holder’s Sanofi ADSs to pay any taxes owed, and the holder will remain liable for any deficiency. If it sells deposited securities, it will, if appropriate, reduce the number of Sanofi ADSs to reflect the sale and pay to the holder any proceeds, or send to the holder any property, remaining after it has paid the taxes. For additional information regarding taxation, see “Item 10. Additional Information — E. Taxation.”
Fees paid to Sanofi by the depositary
JPMorgan, as depositary, has agreed to reimburse Sanofi for certain expenses that Sanofi incurs relating to the establishment and maintenance of the ADR program, as agreed from time to time. Pursuant to a letter agreement dated October 4, 2022 (the “letter agreement”), JPMorgan as our ADS depositary has agreed to make (i) an initial contribution to Sanofi, within 30 days of the commencement date of the letter agreement and (ii) with respect to each 12-month period beginning on the anniversary of the effective date of the agreement (each such 12-month period, a “Contract Year”), a contribution, paid at the end of such Contract Year quarter, equal to the aggregate of the program share (equal to 100% of routine program revenues and 50% of non-routine program revenues) of any program revenues, less the aggregate of any program costs for the applicable Contract Year and any invoiced supplementary costs not paid within 60 days of the date of the applicable invoice.
To the extent in any given Contract Year the depositary does not collect/recoup the entirety of the program costs and unpaid supplementary costs, no contribution shall be payable to Sanofi and such excess will, at the discretion of the depositary, either be deducted from future contributions or be payable to the depositary by Sanofi promptly upon invoicing as supplementary costs under the letter agreement.
JPMorgan has further agreed to waive the $0.05 per ADS issuance fees that would normally be owed by Sanofi in connection with our deposits of shares as part of our employee stock purchase plans. Sanofi is responsible for reimbursing JPMorgan for all taxes and governmental charges in connection with payments to JPMorgan under the letter agreement.
From January 1, 2023 to December 31, 2023, we received a total amount of $24,020,797.28 from JPMorgan pursuant to the letter agreement.
166 | SANOFI FORM 20-F 2023 | ||||
| PART II | ||
| ITEM 13. Defaults, dividend arrearages and delinquencies | ||
Part II
Item 13. Defaults, Dividend Arrearages and Delinquencies
N/A
Item 14. Material Modifications to the Rights of Security Holders
N/A
Item 15. Controls and Procedures
a.Our Chief Executive Officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Exchange Act Rule 13a-15(e)) as of the end of the period covered by this Form 20-F, have concluded that, as of such date, our disclosure controls and procedures were effective to ensure that material information relating to Sanofi was timely made known to them by others within Sanofi.
b.Report of Management on Internal Control Over Financial Reporting.
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Management assessed the effectiveness of internal control over financial reporting as of December 31, 2023 based on the framework in “Internal Control — Integrated Framework” (2013 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Qunol (QRIB Intermediate Holdings, LLC and its affiliates) that was acquired in 2023 has been excluded from the scope of management’s assessment and conclusion on internal control over financial reporting as of December 31, 2023. Qunol (QRIB Intermediate Holdings, LLC and its affiliates) is included in the 2023 consolidated financial statements of the Company and represents less than 1% of total assets as of December 31, 2023 and less than 1% of net sales for the year then ended.
Based on that assessment, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2023 to provide reasonable assurance regarding the reliability of its financial reporting and the preparation of its financial statements for external purposes, in accordance with generally accepted accounting principles.
Due to its inherent limitations, internal control over financial reporting may not prevent or detect misstatements, and can only provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of the Company’s internal control over financial reporting has been audited by PricewaterhouseCoopers Audit (PCAOB ID 1347 ) and Ernst & Young et Autres (PCAOB ID 1704 ) independent registered public accounting firms, as stated in their report on the Company’s internal control over financial reporting as of December 31, 2023, which is included herein. See paragraph (c) of the present Item 15., below.
c.See report of PricewaterhouseCoopers Audit and Ernst & Young et Autres, independent registered public accounting firms, included under “Item 18. Financial Statements” on page F-3.
d.There were no changes to our internal control over financial reporting that occurred during the period covered by this Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
SANOFI FORM 20-F 2023 | 167 | ||||
| PART II | ||
| ITEM 16A. Audit Committee Financial Expert | ||
Item 16A. Audit Committee Financial Expert
The Audit Committee is composed of Fabienne Lecorvaisier, Christophe Babule, Carole Ferrand and Diane Souza.
Our Board of Directors has determined that all directors are independent financial experts within the meaning of Section 407 of the Sarbanes-Oxley Act of 2002.
The Board of Directors deemed Fabienne Lecorvaisier to be a financial expert based on her education and experience in corporate finance in various international banks and as Chief Financial Officer of Essilor and Air Liquide. She is now Executive Vice President, in charge of Sustainable Development, Public and International Affairs as well as the supervision of the Social Programs and the General Secretariat of Air Liquide Group.
The Board of Directors deemed Christophe Babule to be a financial expert based on his education and experience in audit and corporate finance in major corporations and as Executive Vice President and Chief Financial Officer of L’Oréal. He has also served as a director of L’Oréal US Inc.
The Board of Directors deemed Carole Ferrand to be a financial expert based on her education and experience in audit at PricewaterhouseCoopers and as Chief Financial Officer of Sony France, EuropaCorp and Groupe Artémis. She was Chief Financial Officer of Capgemini until end of 2023.
The Board of Directors deemed Diane Souza to be a financial expert based on her education (she is a certified public accountant) and experience in audit and tax in major international corporations, as Chief Financial Officer of Aetna’s Guaranteed Products business, and as Chief Executive Officer of the UnitedHealthcare Specialty Benefits.
The Board of Directors has determined that all four directors meet the independence criteria of US Securities and Exchange Commission Rule 10A-3, although only Fabienne Lecorvaisier, Carole Ferrand and Diane Souza meet the French AFEP-MEDEF Code criteria of independence applied by the Board of Directors for general corporate governance purposes (see Item 16G., below).
Item 16B. Code of Ethics
We have adopted a code of ethics (hereafter the "Code of conduct"), as defined in Item 16B. of Form 20-F under the Exchange Act, containing specific rules relating to financial ethics. Our Code of conduct applies to our Chief Executive Officer, Chief Financial Officer, Chief Accounting Officer and other officers performing similar functions, as designated from time to time. Our Code of conduct is available on our website at www.sanofi.com (information on our website is not incorporated by reference in this annual report). A copy of our Code of conduct may also be obtained free of charge by addressing a written request to the attention of Individual Shareholder Relations at our headquarters in Paris. We will disclose any amendment to the provisions of such financial code of conduct on our website.
Item 16C. Principal Accountants’ Fees and Services
See Note E. to our consolidated financial statements included at Item 18 of this annual report.
Item 16D. Exemptions from the Listing Standards for Audit Committees
N/A
168 | SANOFI FORM 20-F 2023 | ||||
| PART II | ||
| ITEM 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers | ||
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
In 2023, Sanofi made the following purchases of its ordinary shares.
| Period | (A) Total Number of Shares Purchased | (B) Average Price Paid per Share | (C) Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs(a) | (D) Approximate Value of Shares that May Yet Be Purchased Under the Plans or Programs(b) | ||||||||||
January 2023 | 4,000,204 | 90.60 | 4,000,204 | 18,454 | ||||||||||
December 2023 | 2,584,540 | 88.69 | 2,584,540 | 18,683 | ||||||||||
| Total | 6,584,744 | |||||||||||||
(a) The Company was authorized to repurchase up to €18,953,410,350 of shares for a period of eighteen months (i.e. through November 3, 2023) by the Annual Shareholders’ Meeting held on May 3, 2022. Then, the Company was authorized to repurchase up to €18,912,535,950 of shares for a period of eighteen months (i.e. through November 25, 2024) by the Annual Shareholders’ Meeting held on May 25, 2023.
(b) Million of euros.
For more information see “Exhibit 2.2. "Description of Securities" of this annual report”.
Item 16F. Change in Registrant’s Certifying Accountant
The term of office of Ernst & Young et Autres, joint statutory auditor of the Company since 1986, will expire at the end of the Annual Shareholders’ Meeting called in 2024. Ernst & Young et Autres’ term of office cannot be extended because it reached the maximum legal duration.
The selection procedure of the auditors to be appointed by the Annual Shareholders’ Meeting in 2024 was overseen by the Audit Committee, following which a recommendation to the Board of Directors was issued.
The Board of Directors, at its meeting of October 27, 2022 approved the Audit Committee’s recommendation and decided to propose the appointment of Mazars as statutory auditor. Consequently, the Board of Directors will propose to the Annual Shareholders’ Meeting to be held in 2024 to appoint Mazars as new joint statutory auditor for a 6-year term, i.e. until the Annual Shareholders’ Meeting to be held in 2030, which will approve the financial statements for the year 2029.
The report of Ernst & Young et Autres on the consolidated financial statements for each of the years ended December 31, 2023 and 2022 did not contain an adverse opinion or a disclaimer of opinion and was not qualified or modified as to uncertainty, audit scope or accounting principles. During each of the years ended December 31, 2023 and 2022:
•there were no “disagreements” (as that term is described in Item 16F.(a)(1)(iv) of the Instructions to Form 20-F and the instructions to Item 16F.) between Sanofi and Ernst & Young et Autres on any matters of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreement(s), if not resolved to Ernst & Young et Autres’ satisfaction, would have caused Ernst & Young et Autres to make reference to the subject matter of the disagreement(s) in connection with its report; and
•there were no “reportable events” (as that term is defined in Item 16F.(a)(1)(v) of the Instructions to Form 20-F).
A copy of Ernst & Young et Autres’ letter, dated February 23, 2024, is filed as Exhibit 15.3 to this annual report.
Item 16G. Corporate Governance
Sanofi is incorporated under the laws of France, with securities listed on regulated public markets in the United States (Nasdaq Global Select Market – NASDAQ) and France (Euronext Paris). Consequently, as described further in this annual report, our corporate governance framework reflects the mandatory provisions of French corporate law, the securities laws and regulations of France and the United States and the rules of the aforementioned public markets.
As a “foreign private issuer,” as defined in the rules promulgated under the US Securities Exchange Act of 1934, as amended, (the “Exchange Act”), Sanofi is permitted, pursuant to NASDAQ Listing Rule 5615(a)(3), to follow its home country practice in lieu of certain NASDAQ corporate governance requirements applicable to US corporations listed on the NASDAQ. Sanofi has informed NASDAQ that it intends to follow corporate governance standards under French law to the extent permitted by the NASDAQ listing rules and US securities laws, as further discussed below.
We generally follow the “AFEP-MEDEF” corporate governance recommendations for French listed issuers (hereafter referred to as the “AFEP-MEDEF Code”). As a result, our corporate governance framework is similar in many respects to, and provides investor protections that are comparable to – or in some cases, more stringent than – the corresponding rules of the NASDAQ. Nevertheless, there are certain important differences.
SANOFI FORM 20-F 2023 | 169 | ||||
| PART II | ||
Item 16G. Corporate Governance | ||
In line with NASDAQ listing rules applicable to domestic issuers, a majority of Sanofi’s Board of Directors is comprised of independent directors. Sanofi evaluates the independence of members of our Board of Directors using the standards of the French AFEP-MEDEF Code as the principal reference. We believe that AFEP-MEDEF’s overarching criteria for independence – that Board members have no relationship of any kind whatsoever with the Company, its group or the management of either such as to color a Board member’s judgment – is on the whole consistent with the goals of the NASDAQ's listing rules; however, the specific tests proposed under the two standards may vary on some points. Our Audit Committee complies with the independence and other requirements of Rule 10A-3 under the Exchange Act, adopted pursuant to the Sarbanes-Oxley Act of 2002. Our Audit Committee includes one member, Christophe Babule, who is considered non-independent under the AFEP-MEDEF Code, and which is permitted under the AFEP-MEDEF Code. Three out of the four members of our Compensation Committee meet the independence standards of the AFEP-MEDEF Code (the Director representing employees is not considered as independent) and the independence requirements of NASDAQ’s listing rules.
Sanofi follows the recommendation of the AFEP-MEDEF Code that at least one meeting of the Board of Directors not attended by the company’s executive officers be organized each year. Accordingly, Sanofi’s Board Charter provides that the Board of Directors shall organize at least two meetings a year without its executive officers, thereby providing the Chairman with the option of whether to include directors representing employees or any other Group employee, as the case may require, depending on the agenda of the meeting. Sanofi’s practice in that respect departs from NASDAQ Listing Rule 5605(b)(2), which provides that independent directors must have regularly scheduled meetings at which only independent directors are present.
Under French law, the committees of our Board of Directors are advisory only, and where the NASDAQ Listing Rule 5600 series would vest certain decision-making powers with specific committees by delegation (e.g. the appointment of Sanofi’s auditors by the Audit Committee), under French law, our Board of Directors remains the only competent body to take such decisions, albeit taking into account the recommendation of the relevant committees. Additionally, under French corporate law, it is the shareholders of Sanofi voting at the Shareholders’ General Meeting that have the authority to appoint our auditors upon consideration of the proposal of our Board of Directors, although our Board Charter provides that the Board of Directors will make its proposal on the basis of the recommendation of our Audit Committee. We believe that this requirement of French law, together with the additional legal requirement that two sets of statutory auditors be appointed, is in line with the NASDAQ's underlying goal of ensuring that the audit of our accounts be conducted by auditors independent from company management.
NASDAQ Listing Rule 5635 requires a NASDAQ listed company to obtain shareholder approval prior to certain issuances of securities, including: (a) issuances in connection with the acquisition of the stock or assets of another company if upon issuance the issued shares will equal 20% or more of the number of shares or voting power outstanding prior to the issuance, or if certain specified persons have a 5% or greater interest in the assets or company to be acquired (NASDAQ Listing Rule 5635(a)); (b) issuances or potential issuances that will result in a change of control of us (NASDAQ Listing Rule 5635(b)); (c) issuances in connection with equity compensation arrangements (NASDAQ Listing Rule 5635(c)); and (d) 20% or greater issuances in transactions other than public offerings, as defined in the NASDAQ listing rules (NASDAQ Listing Rule 5635(d)). Under French law, our shareholders may approve issuances of equity, as a general matter, through the adoption of delegation of authority resolutions at the Company’s shareholders’ meeting pursuant to which shareholders may delegate their authority to the Board of Directors to increase the Company’s share capital within specified parameters set by the shareholders, which may include a time limitation to carry out the share capital increase, the cancellation of their preferential subscription rights to the benefit of named persons or a category of persons, specified price limitations and/or specific or aggregate limitations on the size of the share capital increase. Due to differences between French law and corporate governance practices and NASDAQ Listing Rule 5635, the Company follows French home country practice, rather than complying with this NASDAQ Listing Rule.
In addition to the oversight role of our Compensation Committee for questions of management compensation including by way of equity, under French law any option or restricted share plans or other share capital increases, whether for the benefit of senior management or employees, may only be adopted by the Board of Directors pursuant to and within the limits of a shareholder resolution approving the related capital increase and delegating to the Board the authority to implement such operations. While NASDAQ rules require shareholder approval when a plan or other equity compensation arrangement is established or materially amended, under French law our shareholders must decide any issuance of equity, as a general matter. We intend to follow our French home country practice and ask our shareholders to delegate their authority to issue incentive equity and define the final terms of any equity compensation plan or arrangements to our Board of Directors. We may, from time to time, ask for our shareholders’ subsequent approval on an equity compensation arrangement in order to obtain advantageous tax treatment or otherwise. In addition, under French law, our Board of Directors must obtain the prior approval of our shareholders before establishing or amending a plan or arrangement that would exceed the limits of the granted delegation.
As described above, a number of issues, which could be resolved directly by a board or its committees in the United States, require the additional protection of direct shareholder consultation in France.
Because we are a “foreign private issuer” as described above, our Chief Executive Officer and our Chief Financial Officer issue the certifications required by Section 302 and Section 906 of the Sarbanes-Oxley Act of 2002 on an annual basis (with the filing of our annual report) rather than on a quarterly basis as would be the case of a US corporation filing quarterly reports on Form 10- Q.
French corporate law provides that the Board of Directors must vote to approve a broadly defined range of transactions that could potentially create conflicts of interest between Sanofi on the one hand and its directors and Chief Executive Officer on the other hand, which are then presented to shareholders for approval at the next annual meeting. This legal safeguard operates in place of certain provisions of the NASDAQ listing rules.
170 | SANOFI FORM 20-F 2023 | ||||
| PART II | ||
ITEM 16G. Corporate Governance | ||
Sanofi is governed by the French Commercial Code, which provides that an ordinary general meeting of the shareholders may validly deliberate when first convened if the shareholders present or represented hold at least one-fifth of the voting shares. If it is reconvened, no quorum is required. The French Commercial Code further provides that the shareholders at an extraordinary general meeting may validly deliberate when first convened only if the shareholders present or represented hold at least one-quarter of the voting shares and, if reconvened, one-fifth of the voting shares. Therefore, Sanofi will not follow NASDAQ Listing Rule 5620(c), which provides that the minimum quorum requirement for a meeting of shareholders is 331⁄3% of the outstanding common voting shares of the company. In accordance with the provisions of the French Commercial Code, the required majority for the adoption of a decision is a simple majority (for an ordinary general meeting of the shareholders) or a two-thirds majority (for an extraordinary general meeting) of the votes cast by the shareholders present or represented.
The Company has, pursuant to Rule 10D-1 under the Exchange Act, introduced a recovery policy for compensation erroneously paid to “executive officers” (as defined in Rule 10D-1(d) under the Exchange Act) based in whole or in part on any financial reporting measures pursuant to the applicable NASDAQ listing rules, Rule 10D-1 under the Exchange Act and applicable interpretive guidance. For more information concerning our recovery policy for compensation erroneously paid to “executive officers”, see also “Item 6. Directors, Senior Management and Employees – B. Compensations”. Our recovery policy for compensation erroneously paid to “executive officers” is reproduced in full as Exhibit 97 to this annual report.
Item 16H. Mine Safety Disclosure
N/A
Item 16I. Disclosure regarding foreign jurisdictions that prevent inspections
N/A
Item 16J. Insider Trading Policies
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16J will be applicable to Sanofi starting the fiscal year ending December 31, 2024.
Item 16K. Cybersecurity
Risk Management and Strategy
Sanofi has implemented a cybersecurity strategy involving various dedicated personnel and resources aimed at preventing, detecting and responding to cyberattacks, as well as being able to recover promptly in the event of material impact following a cyberattack. Additionally, Sanofi has set up various cybersecurity processes applicable to subsidiaries within Sanofi group. Sanofi regularly updates its cybersecurity processes to address cybersecurity trends and threats. Cybersecurity processes have been established to address material cybersecurity risks, including in connection with the following areas:
•information technology and solution usage;
•access control;
•patch management;
•security on specific environments (i.e. cloud, virtualization, SAP, automated systems, IoT, etc.);
•log management;
•network security;
•systems security standards;
•remote access;
•secure development of applications;
•cryptography;
•mobile devices;
•third-party management (including cybersecurity requirements in contracts); and
•incident management.
Sanofi utilizes security standards and frameworks (i.e. the NIST framework) and has established cross-functional risk control capabilities to facilitate operational implementation aligned with its cybersecurity processes.
SANOFI FORM 20-F 2023 | 171 | ||||
| PART II | ||
Item 16K. Cybersecurity | ||
Sanofi regularly analyses its Internet-based services and performs regular penetration tests and attack simulations to assess the protections and the detections capabilities. The cybersecurity compliance status of computing assets connected to Sanofi’s network is routinely consolidated for Sanofi's business units, including within manufacturing, and research and development sites. Monthly dashboards are published and shared within Sanofi’s different business units and global functions. Sanofi implements corrective measures and improvement actions in response to these processes. Data classification and protection tools are in place, such as the implementation of a specific process and technology aimed at detecting and responding to abnormal data flows.
Sanofi has set up a cybersecurity operation center in charge of detecting and responding to cybersecurity threats and attacks, as well as orchestrating Sanofi-wide incident responses. Incident response trainings and simulations are run within Sanofi to be better prepared in case of a cybersecurity incident. In addition, Sanofi’s employees, who are the main users of Sanofi’s digital assets, are regularly trained to face cybersecurity threats and attacks. In the event of a cyberattack, Sanofi has established a plan that includes criteria triggering the notification process for material cybersecurity incidents including from the cybersecurity operation center and the Chief Information Security Officer who can use the internal escalation channels to inform the management and the Board of Directors and, as appropriate, the relevant regulatory bodies.
When dealing with third parties, our main commercial contracts include cybersecurity clauses aimed at ensuring such third parties comply with Sanofi’s cybersecurity rules and requirements, especially when providing services to and processing data from Sanofi. Additionally, Sanofi set up a vendor’s risk assessment program to evaluate the digital maturity of a vendor, which covers their business continuity as well as their related internal regulations, such as data privacy. As part of their contractual commitments major vendors and partners must report to Sanofi any cybersecurity incident which may have a significant impact for Sanofi. A dedicated process has been implemented for third parties’ networks interconnected with Sanofi’s network, aimed at limiting any propagation of a cyberattack to Sanofi’s digital assets.
Sanofi’s cybersecurity risk management processes are integrated into its overall risk management system through its enterprise risk management process, which seeks to identify and address material risks to the organization. Each year, specific risk committees identify the risks that affect Sanofi’s local businesses in each country it operates and Sanofi’s global functions, such as Research and Development or Manufacturing and Supply.
Although Sanofi has put in place the cybersecurity processes described above, Sanofi remains exposed to cybersecurity attacks and incidents and misuse or manipulation of any of its IT systems, which could have a material adverse affect on its business strategy, results of operations or financial condition (see “Item 3. Key Information — D. Risk Factors — Risks relating to our business — Breaches of data security, disruptions of information technology systems and cyber threats could result in financial, legal, business or reputational harm”).
Governance
Sanofi has appointed a Chief Information Security Officer who oversees Sanofi's information, cybersecurity, and technology security. Our current Chief Information Security Officer has been working for Sanofi in this capacity since 2014 and has seventeen years of experience in the cybersecurity industry, including eight years as the global head of cybersecurity at one of France’s largest telecommunications companies. The Chief Information Security Officer is informed about and monitors the prevention, detection, mitigation, and remediation of cybersecurity incidents through the cybersecurity operation center. He develops appropriate plans to mitigate such risks. Such plans are validated by the Chief Digital Officer and shared with the Executive Committee.
The Chief Information Security Officer belongs to the digital division and directly reports to the Chief Digital Officer, a member of the Executive Committee. In addition, the Chief Information Security Officer is a permanent member of the group Risk Committee and reports on the cybersecurity risk to such group Risk Committee, to the Audit Committee and to the Executive Committee regularly. The reporting covers various matters, such as the outcomes of audits on Sanofi’s information systems, the main incidents encountered over the preceding period, Sanofi’s digital transformation or the cybersecurity strategy and framework for the coming years.
The group Risk Committee, comprised of the managers of Sanofi’s global business units, consolidates the risks identified by the specific committees and targets the high priority risks Sanofi is facing. The group Risk Committee then allocates each risk to the relevant Executive Committee member (i.e. the cybersecurity risk is allocated to the Chief Digital Officer as the relevant member of the Executive Committee, who manages the mitigation of such risk with the Chief Information Security Officer) and reports regularly to the Audit Committee. Following this identification and allocation process, the group Risk Committee reports on a quarterly basis to the Executive Committee on the progress of the mitigation plans.
The Audit Committee controls that the cybersecurity risks are well managed and reports on such management to the Board of Directors. The Board of Directors is also informed of such risks, as well as other cybersecurity matters, through periodic reports from the Chief Digital Officer, the Head of the group Risk Committee, or the Chief Information Security Officer.
172 | SANOFI FORM 20-F 2023 | ||||
| PART III | ||
ITEM 17. Financial statements | ||
Part III
Item 17. Financial Statements
See Item 18.
Item 18. Financial Statements
Item 19. Exhibits
| 1.1. | |||||
| 1.2. | |||||
2.1. | The total amount of long-term debt securities authorized under any instrument does not exceed 10% of the total assets of the Company and its subsidiaries on a consolidated basis. We hereby agree to furnish to the SEC, upon its request, a copy of any instrument defining the rights of holders of long-term debt of the Company or of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed. | ||||
2.2. | |||||
8.1. | List of significant subsidiaries, see “Item 4. Information on the Company — C. Organizational Structure” of this annual report. | ||||
12.1. | |||||
12.2. | |||||
13.1. | |||||
13.2. | |||||
15.1. | |||||
15.2. | |||||
15.3. | |||||
97. | |||||
| 101.INS | XBRL Instance Document. | ||||
| 101.SCH | XBRL Taxonomy Extension Schema. | ||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase. | ||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase. | ||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase. | ||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase. | ||||
| 104.1 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | ||||
SANOFI FORM 20-F 2023 | 173 | ||||
Signatures | ||
Signatures
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Sanofi | |||||
| By: | /s/ PAUL HUDSON | ||||
| Name: | Paul Hudson | ||||
| Title: | Chief Executive Officer | ||||
Date: February 23, 2024.
174 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Report of Independent Registered Public Accounting Firms | ||
Report of Independent Registered Public Accounting Firms
To the Shareholders and the Board of Directors of Sanofi,
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Sanofi and its subsidiaries (together the “Company”) as of December 31, 2023, 2022, and 2021, the related consolidated income statements, statements of comprehensive income, statements of changes in equity and statements of cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, 2022, and 2021, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board and in conformity with International Financial Reporting Standards as endorsed by the European Union.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 23, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are public accounting firms registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
SANOFI FORM 20-F 2023 | 175 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Report of Independent Registered Public Accounting Firms | ||
Recoverable amount of other intangible assets
Description of the Matter | Other intangible assets amounted to €24,319 million at December 31, 2023. Management recognized a net loss of €932 million relating to impairment charges and reversals for the year ended December 31, 2023. As described in Notes B.6.1., D.4. and D.5. to the consolidated financial statements, other intangible assets not yet available for use are tested for impairment annually and whenever events or circumstances indicate that impairment might exist. Other intangible assets that generate separate cash flows and assets included in cash-generating units (CGUs) are assessed for impairment when events or changes in circumstances indicate that the asset or CGU may be impaired. Management estimates the recoverable amount of the asset and recognizes an impairment loss if the carrying amount of the asset exceeds its recoverable amount. The recoverable amount of the asset is the higher of its fair value less costs to sell or its value in use. Value in use is determined by management using estimated future cash flows generated by the asset or CGU which are discounted and prepared using the same methods as those used in the initial measurement of the assets and on the basis of medium-term strategic plans. Management cash flow projections include significant assumptions related to mid and long-term sales forecasts; perpetual growth or attrition rate, where applicable; discount rate; and probability of success of current research and development projects. The principal considerations for our determination that auditing the recoverable amount of other intangible assets is especially challenging, subjective, and required complex auditor judgment related to the significant judgments made by management when developing the significant assumptions utilized in the future cash flow projections as described above. | ||||
| How We Addressed the Matter in Our Audit | Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These audit procedures included obtaining an understanding of the process and assessing the design and testing the operating effectiveness of controls relating to management’s other intangible assets impairment assessment, including controls over the significant assumptions used in the impairment testing of the other intangible assets. These audit procedures also included, among others, evaluating the appropriateness of the discounted cash flow model; testing the completeness, accuracy, and relevance of underlying data used in the model; and evaluating the significant assumptions used by management as described above. Evaluating management’s assumptions involved evaluating whether the assumptions used by management were reasonable by considering the current and past performance of other intangible assets in comparison to management’s previous forecasts and current trends, the consistency of certain assumptions with external market and industry data, and whether these assumptions were consistent with evidence obtained in other areas of the audit such as internal company communications and presentations and external communications. We involved our professionals with specialized skills and knowledge to assist us notably in the assessment of the discount rate used by management. | ||||
Valuation of the provisions for rebates relating to Sanofi’s business in the United States – Medicaid, Medicare and Managed Care
Description of the Matter | As described in Notes B.13.1. and D.23. to the consolidated financial statements, products sold in the United States are covered by various Government and State programs (of which Medicaid and Medicare are the most significant) and are subject to commercial agreements with healthcare authorities and certain customers and distributors. Estimates of discounts and rebates incentives (hereinafter the “Rebates”) to be provided to customers under those arrangements are recognized as a reduction of gross sales in the period in which the underlying sales are recognized. Provisions for the Medicaid, Medicare and Managed Care Rebates amounted to €1,421 million, €1,099 million and €1,028 million, respectively, at December 31, 2023. The Rebates estimated by management are based on the nature and patient profile of the underlying product; the applicable regulations or the specific terms and conditions of contracts with governmental authorities, wholesalers and other customers; historical data relating to similar contracts; past experience and sales growth trends for the same or similar products; actual inventory levels in distribution channels, monitored by Sanofi using internal sales data and externally provided data; market trends including competition, pricing and demand. The principal considerations for our determination that auditing the provisions for Rebates relating to the Company’s business in the United States is especially challenging and required complex auditor judgment related to the significant judgment by management due to significant measurement uncertainty involved in developing these provisions. These provisions are estimated based on multiple factors as described above. | ||||
How We Addressed the Matter in Our Audit | Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These audit procedures included obtaining an understanding of the process and assessing the design and testing the operating effectiveness of controls relating to management’s estimates of the provisions for Rebates relating to the Company’s business in the United States, including controls over the assumptions used to estimate these Rebates. These procedures also included, among others, developing an independent estimate of the provisions for Rebates by utilizing third party data on inventory levels in distribution channels, volume, changes to price, the terms of the specific rebate programs, and the historical trend of actual rebate claims paid. The independent estimate was compared to the provisions recorded by the Company. Additionally, these procedures included testing actual rebate claims paid and evaluating the contractual terms of the Company’s rebate agreements. | ||||
176 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Report of Independent Registered Public Accounting Firms | ||
Provisions for product liability risks, litigation and other and contingent liabilities
| Description of the Matter | Provisions for product liability risks, litigation and other were recorded in an amount of €1,283 million at December 31, 2023. As described in Notes B.12., D.19.3. and D.22. to the consolidated financial statements, the Company records such provisions when an outflow of resources is probable and the amount of the outflow can be reliably estimated. The Company also discloses the contingent liabilities in circumstances where management is unable to make a reasonable estimate of the expected financial effect that will result from ultimate resolution of the proceeding, or a cash outflow is not probable. The pharmaceutical industry is highly regulated, which increases the inherent risk of litigation and arbitration. The Company is involved in litigation, arbitration and other legal proceedings. These proceedings are typically related to litigation concerning product liability claims, intellectual property rights, competition law and trade practices, as well as claims under warranties or indemnification arrangements relating to business divestments. The issues raised by these claims are highly complex and subject to substantial uncertainties; therefore, the probability of loss and an estimation of damages are difficult to ascertain. The principal considerations for our determination that auditing the provision for product liability risks, litigation and other, and auditing the contingent liabilities is especially challenging, subjective and required complex auditor judgment resulted from the determination that the measurement of the provisions can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions by management. There is inherent uncertainty related to these cases and in estimating the likelihood and outcome of the cases. | ||||
| How We Addressed the Matter in Our Audit | Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These audit procedures included obtaining an understanding of the process and assessing the design and testing the operating effectiveness of controls relating to management’s evaluation of the provisions for product liability risks, litigation and other, including controls over determining whether a loss is probable and whether the amount of loss can be reasonably estimated, as well as the need for and the level of financial statement disclosures. These procedures also included, among others, obtaining and evaluating the letters of audit inquiry with internal and external legal counsels, evaluating management’s assessment regarding whether an unfavorable outcome is reasonably possible or probable and reasonably estimable through the evaluation of the legal letters and summaries of the proceedings and lawsuit correspondence. We also evaluated the Company’s disclosures for contingent liabilities. | ||||
Uncertain tax positions
| Description of the Matter | As described in Notes B.22. and D.19.4. to the consolidated financial statements, the Company has recorded liabilities pertaining to uncertain tax positions of €1,595 million at December 31, 2023. The Company operates in multiple tax jurisdictions, carrying out potentially complex transactions that require management to make judgments and estimates as to the tax impact of those transactions. The positions adopted by the Company in tax matters are based on its interpretation of tax laws and regulations. Some of those positions may be subject to uncertainty. In such cases, the Company assesses the amount of the tax liability on the basis of the following assumptions: that its position will be examined by one or more tax authorities on the basis of all relevant information; that a technical assessment is carried out with reference to legislation, case law, regulations, and established practice; and that each position is assessed individually (or collectively where appropriate), with no offset or aggregation between positions. Those assumptions are assessed on the basis of facts and circumstances existing at the end of the reporting period. When an uncertain tax liability is regarded as probable, it is measured on the basis of the Company’s best estimate. The principal considerations for our determination that auditing uncertain tax positions is especially challenging, subjective and required complex auditor judgment related to the significant judgment by management when determining the liability for uncertain tax positions, including a high degree of estimation uncertainty of certain assumptions and interpretations of the tax laws and regulations underlying the positions. | ||||
| How We Addressed the Matter in Our Audit | Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These audit procedures included obtaining an understanding of the process and assessing the design and testing the operating effectiveness of controls relating to the identification and recognition of the liability for uncertain tax positions, management’s assessment and interpretation of tax laws and its evaluation of which tax positions may not be sustained upon audit and controls over measurement of the liability. These procedures also included, among others, testing the completeness and accuracy of the underlying data used in the calculation of the liability for uncertain tax positions and evaluating the assumptions used by management when determining its tax positions, the status of tax audits and investigations, and the potential impact of past claims. Our tax professionals assisted in evaluating management’s assessments by comparing the positions taken by management with tax regulations and past decisions from tax authorities and where applicable, evaluating opinions from the Company’s external tax advisors. We also evaluated the disclosures provided in the notes to the consolidated financial statements concerning uncertain tax positions. | ||||
| /s/ PricewaterhouseCoopers Audit | /s/ Ernst & Young et Autres | |||||||
SANOFI FORM 20-F 2023 | 177 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Report of Independent Registered Public Accounting Firms | ||
Report of Independent Registered Public Accounting Firms
To the Shareholders and the Board of Directors of Sanofi,
Opinion on Internal Control over Financial Reporting
We have audited Sanofi and its subsidiaries’ (together the “Company”) internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the “COSO criteria”). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on the COSO criteria.
As indicated in Report of Management on Internal Control Over Financial Reporting appearing under Item 15, management’s assessment and conclusion on the effectiveness of internal control over financial reporting did not include the internal controls of QRIB Intermediate Holdings, LLC and its affiliates, which are included in the 2023 consolidated financial statements of the Company and represented less than 1% of total assets as of December 31, 2023 and less than 1% of net sales for the year then ended. Our audit of internal control over financial reporting of the Company also did not include an evaluation of the internal control over financial reporting of QRIB Intermediate Holdings, LLC and its affiliates.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheets of the Company as of December 31, 2023, 2022, and 2021, the related consolidated income statements, statements of comprehensive income, statements of changes in equity and statements of cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”) and our report dated February 23, 2024 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Report of Management on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are public accounting firms registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| /s/ PricewaterhouseCoopers Audit | /s/ ERNST & YOUNG ET AUTRES | |||||||
Neuilly-sur-Seine and Paris-La Défense, France, February 23, 2024
178 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
2023 Consolidated financial statements
The financial statements are presented in accordance with International Financial Reporting Standards (IFRS).
SANOFI FORM 20-F 2023 | F-1 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated balance sheets - assets | ||
Consolidated balance sheets - assets
| (€ million) | Note | December 31, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||
| Property, plant and equipment | D.3.1. | |||||||||||||
| Right-of-use assets | D.3.2. | |||||||||||||
| Goodwill | D.4. | |||||||||||||
| Other intangible assets | D.4. | |||||||||||||
| Investments accounted for using the equity method | D.6. | |||||||||||||
| Other non-current assets | D.7. | |||||||||||||
| Non-current income tax assets | ||||||||||||||
| Deferred tax assets | D.14. | |||||||||||||
| Non-current assets | ||||||||||||||
| Inventories | D.9. | |||||||||||||
| Accounts receivable | D.10. | |||||||||||||
| Other current assets | D.11. | |||||||||||||
| Current income tax assets | ||||||||||||||
| Cash and cash equivalents | D.13. - D.17.1. | |||||||||||||
| Current assets | ||||||||||||||
| Assets held for sale or exchange | D.8. | |||||||||||||
| Total assets | ||||||||||||||
F-2 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated balance sheets - equity and liabilities | ||
Consolidated balance sheets – equity and liabilities
| (€ million) | Note | December 31, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||
| Equity attributable to equity holders of Sanofi | D.15. | |||||||||||||
| Equity attributable to non-controlling interests | D.16. | |||||||||||||
| Total equity | ||||||||||||||
| Long-term debt | D.17.1. | |||||||||||||
| Non-current lease liabilities | D.17.2. | |||||||||||||
| Non-current liabilities related to business combinations and to non-controlling interests | D.18. | |||||||||||||
| Non-current provisions and other non-current liabilities | D.19. | |||||||||||||
| Non-current income tax liabilities | D.19.4. | |||||||||||||
| Deferred tax liabilities | D.14. | |||||||||||||
| Non-current liabilities | ||||||||||||||
| Accounts payable | ||||||||||||||
| Current liabilities related to business combinations and to non-controlling interests | D.18. | |||||||||||||
| Current provisions and other current liabilities | D.19.5. | |||||||||||||
| Current income tax liabilities | ||||||||||||||
| Current lease liabilities | D.17.2. | |||||||||||||
| Short-term debt and current portion of long-term debt | D.17.1. | |||||||||||||
| Current liabilities | ||||||||||||||
| Liabilities related to assets held for sale or exchange | D.8. | |||||||||||||
| Total equity and liabilities | ||||||||||||||
SANOFI FORM 20-F 2023 | F-3 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated income statements | ||
Consolidated income statements
| (€ million) | Note | 2023 | 2022 | 2021 | ||||||||||
| Net sales | D.35.1. | |||||||||||||
| Other revenues | ||||||||||||||
| Cost of sales | ( | ( | ( | |||||||||||
| Gross profit | ||||||||||||||
| Research and development expenses | ( | ( | ( | |||||||||||
| Selling and general expenses | ( | ( | ( | |||||||||||
| Other operating income | D.25. | |||||||||||||
| Other operating expenses | D.26. | ( | ( | ( | ||||||||||
| Amortization of intangible assets | D.4. | ( | ( | ( | ||||||||||
| Impairment of intangible assets | D.5. | ( | ( | |||||||||||
| Fair value remeasurement of contingent consideration | D.12. - D.18. | ( | ( | |||||||||||
| Restructuring costs and similar items | D.27. | ( | ( | ( | ||||||||||
| Other gains and losses, and litigation | D.28. | ( | ( | ( | ||||||||||
| Operating income | ||||||||||||||
| Financial expenses | D.29. | ( | ( | ( | ||||||||||
| Financial income | D.29. | |||||||||||||
| Income before tax and investments accounted for using the equity method | D.35.1. | |||||||||||||
| Income tax expense | D.30. | ( | ( | ( | ||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | D.31. | ( | ||||||||||||
| Net income | ||||||||||||||
| Net income attributable to non-controlling interests | D.32. | |||||||||||||
| Net income attributable to equity holders of Sanofi | ||||||||||||||
Average number of shares outstanding (million) | D.15.9. | |||||||||||||
Average number of shares after dilution (million) | D.15.9. | |||||||||||||
•Basic earnings per share (€) | ||||||||||||||
•Diluted earnings per share (€) | ||||||||||||||
F-4 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated statements of comprehensive income | ||
Consolidated statements of comprehensive income
| (€ million) | Note | 2023 | 2022 | 2021 | ||||||||||
| Net income | ||||||||||||||
| Attributable to equity holders of Sanofi | ||||||||||||||
| Attributable to non-controlling interests | ||||||||||||||
| Other comprehensive income: | ||||||||||||||
•Actuarial gains/(losses) | D.15.7. | ( | ||||||||||||
•Change in fair value of equity instruments included in financial assets and financial liabilities | D.15.7. | |||||||||||||
•Tax effects | D.15.7. | ( | ( | ( | ||||||||||
| Sub-total: items not subsequently reclassifiable to profit or loss (A) | ( | |||||||||||||
•Change in fair value of debt instruments included in financial assets | D.15.7. | ( | ( | |||||||||||
•Change in fair value of cash flow hedges | D.15.7. | ( | ( | |||||||||||
•Change in currency translation differences | D.15.7. | ( | ||||||||||||
•Tax effects | D.15.7. | ( | ||||||||||||
| Sub-total: items subsequently reclassifiable to profit or loss (B) | ( | |||||||||||||
| Other comprehensive income for the period, net of taxes (A+B) | ( | |||||||||||||
| Comprehensive income | ||||||||||||||
| Attributable to equity holders of Sanofi | ||||||||||||||
| Attributable to non-controlling interests | ||||||||||||||
SANOFI FORM 20-F 2023 | F-5 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated statements of changes in equity | ||
Consolidated statements of changes in equity
| (€ million) | Share capital | Additional paid-in capital | Treasury shares | Reserves and retained earnings | Stock options and other share- based payments | Other comprehensive income | Attributable to equity holders of Sanofi | Attributable to non- controlling interests | Total equity | ||||||||||||||||||||
Balance at January 1, 2021(a) | ( | ( | |||||||||||||||||||||||||||
Other comprehensive income for the period | — | — | — | — | |||||||||||||||||||||||||
Net income for the period | — | — | — | — | — | ||||||||||||||||||||||||
Comprehensive income for the period | — | — | — | — | |||||||||||||||||||||||||
Dividend paid out of 2020 earnings (€ | — | — | — | ( | — | — | ( | — | ( | ||||||||||||||||||||
| Payment of dividends to non-controlling interests | — | — | — | — | — | — | ( | ( | |||||||||||||||||||||
Share repurchase program(b) | — | — | ( | — | — | — | ( | — | ( | ||||||||||||||||||||
| Share-based payment plans: | |||||||||||||||||||||||||||||
•Exercise of stock options(b) | — | — | — | — | — | — | |||||||||||||||||||||||
•Issuance of restricted shares and vesting of existing restricted shares(b)/(d) | ( | ( | — | — | — | ||||||||||||||||||||||||
•Employee share ownership plan(b) | — | — | — | — | — | ||||||||||||||||||||||||
•Value of services obtained from employees | — | — | — | — | — | — | |||||||||||||||||||||||
•Tax effects on share-based payments | — | — | — | — | — | — | |||||||||||||||||||||||
Other changes in non-controlling interests(e) | — | — | — | — | — | — | |||||||||||||||||||||||
Balance at December 31, 2021 | ( | ( | |||||||||||||||||||||||||||
| (€ million) | Share capital | Additional paid-in capital | Treasury shares | Reserves and retained earnings | Stock options and other share- based payments | Other comprehensive income | Attributable to equity holders of Sanofi | Attributable to non- controlling interests | Total equity | ||||||||||||||||||||
Balance at January 1, 2022(a) | ( | ( | |||||||||||||||||||||||||||
Other comprehensive income for the period | — | — | — | — | |||||||||||||||||||||||||
| Net income for the period | — | — | — | — | — | ||||||||||||||||||||||||
| Comprehensive income for the period | — | — | — | — | |||||||||||||||||||||||||
Dividend paid out of 2021 earnings (€ | — | — | — | ( | — | — | ( | — | ( | ||||||||||||||||||||
Effect of the distribution of an exceptional supplementary dividend of | — | — | — | ( | — | — | ( | — | ( | ||||||||||||||||||||
| Payment of dividends to non-controlling interests | — | — | — | — | — | — | ( | ( | |||||||||||||||||||||
Share repurchase program(b) | — | — | ( | — | — | — | ( | — | ( | ||||||||||||||||||||
Reduction in share capital(b) | ( | ( | — | — | — | — | |||||||||||||||||||||||
| Share-based payment plans: | |||||||||||||||||||||||||||||
•Exercise of stock options(b) | — | — | — | — | — | ||||||||||||||||||||||||
•Issuance of restricted shares and vesting of existing restricted shares(b)/(d) | ( | ( | — | — | — | ||||||||||||||||||||||||
•Employee share ownership plan(b) | — | — | — | — | — | ||||||||||||||||||||||||
•Value of services obtained from employees | — | — | — | — | — | — | |||||||||||||||||||||||
•Tax effects on share-based payments | — | — | — | — | — | — | |||||||||||||||||||||||
Other movements | — | — | — | ( | — | — | ( | — | ( | ||||||||||||||||||||
Balance at December 31, 2022 | ( | ||||||||||||||||||||||||||||
F-6 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated statements of changes in equity | ||
| (€ million) | Share capital | Additional paid-in capital | Treasury shares | Reserves and retained earnings | Stock options and other share- based payments | Other comprehensive income | Attributable to equity holders of Sanofi | Attributable to non- controlling interests | Total equity | ||||||||||||||||||||
Balance at January 1, 2023 | ( | ||||||||||||||||||||||||||||
Other comprehensive income for the period | — | — | — | ( | — | ( | ( | ( | ( | ||||||||||||||||||||
| Net income for the period | — | — | — | — | — | ||||||||||||||||||||||||
| Comprehensive income for the period | — | — | — | — | ( | ||||||||||||||||||||||||
Dividend paid out of 2022 earnings (€ | — | — | — | ( | — | — | ( | — | ( | ||||||||||||||||||||
| Payment of dividends to non-controlling interests | — | — | — | — | — | — | ( | ( | |||||||||||||||||||||
Share repurchase program(b) | — | — | ( | — | — | — | ( | — | ( | ||||||||||||||||||||
| Share-based payment plans: | |||||||||||||||||||||||||||||
•Exercise of stock options(b) | — | — | — | — | — | ||||||||||||||||||||||||
•Issuance of restricted shares and vesting of existing restricted shares(b)/(d) | ( | ( | — | — | — | ||||||||||||||||||||||||
•Employee share ownership plan(b) | — | — | — | — | — | ||||||||||||||||||||||||
•Value of services obtained from employees | — | — | — | — | — | — | |||||||||||||||||||||||
•Tax effects on share-based payments | — | — | — | — | — | — | |||||||||||||||||||||||
Other changes arising from issuance of restricted shares(c) | — | — | — | — | — | — | |||||||||||||||||||||||
Other changes in non-controlling interests (e) | — | — | — | — | — | ( | ( | ||||||||||||||||||||||
Balance at December 31, 2023 | ( | ( | |||||||||||||||||||||||||||
(a) Includes the impacts of the IFRIC final agenda decisions of March 2021 on the costs of configuring or customising application software used in a Software as a Service (SaaS) arrangement and of April 2021 on the attribution of benefits to periods of service, as described in Note A.2.1. to the consolidated financial statements for the year ended December 31, 2021.
(b) See Notes D.15.1., D.15.3., D.15.4. and D.15.5.
(c) This line comprises the impact of the issuance of restricted shares to former employees of EUROAPI subsequent to the date on which Sanofi lost control of EUROAPI.
(d) This line includes the use of existing shares to fulfill vested rights under restricted share plans.
(e) This line mainly comprises changes in non-controlling interests arising from divestments and acquisitions.
(f) This amount includes the valuation of the shares distributed as a dividend in kind, at a price of €14.58 per share, as of May 10, 2022 (see note D.2.1.).
SANOFI FORM 20-F 2023 | F-7 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated statements of cash flows | ||
Consolidated statements of cash flows
(€ million) | Note | 2023 | 2022 | 2021 | ||||||||||
| Net income attributable to equity holders of Sanofi | ||||||||||||||
Non-controlling interests | D.32. | |||||||||||||
| Share of undistributed earnings from investments accounted for using the equity method | ( | ( | ||||||||||||
| Depreciation, amortization and impairment of property, plant and equipment, right-of-use assets and intangible assets | ||||||||||||||
Gains and losses on disposals of non-current assets, net of tax(a) | ( | ( | ( | |||||||||||
| Net change in deferred taxes | ( | ( | ( | |||||||||||
Net change in non-current provisions and other non-current liabilities(b) | ( | ( | ||||||||||||
| Cost of employee benefits (stock options and other share-based payments) | D.15.2. - D.15.3. - D.15.8. | |||||||||||||
| Impact of the workdown of acquired inventories remeasured at fair value | D.35.1. | |||||||||||||
Other profit or loss items with no cash effect on cash flows generated by operating activities(c) | ( | |||||||||||||
| Operating cash flow before changes in working capital | ||||||||||||||
| (Increase)/decrease in inventories | ( | ( | ( | |||||||||||
| (Increase)/decrease in accounts receivable | ( | ( | ||||||||||||
| Increase/(decrease) in accounts payable | ||||||||||||||
| Net change in other current assets and other current liabilities | ||||||||||||||
Net cash provided by/(used in) operating activities(d) | ||||||||||||||
| Acquisitions of property, plant and equipment and intangible assets | D.3. - D.4. | ( | ( | ( | ||||||||||
Acquisitions of consolidated undertakings and investments accounted for using the equity method(e) | D.1. - D.18. | ( | ( | ( | ||||||||||
| Acquisitions of other equity investments | D.7. | ( | ( | ( | ||||||||||
Proceeds from disposals of property, plant and equipment, intangible assets and other non-current assets, net of tax(f) | ||||||||||||||
Disposal of consolidated undertakings and investments accounted for using the equity method, net of tax(g) | ||||||||||||||
| Net change in other non-current assets | ( | ( | ( | |||||||||||
| Net cash provided by/(used in) investing activities | ( | ( | ( | |||||||||||
| Issuance of Sanofi shares | D.15.1. | |||||||||||||
| Dividends paid: | ||||||||||||||
•to shareholders of Sanofi | ( | ( | ( | |||||||||||
•to non-controlling interests | ( | ( | ( | |||||||||||
| Payments received/(made) on changes of ownership interest in a subsidiary without loss of control | ( | |||||||||||||
| Additional long-term debt contracted | D.17.1. | |||||||||||||
| Repayments of long-term debt | D.17.1. | ( | ( | ( | ||||||||||
| Repayments of lease liabilities | ( | ( | ( | |||||||||||
Net change in short-term debt and other financial instruments(h) | ( | |||||||||||||
| Acquisitions of treasury shares | D.15.4. | ( | ( | ( | ||||||||||
| Net cash provided by/(used in) financing activities | ( | ( | ( | |||||||||||
| Impact of exchange rates on cash and cash equivalents | ( | |||||||||||||
| Net change in cash and cash equivalents | ( | ( | ||||||||||||
| Cash and cash equivalents, beginning of period | ||||||||||||||
| Cash and cash equivalents, end of period | D.13. | |||||||||||||
(a) Includes non-current financial assets.
(b) This line item includes contributions paid to pension funds (see Note D.19.1.).
(c) This line item mainly comprises unrealized foreign exchange gains and losses arising on the remeasurement of monetary items in non-functional currencies and on instruments used to hedge such items.
F-8 | SANOFI FORM 20-F 2023 | ||||
2023 CONSOLIDATED FINANCIAL STATEMENTS | ||
| Consolidated statements of cash flows | ||
(d) Including:
| 2023 | 2022 | 2021 | |||||||||
•Income tax paid | ( | ( | ( | ||||||||
•Interest paid | ( | ( | ( | ||||||||
•Interest received | |||||||||||
•Dividends received from non-consolidated entities | |||||||||||
(e) This line item includes payments made in respect of contingent consideration identified and recognized as a liability in business combinations. For 2023, it includes the net cash outflow on the acquisitions of Provention Bio and QRIB (see Note D.1.). For 2022, it includes the net cash outflow on the acquisition of Amunix (see Note D.2.1.). For 2021, it includes the net cash outflows on the acquisitions of Kymab, Kiadis, Tidal, Translate Bio, Kadmon and Origimm (see Note D.2.2.).
(f) For 2023, 2022 and 2021, this line item mainly comprises disposals of assets and activities related to portfolio streamlining and disposals of equity and debt instruments.
(g) For 2022, this line item includes the net cash inflows (before taxes) of €101 million on the divestment of EUROAPI (see Note D.2.1.).
(h) For 2023, this line item includes €946 million related to the US commercial paper program. For 2023, 2022 and 2021, it also includes realized foreign exchange differences on (i) cash and cash equivalents in non-functional currencies (primarily the US dollar) and (ii) derivative instruments used to manage such cash and cash equivalents.
SANOFI FORM 20-F 2023 | F-9 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Notes to the Consolidated Financial Statements
Introduction
Sanofi, together with its subsidiaries (collectively “Sanofi”, “the Group” or “the Company”), is a global healthcare leader engaged in the research, development and marketing of therapeutic solutions focused on patient needs.
Sanofi is listed in Paris (Euronext: SAN) and New York (Nasdaq: SNY).
The consolidated financial statements for the year ended December 31, 2023, and the notes thereto, were signed off by the Sanofi Board of Directors on January 31, 2024.
A/ Basis of preparation
A.1. International financial reporting standards (IFRS)
The consolidated financial statements cover the twelve-month periods ended December 31, 2023, 2022 and 2021.
In accordance with Regulation No. 1606/2002 of the European Parliament and Council of July 19, 2002 on the application of international accounting standards, Sanofi has presented its consolidated financial statements in accordance with IFRS since January 1, 2005. The term “IFRS” refers collectively to international accounting and financial reporting standards (IASs and IFRSs) and to interpretations of the interpretations committees (SIC and IFRIC) with mandatory application as of December 31, 2023.
The consolidated financial statements of Sanofi as of December 31, 2023 have been prepared in compliance with IFRS as issued by the International Accounting Standards Board (IASB) and with IFRS as endorsed by the European Union as of December 31, 2023.
IFRS as endorsed by the European Union as of December 31, 2023 are available under the heading “IFRS Financial Statements” via the following web link:
https://www.efrag.org/Endorsement
The consolidated financial statements have been prepared in accordance with the IFRS general principles of fair presentation, going concern, accrual basis of accounting, consistency of presentation, materiality, and aggregation.
A.2. New standards, amendments and interpretations
A.2.1. New standards applicable from January 1, 2023
The following amendments are applicable from January 1, 2023, and have had no material impact: “Disclosure of Accounting Policies” (amendment to IAS 1); “Definition of Accounting Estimates” (amendment to IAS 8); and “Deferred Tax Assets and Liabilities Arising from a Single Transaction” (amendment to IAS 12).
On May 23, 2023, the IASB issued “International Tax Reform—Pillar Two Model Rules”, an immediately applicable amendment to IAS 12 that was endorsed on November 9, 2023. The amendment relates to the effects of the global minimum corporate income tax rate of 15% that will come into force in 2024, in accordance with the model framework of OECD Pillar Two.
The French Finance Bill for 2024 imposes a global minimum corporate income tax rate of 15% and introduces (i) a supplementary multinational tax and (ii) a supplementary national tax for annual accounting periods commencing on or after December 31, 2023. Given that the legislation had not come into force as of December 31, 2023, Sanofi is not liable for any current taxes in that respect. In addition, Sanofi has not recognized any deferred taxes associated with the minimum tax, in accordance with the temporary exemption available under the May 2023 amendment to IAS 12. At this stage, a material impact is expected in respect of Sanofi's operations in France and Singapore, because Sanofi’s average effective tax rate in those countries was less than 15 % at the end of 2023. Other things being equal, applying the Pillar 2 rules in 2023 would have led to an increase of approximately 1.5 percentage points in the effective tax rate based on the Income before tax and investments accounted for using the equity method.
IFRS 17 (Insurance Contracts), issued on May 18, 2017 and applicable on or after January 1, 2023, does not apply to the Sanofi consolidated financial statements because the insurance activities carried on by Sanofi’s captive insurance companies are internal within the Sanofi group (the sole policyholders being subsidiaries of Sanofi), and hence are eliminated on consolidation.
A.2.2. New pronouncements issued by the IASB and applicable from 2024 or later
This note describes standards, amendments and interpretations issued by the IASB that will have mandatory application in 2024 or subsequent years, and Sanofi’s position regarding future application.
On September 22, 2022, the IASB issued an amendment to IFRS 16 (Leases), relating to lease liabilities in a sale-and-leaseback arrangement, which is applicable at the earliest from January 1, 2024; it will not have a material impact on the Sanofi financial statements, and Sanofi has not early adopted it.
F-10 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
On January 23, 2020, the IASB issued “Classification of Liabilities as Current or Non-current”, an amendment to IAS 1, and then on October 31, 2022 issued “Non-current Liabilities with Covenants”, a further amendment to IAS 1. The amendments are applicable at the earliest from January 1, 2024; they will not have a material impact on the Sanofi financial statements, and Sanofi has not early adopted them.
On May 25, 2023, the IASB issued "Supplier Finance Arrangements", an amendment to IAS 7 and IFRS 7, which is applicable at the earliest from January 1, 2024 (subject to endorsement by the European Union) and which relates to disclosures of information about such arrangements. The amendment does not have a material impact on the Sanofi financial statements, and Sanofi will not early adopt it.
On August 15, 2023, the IASB issued "Lack of Exchangeability", an amendment to IAS 21 (The Effect of Changes in Foreign Exchange Rates), relating to how to determine the exchange rate when a currency is not exchangeable. The amendment is applicable at the earliest from January 1, 2025 (subject to endorsement by the European Union); it will not have a material impact on the Sanofi financial statements, and Sanofi will not early adopt it.
A.3. Use of estimates and judgments
The preparation of financial statements requires management to make reasonable estimates and assumptions based on information available at the date of the finalization of the financial statements. Those estimates and assumptions may affect the reported amounts of assets, liabilities, revenues and expenses in the financial statements, and disclosures of contingent assets and contingent liabilities as of the date of the review of the financial statements. Examples of estimates and assumptions include:
•amounts deducted from sales for projected sales returns, chargeback incentives, rebates and price reductions (see Notes B.13. and D.23.);
•impairment of property, plant and equipment and intangible assets (see Notes B.6. and D.5.);
•the valuation of goodwill and the valuation and estimated useful life of acquired intangible assets (see Notes B.3.2., B.4., D.4. and D.5.);
•the measurement of contingent consideration receivable in connection with asset divestments (see Notes B.8.5. and D.12.) and of contingent consideration payable (see Notes B.3. and D.18.);
•the measurement of financial assets and liabilities at amortized cost (see Note B.8.5.);
•the amount of post-employment benefit obligations (see Notes B.23. and D.19.1.);
•the amount of liabilities or provisions for restructuring, litigation, tax risks relating to corporate income taxes, and environmental risks (see Notes B.12., B.19., B.20., D.19. and D.22.); and
•the amount of deferred tax assets resulting from tax losses available for carry-forward and deductible temporary differences (see Notes B.22. and D.14.).
Actual results could differ from these estimates.
In preparing the consolidated financial statements, Sanofi has also taken account of risks related to the effects of climate change and energy transition.
As part of its Planet Care program, Sanofi has committed to move towards carbon neutrality by 2030 and net zero emissions by 2045 for its Scope 1, 2 and 3 emissions. That involves:
•aiming for a 55 % reduction in greenhouse gas (GHG) emissions from Sanofi’s own activities (Scopes 1 & 2) and a 30 % reduction in Scope 3 GHG emissions by 2030 (versus a 2019 baseline), and a 90 % reduction in GHG emissions (all scopes) by 2045. These objectives have been validated by the Science Based Target initiative (STBi);
•supplying all our sites with 100 % renewably-sourced electricity by 2030;
•promoting an eco-friendly vehicle fleet by 2030; and
•engaging the Sanofi supply chain in reducing Scope 3 emissions.
The analysis of climate-related physical and transition risks facing Sanofi was updated in 2023 on the basis of three global warming scenarios out to 2030 and 2050. A number of assumptions – on issues such as carbon costs, natural disasters, water stress, raw material scarcity and logistics disruption – were built into this analysis, which also takes account of certain capital expenditures on mitigations derived from the Planet Care roadmap.
In preparing the consolidated financial statements, that analysis was taken into account as follows:
•the value of intangible assets and property, plant and equipment was subject to impairment testing conducted at CGU level, as described in Note D.5. Certain climate-related assumptions, such as the evolution of energy costs, transitioning to sustainable agriculture, and waste management, are already built into the forecast used for impairment testing purposes. For those assumptions not yet built into budgets, sensitivity analyses can be performed as needed;
•the periodic reviews conducted on the useful lives of property, plant and equipment take account of environmental regulatory constraints, including not only GHG emissions but also physical risks;
•environmental risks are covered by provisions on the basis described in Note D.19.3.; and
•the credit facilities available to Sanofi as of December 31, 2023 incorporate performance objectives, including objectives related to cutting Sanofi’s carbon footprint, which could reduce the cost of debt if they are attained (see Note D.17.).
SANOFI FORM 20-F 2023 | F-11 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
It is important to bear in mind that estimating climate change related risks involves an element of unpredictability. Uncertainties may arise from factors such as changes in government policy, rapid technological change, and varied responses from stakeholders. That high level of uncertainty adds complexity to assessment of the potential impacts on our operations, and to how those impacts are reflected in our budgets. Actual impacts on Sanofi’s profits and financial position could therefore differ from initial estimates.
Finally, in line with its environmental protection objectives, Sanofi has initiated projects to build eco-design into its products so as to limit their environmental impacts over their entire life cycle. Those projects will require Sanofi to redefine all of its production methods, and as such have also been built into definitions of the useful lives of Sanofi production facilities.
A.4. Hyperinflation
In 2023, Sanofi continued to account for subsidiaries based in Venezuela using the full consolidation method, on the basis that the criteria for control as specified in IFRS 10 (Consolidated Financial Statements) are still met. The contribution of the Venezuelan subsidiaries to the consolidated financial statements is immaterial.
In Argentina, the cumulative rate of inflation over the last three years is in excess of 100 %, based on a combination of indices used to measure inflation in that country. Consequently, Sanofi has since July 1, 2018 treated Argentina as a hyperinflationary economy and has applied IAS 29. The impact of the resulting restatements is immaterial at Sanofi group level.
In Turkey, the cumulative rate of inflation over the last three years is in excess of 100 % based on a combination of indices used to measure inflation in that country. Consequently, Sanofi has since January 1, 2022 treated Turkey as a hyperinflationary economy and has applied IAS 29. The impact of the resulting restatements is immaterial at Sanofi group level.
A.5. Agreements relating to the recombinant COVID-19 vaccine candidate developed by Sanofi in collaboration with GSK
On February 18, 2020, Sanofi and the US Department of Health and Human Services extended their research and development partnership to leverage Sanofi’s previous development work on a SARS vaccine to attempt to unlock a fast path forward for developing a COVID-19 vaccine. Under the terms of the collaboration, the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response within the US Department of Health and Human Services, is helping to fund the research and development undertaken by Sanofi.
On April 14, 2020, Sanofi and GlaxoSmithKline (GSK) entered into a collaboration agreement to develop a recombinant COVID-19 vaccine candidate, with Sanofi contributing its S‑protein COVID-19 antigen (based on recombinant DNA technology) and GSK contributing its pandemic adjuvant technology. Sanofi is leading clinical development and the registration process for the vaccine.
On July 31, 2020, the recombinant COVID-19 vaccine candidate developed by Sanofi in collaboration with GSK was selected by the US government’s Operation Warp Speed (OWS) program. Under the OWS, the US government is providing funds to support further development of the vaccine, including clinical trials and scaling-up of manufacturing capacity. Initially, the agreement also provided for the supply of 100 million doses of the vaccine. In light of the evolving context of the pandemic (including variants of the virus) and the availability of vaccines on the market, the parties decided to review the initial supply contract. At the end of 2023, the agreement was amended in respect of the supply clause, confirming that Sanofi had fulfilled its contractual obligations and setting the amount of compensation paid to Sanofi. On the basis of that signed amendment, Sanofi recognized an amount of €411 million within the line item Other revenues; that amount was paid to Sanofi in December 2023.
Sanofi has recognized the funding received from the US government as a deduction from the development expenses incurred, in accordance with IAS 20 (Accounting for Government Grants and Disclosure of Government Assistance).
The amount of government aid received from the US federal government and BARDA and recognized as a deduction from development expenses and other operating expenses was €59 million in 2023, compared with €265 million in 2022 and €147 million in 2021.
In September 2020, Sanofi and GSK signed pre-order contracts with the Canadian and UK governments and with the European Union for doses of the vaccine candidate. During 2021, Sanofi and GSK contractualized with the Canadian and UK governments and with the European Union on the number of doses ordered.
On December 15, 2021, Sanofi and GSK announced positive preliminary data on their COVID-19 booster vaccine candidate and indicated that their Phase 3 trial was to continue, based on recommendations from an independent monitoring board.
On November 10, 2022, in line with the positive opinion issued by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency, the European Commission approved VIDPREVTYN Beta vaccine as booster for the prevention of COVID-19 in adults aged 18 years and older. Designed to provide broad protection against multiple variants, this protein-based COVID-19 booster vaccine is based on the Beta variant antigen and includes GSK’s pandemic adjuvant. VIDPREVTYN Beta is indicated as a booster for active immunization against SARS-CoV-2 in adults who have previously received an mRNA or adenoviral COVID-19 vaccine.
On December 21, 2022, following the European Commission approval, the Medicines and Healthcare Products Regulatory Agency (MHRA) approved VIDPREVTYN Beta vaccine for the prevention of COVID-19 in adults aged 18 and over within the UK.
F-12 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
In accordance with IFRS 15 (see Note B.13.1.), Sanofi recognizes revenue when control over the product is transferred to the customer (for vaccines, transfer of control is determined by reference to the terms of release and acceptance of batches of vaccine). Payments received subsequent to signature of vaccine pre-order contracts relating to doses not yet delivered are customer contract liabilities (i.e. an obligation for the entity to supply goods to a customer, for which consideration has been received from the customer). They are presented within “Customer contract liabilities” in the balance sheet (see Note D.19.5.), and within “Net change in other current assets and other current liabilities” in the statement of cash flows.
The pre-order contracts for Canada, the United Kingdom and the European Union expired in 2023. The customer contract liabilities, which amounted to €269 million as of December 31, 2022 and €319 million as of December 31, 2021 (see Note D.19.5., “Current provisions and other current liabilities”) were released to profit or loss in 2023, including an amount of €94 million classified in Other revenue in respect of doses which there was no longer an obligation to deliver as of December 31, 2023.
B/ Summary of significant accounting policies
B.1. Basis of consolidation
In accordance with IFRS 10 (Consolidated Financial Statements), the consolidated financial statements of Sanofi include the financial statements of entities that Sanofi controls directly or indirectly, regardless of the level of the equity interest in those entities. An entity is controlled when Sanofi has power over the entity, exposure or rights to variable returns from its involvement with the entity, and the ability to affect those returns through its power over the entity. In determining whether control exists, potential voting rights must be taken into account if those rights are substantive, in other words they can be exercised on a timely basis when decisions about the relevant activities of the entity are to be taken.
Entities consolidated by Sanofi are referred to as “subsidiaries”. Entities that Sanofi controls by means other than voting rights are referred to as “consolidated structured entities”.
In accordance with IFRS 11 (Joint Arrangements), Sanofi classifies its joint arrangements (i.e. arrangements in which Sanofi exercises joint control with one or more other parties) either as a joint operation (in which case, Sanofi recognizes the assets and liabilities of the operation in proportion to its rights and obligations relating to those assets and liabilities) or as a joint venture.
Sanofi exercises joint control over a joint arrangement when decisions relating to the relevant activities of the arrangement require the unanimous consent of Sanofi and the other parties with whom control is shared.
Sanofi exercises significant influence over an entity when it has the power to participate in the financial and operating policy decisions of that entity, but does not have the power to exercise control or joint control over those policies.
In accordance with IAS 28 (Investments in Associates and Joint Ventures), the equity method is used to account for joint ventures (i.e. entities over which Sanofi exercises joint control) and for associates (i.e. entities over which Sanofi exercises significant influence).
Under the equity method, the investment is initially recognized at cost, and subsequently adjusted to reflect changes in the net assets of the associate or joint venture. IAS 28 does not specify the treatment to be adopted on first-time application of the equity method to an investee following a step acquisition. Consequently, by reference to paragraph 10 of IAS 28, Sanofi has opted to apply the cost method, whereby the carrying amount of the investment represents the sum of the historical cost amounts for each step in the acquisition. As of the date on which the equity method is first applied, goodwill (which is included in the carrying amount of the investment) is determined for each acquisition step. The same applies to subsequent increases in the percentage interest in the equity-accounted investment.
When the criteria of IFRS 5 are met, Sanofi recognizes the equity interest within the balance sheet line item Assets held for sale or exchange. The equity method is not applied to equity interests that are classified as held-for-sale assets.
Transactions between consolidated companies are eliminated, as are intragroup profits.
A list of the principal companies included in the consolidation in 2023 is presented in Note F.
B.2. Foreign currency translation
B.2.1. Accounting for foreign currency transactions in the financial statements of consolidated entities
Non-current assets (other than receivables) and inventories acquired in foreign currencies are translated into the functional currency using the exchange rate prevailing at the acquisition date.
Monetary assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the end of the reporting period. The gains and losses resulting from foreign currency translation are recorded in the income statement. However, foreign exchange gains and losses arising from the translation of advances between consolidated subsidiaries for which settlement is neither planned nor likely to occur in the foreseeable future are recognized in equity, in the line item Change in currency translation differences.
SANOFI FORM 20-F 2023 | F-13 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.2.2. Foreign currency translation of the financial statements of foreign entities
Sanofi presents its consolidated financial statements in euros (€). In accordance with IAS 21 (The Effects of Changes in Foreign Exchange Rates), each subsidiary accounts for its transactions in the currency that is most representative of its economic environment (the functional currency).
All assets and liabilities are translated into euros using the exchange rate of the subsidiary’s functional currency prevailing at the end of the reporting period. Income statements are translated using a weighted average exchange rate for the period, except in the case of foreign subsidiaries in a hyperinflationary economy. The resulting currency translation difference is recognized as a separate component of equity in the consolidated statement of comprehensive income, and is recognized in the income statement only when the subsidiary is sold or is wholly or partially liquidated.
B.3. Business combinations and transactions with non-controlling interests
B.3.1. Accounting for business combinations, transactions with non-controlling interests and loss of control
Business combinations are accounted for in accordance with IFRS 3 (Business Combinations) and IFRS 10 (Consolidated Financial Statements).
Business combinations are accounted for using the acquisition method. Under this method, the acquiree’s identifiable assets and liabilities that satisfy the recognition criteria of IFRS 3 (Business Combinations) are measured initially at their fair values at the date of acquisition, except for (i) non-current assets classified as held for sale (which are measured at fair value less costs to sell) and (ii) assets and liabilities that fall within the scope of IAS 12 (Income Taxes) and IAS 19 (Employee Benefits). Restructuring liabilities are recognized as a liability of the acquiree only if the acquiree has an obligation as of the acquisition date to carry out the restructuring.
The principal accounting rules applicable to business combinations and transactions with non-controlling interests include:
•acquisition-related costs are recognized as an expense, as a component of Operating income;
•contingent consideration is recognized in equity if the contingent payment is settled by delivery of a fixed number of the acquirer’s equity instruments; otherwise, it is recognized in liabilities related to business combinations. Contingent consideration is recognized at fair value at the acquisition date irrespective of the probability of payment. If the contingent consideration was originally recognized as a financial liability, subsequent adjustments to the liability are recognized in profit or loss in the line item Fair value remeasurement of contingent consideration, unless the adjustment is made within the 12 months following the acquisition date and relates to facts and circumstances existing as of that date;
•goodwill may be calculated on the basis of either (i) the entire fair value of the acquiree, or (ii) a share of the fair value of the acquiree proportionate to the interest acquired. This option is elected for each acquisition individually.
Purchase price allocations are performed under the responsibility of management, with assistance from an independent valuer in the case of major acquisitions. IFRS 3 does not specify an accounting treatment for contingent consideration arising from a business combination made by an entity prior to the acquisition of control in that entity and carried as a liability in the acquired entity’s balance sheet. The accounting treatment applied by Sanofi to such a liability is to measure it at fair value as of the acquisition date and to report it in the line item Liabilities related to business combinations and to non-controlling interests, with subsequent remeasurements recognized in profit or loss. This treatment is consistent with the accounting applied to contingent consideration in the books of the acquirer.
Finally, management may where it deems fit elect to apply the optional test to identify concentration of fair value permitted under IFRS 3 in order to determine whether a transaction is a business combination within the meaning of IFRS 3, or merely the acquisition of an asset or of a group of similar assets.
B.3.2. Goodwill
The excess of the cost of an acquisition over Sanofi’s interest in the fair value of the identifiable assets and liabilities of the acquiree is recognized as goodwill at the date of the business combination. Goodwill arising on the acquisition of subsidiaries is shown in a separate balance sheet line item, whereas goodwill arising on the acquisition of investments accounted for using the equity method is recorded in Investments accounted for using the equity method.
Goodwill arising on foreign operations is expressed in the functional currency of the country concerned and translated into euros using the exchange rate prevailing at the end of the reporting period.
In accordance with IAS 36 (Impairment of Assets), goodwill is carried at cost less accumulated impairment (see Note B.6.).
Goodwill is tested for impairment annually and whenever events or circumstances indicate that impairment might exist. Such events or circumstances include significant changes more likely than not to have an other-than-temporary impact on the substance of the original investment.
F-14 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.4. Other intangible assets
Other intangible assets are initially measured at acquisition cost or production cost, including any directly attributable costs of preparing the asset for its intended use, or (in the case of assets acquired in a business combination) at fair value as of the date of the business combination. Intangible assets are amortized on a straight line basis over their useful lives.
The useful lives of other intangible assets are reviewed at the end of each reporting period. The effect of any adjustment to useful lives is recognized prospectively as a change in accounting estimate.
Amortization of other intangible assets is recognized in the income statement within Amortization of intangible assets except for amortization charged against (i) acquired or internally-developed software and (ii) other rights of an industrial or operational nature, which is recognized in the relevant classification of expense by function.
Sanofi does not own any intangible assets with an indefinite useful life, other than goodwill.
Intangible assets (other than goodwill) are carried at cost less accumulated amortization and accumulated impairment, if any, in accordance with IAS 36 (see Note B.6.).
B.4.1. Research and development not acquired in a business combination
Internally generated research and development
Under IAS 38, research expenses are recognized in profit or loss when incurred.
Internally generated development expenses are recognized as an intangible asset if, and only if, all the following six criteria can be demonstrated: (a) the technical feasibility of completing the development project; (b) Sanofi’s intention to complete the project; (c) Sanofi’s ability to use the project; (d) the probability that the project will generate future economic benefits; (e) the availability of adequate technical, financial and other resources to complete the project; and (f) the ability to measure the development expenditure reliably.
Due to the risks and uncertainties relating to regulatory approval and to the research and development process, the six criteria for capitalization are usually considered not to have been met until the product has obtained marketing approval from the regulatory authorities. Consequently, internally generated development expenses arising before marketing approval has been obtained, mainly the cost of clinical trials, are generally expensed as incurred within Research and development expenses.
Some industrial development expenses (such as those incurred in developing a second-generation synthesis process) are incurred after marketing approval has been obtained, in order to improve the industrial process for an active ingredient. To the extent that the six IAS 38 criteria are considered as having been met, such expenses are recognized as an asset in the balance sheet within Other intangible assets as incurred. Similarly, some clinical trials, for example those undertaken to obtain a geographical extension for a molecule that has already obtained marketing approval in a major market, may in certain circumstances meet the six capitalization criteria under IAS 38, in which case the related expenses are recognized as an asset in the balance sheet within Other intangible assets.
Separately acquired research and development
Payments for separately acquired research and development are capitalized within Other intangible assets provided that they meet the definition of an intangible asset: a resource that is (i) controlled by Sanofi, (ii) expected to provide future economic benefits for Sanofi, and (iii) identifiable (i.e. it is either separable or arises from contractual or legal rights). Under paragraph 25 of IAS 38, the first condition for capitalization (the probability that the expected future economic benefits from the asset will flow to the entity) is considered to be satisfied for separately acquired research and development. Consequently, upfront and milestone payments to third parties related to pharmaceutical products for which marketing approval has not yet been obtained are recognized as intangible assets, and amortized on a straight line basis over their useful lives beginning when marketing approval is obtained.
Payments under research and development arrangements relating to access to technology or to databases, and payments made to purchase generics dossiers, are also capitalized, and amortized over the useful life of the intangible asset.
Subcontracting arrangements, payments for research and development services, and continuous payments under research and development collaborations which are unrelated to the outcome of that collaboration, are expensed over the service term.
B.4.2. Other intangible assets not acquired in a business combination
Licenses other than those related to pharmaceutical products and research projects, in particular software licenses, are capitalized at acquisition cost, including any directly attributable cost of preparing the software for its intended use. Software licenses are amortized on a straight line basis over their useful lives for Sanofi ( to five years ).
Internally generated costs incurred to develop or upgrade software are capitalized if the IAS 38 recognition criteria are satisfied, and amortized on a straight line basis over the useful life of the software from the date on which the software is ready for use.
SANOFI FORM 20-F 2023 | F-15 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.4.3. Other intangible assets acquired in a business combination
Other intangible assets acquired in a business combination (in-process research and development, technology platforms, and currently marketed products) that are reliably measurable are identified separately from goodwill, measured at fair value, and capitalized within Other intangible assets in accordance with IFRS 3 (Business Combinations) and IAS 38 (Intangible Assets). The related deferred tax liability is also recognized if a deductible or taxable temporary difference exists.
In-process research and development acquired in a business combination is amortized on a straight line basis over its useful life from the date of receipt of marketing approval.
Rights to technology platforms and to products currently marketed by Sanofi are amortized on a straight line basis over their useful lives, determined (in particular for marketed products) on the basis of cash flow forecasts which take into account the patent protection period of the marketed product.
B.5. Property, plant and equipment owned and leased
B.5.1. Property, plant and equipment owned
Property, plant and equipment is initially measured and recognized at acquisition cost, including any directly attributable cost of preparing the asset for its intended use, or (in the case of assets acquired in a business combination) at fair value as of the date of the business combination. The component-based approach to accounting for property, plant and equipment is applied. Under this approach, each component of an item of property, plant and equipment with a cost which is significant in relation to the total cost of the item and which has a different useful life from the other components must be depreciated separately.
After initial measurement, property, plant and equipment is carried at cost less accumulated depreciation and impairment, except for land which is carried at cost less impairment.
Subsequent costs are not recognized as assets unless (i) it is probable that future economic benefits associated with those costs will flow to Sanofi and (ii) the costs can be measured reliably.
Borrowing costs attributable to the financing of items of property, plant and equipment, and incurred during the construction period, are capitalized as part of the acquisition cost of the item.
Government grants relating to property, plant and equipment are deducted from the acquisition cost of the asset to which they relate.
The depreciable amount of items of property, plant and equipment, net of any residual value, is depreciated on a straight line basis over the useful life of the asset. The useful life of an asset is usually equivalent to its economic life.
The customary useful lives of property, plant and equipment are as follows:
| Buildings | |||||
| Fixtures | |||||
| Machinery and equipment | |||||
| Other | |||||
Useful lives and residual values of property, plant and equipment are reviewed annually. The effect of any adjustment to useful lives or residual values is recognized prospectively as a change in accounting estimate.
Depreciation of property, plant and equipment is recognized as an expense in the income statement, in the relevant classification of expense by function.
B.5.2. Property, plant and equipment leased
Effective from January 1, 2019 leases contracted by Sanofi have been accounted for in accordance with IFRS 16 (Leases). Sanofi recognizes a right-of-use asset and a lease liability for all of its lease contracts, except for (i) leases relating to low-value assets and (ii) short-term leases (12 months or less). Payments made in respect of leases not recognized on the balance sheet are recognized as an operating expense on a straight line basis over the lease term.
On commencement of a lease, the liability for future lease payments is discounted at the incremental borrowing rate, which is a risk-free rate adjusted to reflect the specific risk profile of each Sanofi entity. Because lease payments are spread over the lease term, Sanofi applies a discount rate based on the duration of those payments.
The payments used to determine the liability for future lease payments exclude non-lease components, but include fixed payments that Sanofi expects to make to the lessor over the estimated lease term.
After commencement of the lease, the liability for future lease payments is reduced by the amount of the lease payments made, and increased to reflect interest on the liability. In the event of a reassessment or modification of future lease payments, the lease liability is remeasured. The right-of-use asset – which is initially measured at cost including direct costs of the lessee, prepayments made at or prior to the commencement date, less lease incentives received and restoration costs – is depreciated on a straight line basis over the lease term, and tested for impairment as required.
Sanofi recognizes deferred taxes in respect of right-of-use assets and lease liabilities.
Leasehold improvements are depreciated over their economic life, which is capped at the lease term as determined under IFRS 16.
F-16 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.6. Impairment of property, plant and equipment, intangible assets, and investments accounted for using the equity method
B.6.1. Impairment of property, plant and equipment and intangible assets
In accordance with IAS 36 (Impairment of Assets), assets that generate separate cash flows and assets included in cash-generating units (CGUs) are assessed for impairment when events or changes in circumstances indicate that the asset or CGU may be impaired. A CGU is the smallest identifiable group of assets that generates cash inflows that are largely independent of the cash inflows from other assets or groups of assets.
Under IAS 36, each CGU or group of CGUs to which goodwill is allocated must (i) represent the lowest level within the entity at which the goodwill is monitored for internal management purposes, and (ii) not be larger than an operating segment determined in accordance with IFRS 8 (Operating Segments), before application of the IFRS 8 aggregation criteria (see Note B.26.).
Quantitative and qualitative indications of impairment (primarily relating to the status of the research and development portfolio, pharmacovigilance, patent litigation, and the launch of competing products) are reviewed at the end of each reporting period. If there is any internal or external indication of impairment, Sanofi estimates the recoverable amount of the asset or CGU.
Other intangible assets not yet available for use (such as capitalized in-process research and development), and CGUs or groups of CGUs that include goodwill, are tested for impairment annually whether or not there is any indication of impairment, and more frequently if any event or circumstance indicates that they might be impaired. Such assets are not amortized.
When there is an internal or external indication of impairment, Sanofi estimates the recoverable amount of the asset and recognizes an impairment loss if the carrying amount of the asset exceeds its recoverable amount. The recoverable amount of the asset is the higher of its fair value less costs to sell or its value in use. To determine value in use, Sanofi uses estimates of future cash flows generated by the asset or CGU, prepared using the same methods as those used in the initial measurement of the asset or CGU on the basis of medium-term strategic plans.
In the case of goodwill, estimates of future cash flows are based on a six-year strategic plan and a terminal value. In the case of other intrangible assets, the period used is based on the economic life of the asset.
Estimated cash flows are discounted at long-term market interest rates that reflect the best estimate by Sanofi of the time value of money, the risks specific to the asset or CGU, and economic conditions in the geographical regions in which the business activity associated with the asset or CGU is located.
Certain assets and liabilities that are not directly attributable to a specific CGU are allocated between CGUs on a basis that is reasonable, and consistent with the allocation of the corresponding goodwill.
Impairment losses arising on property, plant and equipment, software and certain rights, are recognized within the appropriate income statement line item according to the origin of the impairment.
Impairment losses arising on other intangible assets (products, trademarks, technology platforms, acquired R&D) are recognized within Impairment of intangible assets in the income statement.
B.6.2. Impairment of investments accounted for using the equity method
In accordance with IAS 28 (Investments in Associates and Joint Ventures), Sanofi determines whether investments accounted for using the equity method may be impaired based on indicators such as default in contractual payments, significant financial difficulties, probability of bankruptcy, or a prolonged or significant decline in quoted market price. If an investment is impaired, the amount of the impairment loss is determined by applying IAS 36 (see Note B.6.1.) and recognized in Share of profit/(loss) from investments accounted for using the equity method.
B.6.3. Reversals of impairment losses charged against property, plant and equipment, intangible assets, and investments accounted for using the equity method
At the end of each reporting period, Sanofi assesses whether events or changes in circumstances indicate that an impairment loss recognized in a prior period in respect of an asset (other than goodwill) or an investment accounted for using the equity method can be reversed. If this is the case, and the recoverable amount as determined based on the revised estimates exceeds the carrying amount of the asset, Sanofi reverses the impairment loss only to the extent of the carrying amount that would have been determined had no impairment loss been recognized for the asset.
Reversals of impairment losses in respect of other intangible assets are recognized within the income statement line item Impairment of intangible assets, while reversals of impairment losses in respect of investments accounted for using the equity method are recognized within the income statement line item Share of profit/(loss) from investments accounted for using the equity method. Impairment losses taken against goodwill are never reversed, unless the goodwill is part of the carrying amount of an investment accounted for using the equity method.
SANOFI FORM 20-F 2023 | F-17 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.7. Assets held for sale or exchange and liabilities related to assets held for sale or exchange
In accordance with IFRS 5 (Non-Current Assets Held for Sale and Discontinued Operations), non-current assets and groups of assets are classified as held for sale in the balance sheet if their carrying amount will be recovered principally through a sale transaction rather than through continuing use. Within the meaning of IFRS 5, the term “sale” also includes exchanges for other assets.
Non-current assets or asset groups held for sale must be available for immediate sale in their present condition, subject only to terms that are usual and customary for sales of such assets, and a sale must be highly probable. Criteria used to determine whether a sale is highly probable include:
•the appropriate level of management must be committed to a plan to sell;
•an active program to locate a buyer and complete the plan must have been initiated;
•the asset must be actively marketed for sale at a price that is reasonable in relation to its current fair value;
•completion of the sale should be foreseeable within the 12 months following the date of reclassification to Assets held for sale or exchange; and
•actions required to complete the plan should indicate that it is unlikely that significant changes to the plan will be made or that the plan will be withdrawn.
Before initial reclassification of the non-current asset (or asset group) to Assets held for sale or exchange, the carrying amounts of the asset (or of all the assets and liabilities in the asset group) must be measured in accordance with the applicable standards.
Subsequent to reclassification to Assets held for sale or exchange, the non-current asset (or asset group) is measured at the lower of carrying amount or fair value less costs to sell, with any write-down recognized by means of an impairment loss. Once a non-current asset has been reclassified as held for sale or exchange, it is no longer depreciated or amortized.
In a disposal of an equity interest leading to loss of control, all the assets and liabilities of the entity involved are classified as held-for-sale assets or liabilities within the balance sheet line items Assets held for sale or exchange or Liabilities related to assets held for sale or exchange, provided that the disposal satisfies the IFRS 5 classification criteria.
The profit or loss generated by a held-for-sale asset group is reported in a separate line item in the income statement for the current period and for the comparative periods presented, provided that the asset group:
•represents a separate major line of business or geographical area of operations; or
•is part of a single coordinated plan to dispose of a separate major line of business or geographical area of operations; or
•is a subsidiary acquired exclusively with a view to resale.
In accordance with IFRS 10, transactions between companies that are held for sale or treated as discontinued operations and other consolidated companies are eliminated.
Events or circumstances beyond Sanofi’s control may extend the period to complete the sale or exchange beyond one year without precluding classification of the asset (or disposal group) in Assets held for sale or exchange provided that there is sufficient evidence that Sanofi remains committed to the planned sale or exchange. Finally, in the event of changes to a plan of sale that requires an asset no longer to be classified as held for sale, IFRS 5 specifies the following treatment:
•the assets and liabilities previously classified as held for sale are reclassified to the appropriate balance sheet line items, with no restatement of comparative periods;
•each asset is measured at the lower of (a) its carrying amount before the asset was reclassified as held for sale, adjusted for any depreciation, amortization or revaluation that would have been recognized if the asset had not been reclassified as held for sale, or (b) its recoverable amount at the date of reclassification;
•the backlog of depreciation, amortization and impairment not recognized while non-current assets were classified as held for sale must be reported in the same income statement line item that was used to report impairment losses arising on initial reclassification of assets as held for sale and gains or losses arising on the sale of such assets. In the consolidated income statement, those impacts are reported within the line item Other gains and losses, and litigation;
•the net income of a business previously classified as discontinued or as held for sale or exchange and reported on a separate line in the income statement must be reclassified and included in net income from continuing operations, for all periods presented;
•in addition, segment information relating to the income statement and the statement of cash flows (acquisitions of non-current assets) must be disclosed in the notes to the financial statements in accordance with IFRS 8 (Operating Segments), and must also be restated for all prior periods presented.
F-18 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.8. Financial instruments
B.8.1. Non-derivative financial assets
In accordance with IFRS 9 (Financial Instruments) and IAS 32 (Financial Instruments: Presentation), Sanofi has adopted the classification of non-derivative financial assets described below. The classification used depends on (i) the characteristics of the contractual cash flows (i.e. whether they represent interest or principal) and (ii) the business model for managing the asset applied at the time of initial recognition.
Financial assets at fair value through other comprehensive income
These mainly comprise:
•quoted and unquoted equity investments that Sanofi does not hold for trading purposes and that management has designated at “fair value through other comprehensive income” on initial recognition. Gains and losses arising from changes in fair value are recognized in equity within the statement of comprehensive income in the period in which they occur. When such instruments are derecognized, the previously-recognized changes in fair value remain within Other comprehensive income, as does the gain or loss on divestment. Dividends received are recognized in profit or loss for the period, within the line item Financial income; and
•debt instruments whose contractual cash flows represent payments of interest or repayments of principal, and which are managed with a view to collecting cash flows and selling the asset. Gains and losses arising from changes in fair value are recognized in equity within the statement of comprehensive income in the period in which they occur. When such assets are derecognized, the cumulative gains and losses previously recognized in equity are reclassified to profit or loss for the period within the line items Financial income or Financial expenses.
Financial assets at fair value through profit or loss
These mainly comprise:
•contingent consideration already carried in the books of an acquired entity or granted in connection with a business combination;
•instruments whose contractual cash flows represent payments of interest and repayments of principal, which are managed with a view to selling the asset;
•instruments that management has designated at “fair value through profit or loss” on initial recognition; and
•quoted and unquoted equity investments: equity instruments that are not held for trading and which management did not designate at “fair value through other comprehensive income” on initial recognition, and instruments that do not meet the IFRS definition of “equity instruments”.
Gains and losses arising from changes in fair value are recognized in profit or loss within the line items Financial income or Financial expenses. Dividends received are recognized in profit or loss for the period, within the line item Financial income.
Fair value of equity investments in unquoted entities
On initial recognition of an equity investment in an entity not quoted in an active market, the fair value of the investment is the acquisition cost. Cost ceases to be a representative measure of the fair value of an unquoted equity investment when Sanofi identifies significant changes in the investee, or in the environment in which it operates. In such cases, an internal valuation is carried out, based mainly on growth forecasts or by reference to similar transactions contracted with third parties.
Financial assets measured at amortized cost
Financial assets at amortized cost comprise instruments whose contractual cash flows represent payments of interest and repayments of principal and which are managed with a view to collecting cash flows. The main assets in this category are loans and receivables. They are presented within the line items Other non-current assets, Other current assets, Accounts receivable and Cash and cash equivalents. Loans with a maturity of more than 12 months are presented in “Long-term loans and advances” within Other non-current assets. These financial assets are measured at amortized cost using the effective interest method.
Impairment of financial assets measured at amortized cost
The main assets involved are accounts receivable. Accounts receivable are initially recognized at the amount invoiced to the customer. Impairment losses on trade accounts receivable are estimated using the expected loss method, in order to take account of the risk of payment default throughout the lifetime of the receivables. The expected credit loss is estimated collectively for all accounts receivable at each reporting date using an average expected loss rate, determined primarily on the basis of historical credit loss rates. However, that average expected loss rate may be adjusted if there are indications of a likely significant increase in credit risk. If a receivable is subject to a known credit risk, a specific impairment loss is recognized for that receivable. The amount of expected losses is recognized in the balance sheet as a reduction in the gross amount of accounts receivable. Impairment losses on accounts receivable are recognized within Selling and general expenses in the income statement.
SANOFI FORM 20-F 2023 | F-19 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.8.2. Derivative instruments
Derivative instruments that do not qualify for hedge accounting are initially and subsequently measured at fair value, with changes in fair value recognized in the income statement in Other operating income or in Financial income or Financial expenses, depending on the nature of the underlying economic item which is hedged.
Derivative instruments that qualify for hedge accounting are measured using the policies described in Note B.8.3. below.
IFRS 13 (Fair Value Measurement) requires counterparty credit risk to be taken into account when measuring the fair value of financial instruments. That risk is estimated on the basis of observable, publicly-available statistical data.
Policy on offsetting
In order for a financial asset and a financial liability to be presented as a net amount in the balance sheet under IAS 32, there must be:
(a) a legally enforceable right to offset; and
(b) the intention either to settle on a net basis, or to realize the asset and settle the liability simultaneously.
B.8.3. Hedging
As part of its overall market risk management policy, Sanofi enters into various hedging transactions involving derivative or non-derivative instruments; these may include forward contracts, currency swaps or options, interest rate swaps or options, cross-currency swaps, and debt placings or issues.
Such financial instruments are designated as hedging instruments and recognized using the hedge accounting principles of IFRS 9 when (a) there is formal designation and documentation of the hedging relationship, of how the effectiveness of the hedging relationship will be assessed, and of the underlying market risk management objective and strategy; (b) the hedged item and the hedging instrument are eligible for hedge accounting; and (c) there is an economic relationship between the hedged item and the hedging instrument, defined on the basis of a hedge ratio that is consistent with the underlying market risk management strategy, and the residual credit risk does not dominate the value changes that result from that economic relationship.
Fair value hedge
A fair value hedge is a hedge of the exposure to changes in fair value of an asset, liability or firm commitment that is attributable to one or more risk components and could affect profit or loss.
Changes in fair value of the hedging instrument and changes in fair value of the hedged item attributable to the hedged risk components are generally recognized in the income statement, within Other operating income for hedges related to operating activities, or within Financial income or Financial expenses for hedges related to investing or financing activities.
Cash flow hedge
A cash flow hedge is a hedge of the exposure to variability in cash flows from an asset, liability or highly probable forecast transaction that is attributable to one or more risk components and could affect profit or loss.
Changes in fair value of the hedging instrument attributable to the effective portion of the hedge are recognized directly in equity in the consolidated statement of comprehensive income. Changes in fair value attributable to the ineffective portion of the hedge are recognized in the income statement within Other operating income for hedges related to operating activities, and within Financial income or Financial expenses for hedges related to investing or financing activities.
Cumulative changes in fair value of the hedging instrument previously recognized in equity are reclassified to the income statement when the hedged transaction affects profit or loss. Those reclassified gains and losses are recognized within Other operating income for hedges related to operating activities, and within Financial income or Financial expenses for hedges related to investing or financing activities.
When a forecast transaction results in the recognition of a non-financial asset or liability, cumulative changes in the fair value of the hedging instrument previously recognized in equity are incorporated in the initial carrying amount of that asset or liability.
When the hedging instrument expires or is sold, terminated or exercised, the cumulative gain or loss previously recognized in equity remains separately recognized in equity and is not reclassified to the income statement, or recognized as an adjustment to the initial cost of the related non-financial asset or liability, until the forecast transaction occurs. However, if Sanofi no longer expects the forecast transaction to occur, the cumulative gain or loss previously recognized in equity is recognized immediately in profit or loss.
Hedge of a net investment in a foreign operation
In a hedge of a net investment in a foreign operation, changes in the fair value of the hedging instrument attributable to the effective portion of the hedge are recognized directly in equity in the consolidated statement of comprehensive income. Changes in fair value attributable to the ineffective portion of the hedge are recognized in the income statement within Financial income or Financial expenses. When the investment in the foreign operation is sold, the changes in the fair value of the hedging instrument previously recognized in equity are reclassified to the income statement within Financial income or Financial expenses.
F-20 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Cost of hedging
As part of its market risk management policy, Sanofi may designate currency options or interest rate options as hedging instruments, the effectiveness of which is measured on the basis of changes in intrinsic value. In such cases, the time value of the option is treated as a hedging cost and accounted for as follows:
•if the option includes a component that is not aligned on the critical features of the hedged item, the corresponding change in the time value is taken to profit or loss;
•otherwise, the change in the time value is taken to equity within the statement of comprehensive income, and then:
–if the hedged item is linked to a transaction that results in the recognition of a financial asset or liability, the change in the time value is reclassified to profit or loss symmetrically with the hedged item, or
–if the hedged item is linked to a transaction that results in the recognition of a non-financial asset or liability, the change in the time value is incorporated in the initial carrying amount of that asset or liability, or
–if the hedged item is linked to a period of time, the change in time value is reclassified to profit or loss on a straight line basis over the life of the hedging relationship.
In the case of forward contracts and foreign exchange swaps, and of cross-currency swaps that qualify for hedge accounting on the basis of changes in spot rates, Sanofi may elect for each transaction to use the option whereby the premium/discount or foreign currency basis spread are treated in the same way as the time value of an option.
Discontinuation of hedge accounting
Hedge accounting is discontinued when the eligibility criteria are no longer met (in particular, when the hedging instrument expires or is sold, terminated or exercised), or if there is a change in the market risk management objective of the hedging relationship.
B.8.4. Non-derivative financial liabilities
Borrowings and debt
Bank borrowings and debt instruments are initially measured at fair value of the consideration received, net of directly attributable transaction costs.
Subsequently, they are measured at amortized cost using the effective interest method. All costs related to the issuance of borrowings or debt instruments, and all differences between the issue proceeds net of transaction costs and the value on redemption, are recognized within Financial expenses in the income statement over the term of the debt using the effective interest method.
Liabilities related to business combinations and to non-controlling interests
These line items record the fair value of (i) contingent consideration payable in connection with business combinations and (ii) commitments to buy out equity holders of subsidiaries, including put options granted to non-controlling interests.
Adjustments to the fair value of commitments to buy out equity holders of subsidiaries, including put options granted to non-controlling interests, are recognized in equity.
Other non-derivative financial liabilities
Other non-derivative financial liabilities include trade accounts payable, which are measured at fair value (which in most cases equates to face value) on initial recognition, and subsequently at amortized cost.
B.8.5. Fair value of financial instruments
Under IFRS 13 (Fair Value Measurement) and IFRS 7 (Financial Instruments: Disclosures), fair value measurements must be classified using a hierarchy based on the inputs used to measure the fair value of the instrument. This hierarchy has three levels:
a.level 1: quoted prices in active markets for identical assets or liabilities (without modification or repackaging);
b.level 2: quoted prices in active markets for similar assets and liabilities, or valuation techniques in which all important inputs are derived from observable market data; and
c.level 3: valuation techniques in which not all important inputs are derived from observable market data.
SANOFI FORM 20-F 2023 | F-21 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below shows the disclosures required under IFRS 7 relating to the measurement principles applied to financial instruments.
| Note | Type of financial instrument | Measurement principle | Level in fair value hierarchy | Valuation technique | Method used to determine fair value | ||||||||||||||||||
| Valuation model | Market data | ||||||||||||||||||||||
| Exchange rate | Interest rate | ||||||||||||||||||||||
| D.7. | Financial assets measured at fair value (quoted equity instruments) | Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||
| D.7. | Financial assets measured at fair value (quoted debt instruments) | Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||
| D.7. | Financial assets measured at fair value (unquoted equity instruments) | Fair value | 3 | Cost/Approach based on comparables | If cost ceases to be a representative measure of fair value, an internal valuation is carried out, based mainly on comparables. | ||||||||||||||||||
| D.7. | Financial assets measured at fair value (contingent consideration receivable) | Fair value | 3 | Revenue-based approach | The fair value of contingent consideration receivable is determined by adjusting the contingent consideration at the end of the reporting period using the method described in Note D.7.3. | ||||||||||||||||||
| D.7. | Financial assets measured at fair value held to meet obligations under post-employment benefit plans | Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||
| D.7. | Financial assets designated at fair value held to meet obligations under deferred compensation plans | Fair value | 1 | Market value | Quoted market price | N/A | |||||||||||||||||
| D.7. | Long-term loans and advances and other non-current receivables | Amortized cost | N/A | N/A | The amortized cost of long-term loans and advances and other non-current receivables at the end of the reporting period is not materially different from their fair value. | ||||||||||||||||||
| D.13. | Investments in mutual funds | Fair value | 1 | Market value | Net asset value | N/A | |||||||||||||||||
| D.13. | Negotiable debt instruments, commercial paper, instant access deposits and term deposits | Amortized cost | N/A | N/A | Because these instruments have a maturity of less than three months, amortized cost is regarded as an acceptable approximation of fair value as disclosed in the notes to the consolidated financial statements. | ||||||||||||||||||
D.17.1., D.19. | Debt | Amortized cost(a) | N/A | N/A | In the case of debt with a maturity of less than three months, amortized cost is regarded as an acceptable approximation of fair value as reported in the notes to the consolidated financial statements. For debt with a maturity of more than three months, fair value as reported in the notes to the consolidated financial statements is determined either by reference to quoted market prices at the end of the reporting period (quoted instruments) or by discounting the future cash flows based on observable market data at the end of the reporting period (unquoted instruments). For financial liabilities based on variable payments such as royalties, fair value is determined on the basis of discounted cash flow projections. | ||||||||||||||||||
| D.17.2. | Lease liabilities | Amortized cost | N/A | N/A | The liability for future lease payments is discounted using the incremental borrowing rate. | ||||||||||||||||||
| D.20. | Forward currency contracts | Fair value | 2 | Present value of future cash flows | Mid Market | < 1 year: Mid Money Market > 1 year: Mid Zero Coupon | |||||||||||||||||
| D.20. | Interest rate swaps | Fair value | 2 | Revenue-based approach | Present value of future cash flows | Mid Market Spot | < 1 year: Mid Money Market and LIFFE interest rate futures > 1 year: Mid Zero Coupon | ||||||||||||||||
| D.20. | Cross-currency swaps | Fair value | 2 | Present value of future cash flows | Mid Market Spot | < 1 year: Mid Money Market and LIFFE interest rate futures > 1 year: Mid Zero Coupon | |||||||||||||||||
| D.18. | Liabilities related to business combinations and to non-controlling interests (CVRs) | Fair value | 1 | Market value | Quoted market price | ||||||||||||||||||
| D.18. | Liabilities related to business combinations and to non-controlling interests (other than CVRs) | Fair value | 3 | Revenue-based approach | Under IAS 32, contingent consideration payable in a business combination is a financial liability. The fair value of such liabilities is determined by adjusting the contingent consideration at the end of the reporting period using the method described in Note B.8.4. | ||||||||||||||||||
(a) In the case of debt designated as a hedged item in a fair value hedging relationship, the carrying amount in the consolidated balance sheet includes changes in fair value attributable to the hedged risk(s).
F-22 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.8.6. Derecognition of financial instruments
Financial assets are derecognized when the contractual rights to cash flows from the asset have ended or have been transferred and when Sanofi has transferred substantially all the risks and rewards of ownership of the asset. If Sanofi has neither transferred nor retained substantially all the risks and rewards of ownership of a financial asset, it is derecognized if Sanofi does not retain control of the asset.
A financial liability is derecognized when Sanofi’s contractual obligations in respect of the liability are discharged, cancelled or extinguished.
B.8.7. Risks relating to financial instruments
Market risks in respect of non-current financial assets, cash equivalents, derivative instruments and debt are described in the discussions of risk factors presented in Item 3.D. and Item 11. of Sanofi’s annual report on Form 20-F for 2023.
Credit risk is the risk that customers may fail to pay their debts. For a description of credit risk, refer to “We are subject to the risk of non-payment by our customers” within Item 3.D. and Item 11. of Sanofi’s annual report on Form 20-F for 2023.
B.9. Inventories
Inventories are measured at the lower of cost or net realizable value. Cost is calculated using the weighted average cost method or the first-in, first-out method, depending on the nature of the inventory.
The cost of finished goods inventories includes costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition.
Net realizable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
During the launch phase of a new product, any inventories of that product are written down to zero pending regulatory approval, other than in specific circumstances which make it possible to estimate that there is a high probability at the end of the reporting period that the carrying amount of the inventories will be recoverable. The write-down is reversed once it becomes highly probable that marketing approval will be obtained.
B.10. Cash and cash equivalents
Cash and cash equivalents as shown in the consolidated balance sheet and statement of cash flows comprise cash, plus liquid short-term investments that are readily convertible into cash and are subject to an insignificant risk of changes in value in the event of movements in interest rates.
B.11. Treasury shares
In accordance with IAS 32, Sanofi treasury shares are deducted from equity, irrespective of the purpose for which they are held. No
B.12. Provisions for risks
In accordance with IAS 37 (Provisions, Contingent Liabilities and Contingent Assets), Sanofi records a provision when it has a present obligation, whether legal or constructive, as a result of a past event; it is probable that an outflow of resources embodying economic benefits will be required to settle the obligation; and a reliable estimate can be made of the amount of the outflow of resources.
If the obligation is expected to be settled more than 12 months after the end of the reporting period, or has no definite settlement date, the provision is recorded within Non-current provisions and other non-current liabilities.
Provisions relating to the insurance programs in which Sanofi’s captive insurance company participates are based on risk exposure estimates calculated by management, with assistance from independent actuaries, using IBNR (Incurred But Not Reported) techniques. Those techniques use past claims experience, within Sanofi and in the market, to estimate future trends in the cost of claims.
Contingent liabilities are not recognized, but are disclosed in the notes to the financial statements unless the possibility of an outflow of economic resources is remote.
Sanofi estimates provisions on the basis of events and circumstances related to present obligations at the end of the reporting period and of past experience, and to the best of management’s knowledge at the date of preparation of the financial statements.
Reimbursements offsetting the probable outflow of resources are recognized as assets only if it is virtually certain that they will be received. Contingent assets are not recognized.
Restructuring provisions are recognized if Sanofi has a detailed, formal restructuring plan at the end of the reporting period and has announced its intention to implement this plan to those affected by it.
SANOFI FORM 20-F 2023 | F-23 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Sanofi records non-current provisions for certain obligations, such as legal or constructive obligations, where an outflow of resources is probable and the amount of the outflow can be reliably estimated.
In the case of environmental risks, including at sites where operations are ongoing, Sanofi recognizes a provision where there is a violation of integrity in respect of human health or the environment resulting from past contamination at a site that requires remediation. The amount of the provision is a best estimate of the future expenditures to be incurred on the remediation plan.
Where the effect of the time value of money is material, those provisions are measured at the present value of the expenditures expected to be required to settle the obligation, calculated using a discount rate that reflects an estimate of the time value of money and the risks specific to the obligation.
Increases in provisions to reflect the effects of the passage of time are recognized within Financial expenses.
B.13. Revenue recognition
B.13.1. Net sales
Revenue arising from the sale of goods is presented in the income statement within Net sales. Net sales comprise revenue from sales of pharmaceutical products, consumer healthcare products, active ingredients and vaccines, net of sales returns, of customer incentives and discounts, and of certain sales-based payments paid or payable to the healthcare authorities. Analyses of net sales are provided in Note D.35.1. “Segment Information”.
In accordance with IFRS 15 (Revenue from Contracts with Customers), such revenue is recognized when Sanofi transfers control over the product to the customer; control of an asset refers to the ability to direct the use of, and obtain substantially all of the remaining benefits from that asset. For the vast majority of contracts, revenue is recognized when the product is physically transferred, in accordance with the delivery and acceptance terms agreed with the customer.
For contracts entered into by Sanofi Pasteur, transfer of control is usually determined by reference to the terms of release (immediate or deferred) and acceptance of batches of vaccine.
In the case of contracts with distributors, Sanofi does not recognize revenue when the product is physically transferred to the distributor if the products are sold on consignment, or if the distributor acts as agent. In such cases, revenue is recognized when control is transferred to the end customer, and the distributor’s commission is presented within the line item Selling and general expenses in the income statement.
The amount of revenue recognized reflects the various types of price reductions or rights of return offered by Sanofi to its customers on certain products. Such price reductions and rights of return qualify as variable consideration under IFRS 15.
In particular, products sold in the United States are covered by various Government and State programs (such as Medicare and Medicaid) under which products are sold at a discount. Rebates are granted to healthcare authorities, and under contractual arrangements with certain customers. Some wholesalers are entitled to chargeback incentives based on the selling price to the end customer, under specific contractual arrangements. Cash discounts may also be granted for prompt payment. Returns, discounts, incentives and rebates, as described above, are recognized in the period in which the underlying sales are recognized as a reduction of gross sales.
These amounts are calculated as follows:
•the amount of chargeback incentives is estimated on the basis of the relevant subsidiary’s standard sales terms and conditions, and in certain cases on the basis of specific contractual arrangements with the customer;
•the amount of rebates based on attainment of sales targets is estimated and accrued as each of the underlying sales transactions is recognized;
•the amount of price reductions under Government and State programs, largely in the United States, is estimated on the basis of the specific terms of the relevant regulations or agreements, and accrued as each of the underlying sales transactions is recognized;
•the amount of sales returns is calculated on the basis of management’s best estimate of the amount of product that will ultimately be returned by customers. In countries where product returns are possible, Sanofi operates a returns policy that allows the customer to return products within a certain period either side of the expiry date (usually 12 months after the expiry date). The amount recognized for returns is estimated on the basis of past experience of sales returns. Sanofi also takes into account factors such as levels of inventory in its various distribution channels, product expiry dates, information about potential discontinuation of products, the entry of competing generics into the market, and the launch of over-the-counter medicines. Most product return clauses relate solely to date-expired products, which cannot be resold and are destroyed. Sanofi does not recognize a right of return asset in the balance sheet for contracts that allow for the return of time-expired products, since those products have no value.
F-24 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The estimated amounts described above are recognized in the income statement within Net sales as a reduction of gross sales, and within Other current liabilities in the balance sheet. They are subject to regular review and adjustment as appropriate based on the most recent data available to management. Sanofi believes that it has the ability to measure each of the above amounts reliably, using the following factors in developing its estimates:
•the nature and patient profile of the underlying product;
•the applicable regulations or the specific terms and conditions of contracts with governmental authorities, wholesalers and other customers;
•historical data relating to similar contracts, in the case of qualitative and quantitative rebates and chargeback incentives;
•past experience and sales growth trends for the same or similar products;
•actual inventory levels in distribution channels, monitored by Sanofi using internal sales data and externally provided data;
•the shelf life of Sanofi products; and
•market trends including competition, pricing and demand.
An analysis of provisions for discounts, rebates and sales returns is provided in Note D.23.
B.13.2. Other revenues
The line item Other revenues is used to recognize all revenue that falls within the scope of IFRS 15 but does not relate to sales of Sanofi products.
It mainly comprises (i) royalties received from licensing intellectual property rights to third parties; (ii) VaxServe sales of products sourced from third-party manufacturers; and (iii) revenue received under agreements for Sanofi to provide manufacturing services to third parties.
Royalties received under licensing arrangements are recognized over the period during which the underlying sales are recognized.
VaxServe is a Vaccines segment entity whose operations include the distribution within the United States of vaccines and other products manufactured by third parties. VaxServe sales of products sourced from third-party manufacturers are presented within Other revenues.
B.14. Cost of sales
Cost of sales consists primarily of the industrial cost of goods sold, payments made under licensing agreements, and distribution costs. The industrial cost of goods sold includes the cost of materials, depreciation of property, plant and equipment, amortization of software, personnel costs, and other expenses attributable to production.
B.15. Research and development
Note B.4.1. “Research and development not acquired in a business combination” and Note B.4.3. “Other intangible assets acquired in a business combination” describe the principles applied to the recognition of research and development costs.
Contributions or reimbursements received from alliance partners are recorded as a reduction of Research and development expenses.
B.16. Other operating income and expenses
B.16.1. Other operating income
Other operating income includes the share of profits that Sanofi is entitled to receive from alliance partners in respect of product marketing agreements. It also includes revenues generated under certain agreements, which may include partnership, co-promotion arrangements and licenses not included in Other revenues.
This line item also includes realized and unrealized foreign exchange gains and losses on operating activities (see Note B.8.3.), and operating gains on disposals not regarded as major disposals (see Note B.20.).
B.16.2. Other operating expenses
Other operating expenses mainly comprise the share of profits that alliance partners are entitled to receive from Sanofi under product marketing agreements.
B.17. Amortization and impairment of intangible assets
B.17.1. Amortization of intangible assets
The expenses recorded in this line item comprise amortization charged against intangible assets (products, trademarks and technology platforms, see Note D.4.) whose contribution to Sanofi’s commercial, industrial and development functions cannot be separately identified.
SANOFI FORM 20-F 2023 | F-25 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Amortization of software, and of other rights of an industrial or operational nature, is recognized as an expense in the income statement, in the relevant line items of expense by function.
B.17.2. Impairment of intangible assets
This line item records impairment losses taken against intangible assets (products, trademarks, technology platforms and acquired research), and any reversals of such impairment losses.
B.18. Fair value remeasurement of contingent consideration
Changes in the fair value of contingent consideration that was (i) already carried in the books of an acquired entity, or (ii) granted in connection with a business combination and initially recognized as a liability in accordance with IFRS 3, are reported in profit or loss. Such adjustments are reported separately in the income statement, in the line item Fair value remeasurement of contingent consideration.
This line item also includes changes in the fair value of contingent consideration receivable in connection with a divestment and classified as a financial asset at fair value through profit or loss.
Finally, it includes the effect of the unwinding of discount, and of exchange rate movements where the asset or liability is expressed in a currency other than the functional currency of the reporting entity.
B.19. Restructuring costs and similar items
Restructuring costs are expenses incurred in connection with the transformation or reorganization of Sanofi’s operations or support functions. Such costs include collective redundancy plans, compensation to third parties for early termination of contracts, and commitments made in connection with transformation or reorganization decisions. They also include accelerated depreciation charges arising from site closures (including closures of leased sites), and losses on asset disposals resulting from such decisions.
In addition, this line item includes expenses incurred in connection with programs implemented as part of the transformation strategy announced in December 2019 (and previously in November 2015), and intended primarily (i) to deliver a global information systems solution, further supported by the implementation from 2021 of Sanofi’s new digital strategy; and (ii) to create a standalone CHC entity and proceed to the proposed separation of the CHC business as announced when the new chapter in the Play to Win strategy was presented in October 2023.
B.20. Other gains and losses, and litigation
The line item Other gains and losses, and litigation includes the impact of material transactions of an unusual nature or amount which Sanofi believes it necessary to report separately in the income statement in order to improve the relevance of the financial statements, such as:
•gains and losses on major disposals of property, plant and equipment, of intangible assets, of assets (or groups of assets and liabilities) held for sale, or of a business within the meaning of IFRS 3, other than those considered to be restructuring costs;
•impairment losses and reversals of impairment losses on assets (or groups of assets and liabilities) held for sale, other than those considered to be restructuring costs;
•gains on bargain purchases;
•costs relating to major litigation; and
•pre-tax separation costs associated with the process of disinvesting from operations in the event of a major divestment.
B.21. Financial expenses and income
B.21.1. Financial expenses
Financial expenses mainly comprise interest charges on debt financing; negative changes in the fair value of certain financial instruments (where changes in fair value are recognized in profit or loss); realized and unrealized foreign exchange losses on financing and investing activities; impairment losses on financial instruments; and any reversals of impairment losses on financial instruments.
Financial expenses also include expenses arising from the unwinding of discount on long-term provisions, and the net interest cost related to employee benefits. This line item does not include commercial cash discounts, which are deducted from net sales.
B.21.2. Financial income
Financial income includes interest and dividend income; positive changes in the fair value of certain financial instruments (where changes in fair value are recognized in profit or loss); realized and unrealized foreign exchange gains on financing and investing activities; and gains on disposals of financial assets at fair value through profit or loss.
F-26 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.22. Income tax expense
Income tax expense includes all current and deferred taxes of consolidated companies.
Sanofi accounts for deferred taxes in accordance with IAS 12 (Income Taxes), using the methods described below:
•deferred tax assets and liabilities are recognized on taxable and deductible temporary differences, and on tax loss carry-forwards. Temporary differences are differences between the carrying amount of an asset or liability in the balance sheet and its tax base;
•French business taxes include a value added based component: “CVAE” (Cotisation sur la Valeur Ajoutée des Entreprises). Given that CVAE is (i) calculated as the amount by which certain revenues exceed certain expenses and (ii) borne primarily by companies that own intellectual property rights on income derived from those rights (royalties, and margin on sales to third parties and to Sanofi entities), it is regarded as meeting the definition of income taxes specified in IAS 12, paragraph 2 (“taxes which are based on taxable profits”);
•deferred tax assets and liabilities are calculated using the tax rate expected to apply in the period when the corresponding temporary differences are expected to reverse, based on tax rates enacted or substantively enacted at the end of the reporting period;
•deferred tax assets are recognized in respect of deductible temporary differences, tax losses available for carry-forward and unused tax credits to the extent that future recovery is regarded as probable. The recoverability of deferred tax assets is assessed on a case-by-case basis, taking into account the profit forecasts contained in Sanofi’s medium-term business plan;
•a deferred tax liability is recognized for temporary differences relating to interests in subsidiaries, associates and joint ventures, except in cases where Sanofi is able to control the timing of the reversal of the temporary differences. This applies in particular when Sanofi is able to control dividend policy and it is probable that the temporary differences will not reverse in the foreseeable future;
•no deferred tax is recognized on eliminations of intragroup transfers of interests in subsidiaries, associates or joint ventures;
•each tax entity calculates its own net deferred tax position. All net deferred tax asset and liability positions are then aggregated and shown in separate line items on the relevant side of the consolidated balance sheet. Deferred tax assets and liabilities are offset only if (i) Sanofi has a legally enforceable right to offset current tax assets and current tax liabilities, and (ii) the deferred tax assets and deferred tax liabilities relate to income taxes levied by the same taxation authority;
•deferred taxes are not discounted, except implicitly in the case of deferred taxes on assets and liabilities which are already impacted by discounting. In addition, Sanofi has elected not to discount current taxes payable or receivable where the amounts in question are payable or receivable in the long term;
•withholding taxes on intragroup royalties and dividends, and on royalties and dividends collected from third parties, are accounted for as current income taxes.
In accounting for business combinations, Sanofi complies with IFRS 3 as regards the recognition of deferred tax assets after the initial accounting period. Consequently, any deferred tax assets recognized by the acquiree after the end of that period in respect of temporary differences or tax loss carry-forwards existing at the acquisition date are recognized in profit or loss.
The positions adopted by Sanofi in tax matters are based on its interpretation of tax laws and regulations. Some of those positions may be subject to uncertainty. In such cases, Sanofi assesses the amount of the tax liability on the basis of the following assumptions: that its position will be examined by one or more tax authorities on the basis of all relevant information; that a technical assessment is carried out with reference to legislation, case law, regulations, and established practice; and that each position is assessed individually (or collectively where appropriate), with no offset or aggregation between positions. Those assumptions are assessed on the basis of facts and circumstances existing at the end of the reporting period. When an uncertain tax liability is regarded as probable, it is measured on the basis of Sanofi’s best estimate and recognized as a liability; uncertain tax assets are not recognized. The amount of the liability includes any penalties and late payment interest. The line item Income tax expense includes the effects of tax reassessments and tax disputes, and any penalties and late payment interest arising from such disputes that have the characteristics of income taxes within the meaning of paragraph 2 of IAS 12 (“taxes which are based on taxable profits”). Tax exposures relating to corporate income taxes are presented separately within Non-current income tax liabilities (see Note D.19.4.).
In accordance with IAS 1 (Presentation of Financial Statements), current income tax assets and liabilities are presented as separate line items in the consolidated balance sheet.
B.23. Employee benefit obligations
Sanofi offers retirement benefits to employees and retirees. Such benefits are accounted for in accordance with IAS 19 (Employee Benefits).
Benefits are provided in the form of either defined contribution plans or defined benefit plans. In the case of defined contribution plans, the cost is recognized immediately in the period in which it is incurred, and equates to the amount of the contributions paid by Sanofi. For defined benefit plans, Sanofi generally recognizes its obligations to pay pensions and similar benefits to employees as a liability, based on an actuarial estimate of the rights vested or currently vesting in employees and retirees, using the projected unit credit method. Estimates are performed at least once a year, and rely on financial assumptions (such as discount
SANOFI FORM 20-F 2023 | F-27 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
rates) and demographic assumptions (such as life expectancy, retirement age, employee turnover, and the rate of salary increases).
Obligations relating to other post-employment benefits (healthcare and life insurance) offered by Sanofi companies to employees are also recognized as a liability based on an actuarial estimate of the rights vested or currently vesting in employees and retirees at the end of the reporting period.
Such liabilities are recognized net of the fair value of plan assets.
In the case of multi-employer defined benefit plans where plan assets cannot be allocated to each participating employer with sufficient reliability, the plan is accounted for as a defined contribution plan, in accordance with paragraph 34 of IAS 19.
The benefit cost for the period consists primarily of current service cost, past service cost, net interest cost, gains or losses arising from plan settlements not specified in the terms of the plan, and actuarial gains or losses arising from plan curtailments. Net interest cost for the period is determined by applying the discount rate specified in IAS 19 to the net liability (i.e. the amount of the obligation, net of plan assets) recognized in respect of defined benefit plans. Past service cost is recognized immediately in profit or loss in the period in which it is incurred, regardless of whether or not the rights have vested at the time of adoption (in the case of a new plan) or of amendment (in the case of an existing plan).
Actuarial gains and losses on defined benefit plans (pensions and other post-employment benefits), also referred to as “Remeasurements of the net defined benefit liability (asset)”, arise as a result of changes in financial and demographic assumptions, experience adjustments, and the difference between the actual return and interest cost on plan assets. The impacts of those remeasurements are recognized in Other comprehensive income, net of deferred taxes; they are not subsequently reclassifiable to profit or loss.
B.24. Share-based payment
Share-based payment expense is recognized as a component of operating income, in the relevant classification of expense by function. In measuring the expense, the level of attainment of any performance conditions is taken into account.
B.24.1. Stock option plans
Sanofi has granted a number of equity-settled share-based payment plans (stock option plans) to some of its employees. The terms of those plans may make the award contingent on the attainment of performance criteria for some of the grantees.
In accordance with IFRS 2 (Share-Based Payment), services received from employees as consideration for stock options are recognized as an expense in the income statement, with the opposite entry recognized in equity. The expense corresponds to the fair value of the stock option plans, and is charged to income on a straight-line basis over the four-year vesting period of the plan.
The fair value of stock option plans is measured at the date of grant using the Black-Scholes valuation model, taking into account the expected life of the options. The resulting expense also takes into account the expected cancellation rate of the options. The expense is adjusted over the vesting period to reflect actual cancellation rates resulting from option-holders ceasing to be employed by Sanofi.
B.24.2. Employee share ownership plans
Sanofi may offer its employees the opportunity to subscribe to reserved share issues at a discount to the reference market price. Shares awarded to employees under such plans fall within the scope of IFRS 2. Consequently, an expense is recognized at the subscription date, based on the value of the discount offered to employees.
B.24.3. Restricted share plans
Sanofi may award restricted share plans to certain of its employees. The terms of those plans may make the award contingent on the attainment of performance criteria for some of the grantees.
In accordance with IFRS 2, an expense equivalent to the fair value of such plans is recognized in profit or loss on a straight line basis over the vesting period of the plan, with the opposite entry recognized in equity. The vesting period is three years .
The fair value of restricted share plans is based on the quoted market price of Sanofi shares at the date of grant, adjusted for expected dividends during the vesting period; it also takes account of any vesting conditions contingent on stock market performance, measured using the Monte-Carlo valuation model. Other vesting conditions are taken into account in the estimate of the number of shares awarded during the vesting period; that number is then definitively adjusted based on the actual number of shares awarded on the vesting date.
B.25. Earnings per share
Basic earnings per share is calculated using the weighted average number of shares outstanding during the reporting period, adjusted on a time-weighted basis from the acquisition date to reflect the number of own shares held by Sanofi. Diluted earnings per share is calculated on the basis of the weighted average number of ordinary shares, computed using the treasury stock method.
This method assumes that (i) all outstanding dilutive options and warrants are exercised, and (ii) Sanofi acquires its own shares at the quoted market price for an amount equivalent to the cash received as consideration for the exercise of the options or warrants, plus the expense arising on unamortized stock options.
F-28 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
B.26. Segment information
In accordance with IFRS 8 (Operating Segments), the segment information reported by Sanofi is prepared on the basis of internal management data provided to our Chief Executive Officer, who is the chief operating decision maker of Sanofi. The performance of those segments is monitored individually using internal reports and common indicators.
In 2022, Sanofi reported three operating segments (Pharmaceuticals, Vaccines and Consumer Healthcare). The costs of the global support functions (Corporate Affairs, Finance, People & Culture, Legal, Ethics, Business Integrity & Global Security, Information Solutions & Technology, Sanofi Business Services, etc.), which are mainly managed centrally at group-wide level, were presented within the “Other” category.
In 2023, Sanofi reviewed the presentation of its segment information following adjustments to its internal reporting systems in order to reflect (i) progress on the “Play to Win” strategy leading to the creation of the standalone Consumer Healthcare Global Business Unit (GBU) which, in addition to integrated research, development and production functions now also has its own dedicated global support functions (including Finance, People & Culture, Legal, Ethics, Business Integrity & Global Security, Information Solutions & Technology, Global Business Services, etc.); and (ii) organizational changes to Sanofi’s Manufacturing & Supply global function (previously known as Industrial Affairs).
Consequently, with effect from January 1, 2023, Sanofi reports two operating segments: Biopharma and Consumer Healthcare.
The Biopharma operating segment comprises commercial operations and research, development and production activities relating to the Specialty Care, General Medicines and Vaccines franchises, for all geographical territories. The segment’s results include the costs of global support functions that are not within the managerial responsibility of the Consumer Healthcare GBU.
The Consumer Healthcare operating segment comprises commercial operations relating to Consumer Healthcare products, and research, development and production activities and global support functions (as listed above) dedicated to the segment, for all geographical territories. The Consumer Healthcare GBU segment’s results reflect all incurred costs of global support functions attributable to its business.
The “Other” category comprises reconciling items, primarily but not limited to (i) gains and losses on centralized foreign exchange risk hedging transactions that cannot be allocated to the operating segments and (ii) gains and losses on retained commitments in respect of previously divested operations.
Information about operating segments for the years ended December 31, 2023, 2022 and 2021 is presented in Note D.35., “Segment information”.
B.27. Management of capital
In order to maintain or adjust the capital structure, Sanofi can adjust the amount of dividends paid to shareholders, repurchase its own shares, issue new shares, or issue securities giving access to its capital.
The following objectives are defined under the terms of Sanofi’s share repurchase programs:
•the implementation of any stock option plan giving entitlement to purchase shares in the Sanofi parent company;
•the allotment or sale of shares to employees under statutory profit sharing schemes and employee savings plans;
•the consideration-free allotment of shares (i.e. restricted share plans);
•the cancellation of some or all of the repurchased shares;
•market-making in the secondary market by an investment services provider under a liquidity contract in compliance with the ethical code recognized by the Autorité des marchés financiers (AMF);
•the delivery of shares on the exercise of rights attached to securities giving access to the capital by redemption, conversion, exchange, presentation of a warrant or any other means;
•the delivery of shares (in exchange, as payment, or otherwise) in connection with mergers and acquisitions;
•the execution by an investment services provider of purchases, sales or transfers by any means, in particular via off-market trading; or
•any other purpose that is or may in the future be authorized under the applicable laws and regulations.
Sanofi is not subject to any constraints on equity capital imposed by third parties.
Total equity includes Equity attributable to equity holders of Sanofi and Equity attributable to non-controlling interests, as shown in the consolidated balance sheet.
Sanofi defines “Net debt” as (i) the sum of short-term debt, long-term debt and interest rate derivatives and currency derivatives used to hedge debt, minus (ii) the sum of cash and cash equivalents and interest rate derivatives and currency derivatives used to hedge cash and cash equivalents.
SANOFI FORM 20-F 2023 | F-29 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
C/ Principal alliances
C.1. Alliance arrangements with Regeneron Pharmaceuticals, Inc. (Regeneron)
Collaboration agreements on human therapeutic antibodies
In November 2007, Sanofi and Regeneron signed two agreements (amended in November 2009) relating to human therapeutic antibodies: (i) the Discovery and Preclinical Development Agreement, and (ii) the License and Collaboration Agreement, relating to clinical development and commercialization. Under the License and Collaboration Agreement, Sanofi had an option to develop and commercialize antibodies discovered by Regeneron under the Discovery and Preclinical Development Agreement.
Discovery and development
Because Sanofi decided not to exercise its option to extend the Discovery and Preclinical Development Agreement, that agreement expired on December 31, 2017.
As a result of Sanofi’s exercise of an option with respect to an antibody under the Discovery and Preclinical Development Agreement, such antibody became a “Licensed Product” under the License and Collaboration Agreement, pursuant to which Sanofi and Regeneron co-develop the antibody with Sanofi initially being wholly responsible for funding the development program. On receipt of the first positive Phase 3 trial results for any antibody being developed under the License and Collaboration Agreement, the subsequent development costs for that antibody are split 80 % Sanofi, 20 % Regeneron. Amounts received from Regeneron under the License and Collaboration Agreement are recognized by Sanofi as a reduction in the line item Research and development expenses. Co-development with Regeneron of the antibodies DUPIXENT, KEVZARA and REGN3500 (SAR440340 - itepekimab) is ongoing under the License and Collaboration Agreement as of December 31, 2023.
Once a product begins to be commercialized, and provided that the share of quarterly results under the agreement represents a profit, Sanofi is entitled to an additional portion of Regeneron’s profit-share (capped at 20 % of Regeneron’s share of quarterly profits since April 1, 2022, and at 10 % until March 31, 2022) until Regeneron has paid 50 % of the cumulative development costs incurred by the parties in the collaboration (see Note D.21.1.).
On the later of (i) 24 months before the scheduled launch date or (ii) the first positive Phase 3 trial results, Sanofi and Regeneron share the commercial expenses of the antibodies co-developed under the License and Collaboration Agreement.
Commercialization
Sanofi is the lead party with respect to the commercialization of all co-developed antibodies, and Regeneron has certain option rights to co-promote the antibodies. Regeneron has exercised its co-promotion rights in the United States and in certain other countries. Sanofi recognizes all sales of the antibodies. Profits and losses arising from commercial operations in the United States are split 50 /50. Outside the United States, Sanofi is entitled to between 55 % and 65 % of profits depending on sales of the antibodies, and bears 55 % of any losses. The share of profits and losses due to or from Regeneron under the agreement is recognized within the line items Other operating income or Other operating expenses, which are components of Operating income.
In addition, Regeneron is entitled to receive payments contingent on the attainment of specified levels of aggregate sales on all antibodies outside the United States, on a rolling twelve-month basis. A liability for those payments is recognized on the balance sheet when it is probable that the specified level of aggregate sales will be met. The opposite entry for that liability is capitalized within Other intangible assets on the balance sheet. Two payments of $50 2.0 billion and then of $2.5 billion in sales of all antibodies outside the United States on a rolling twelve-month basis. The final milestone payment of $50 million, payable to Regeneron in the event that $3.0 billion in sales on a rolling twelve-month basis is attained, was made in 2023.
Amendments to the collaboration agreements
In January 2018, Sanofi and Regeneron signed a set of amendments to their collaboration agreements, including an amendment that allowed for the funding of additional programs on DUPIXENT and REGN3500 (SAR440340 – itepekimab) with an intended focus on extending the current range of indications, finding new indications, and improving co-morbidity between multiple pathologies.
Effective April 1, 2020, Sanofi and Regeneron signed a Cross License and Commercialization Agreement for PRALUENT, whereby Sanofi obtained sole ex-US rights to PRALUENT, and Regeneron obtained sole US rights to PRALUENT along with a right to 5 % royalties on Sanofi’s sales of PRALUENT outside the United States. Each party is solely responsible for funding the development, manufacturing and commercialization of PRALUENT in their respective territories. Although each party has sole responsibility for supplying PRALUENT in its respective territory, Sanofi and Regeneron entered into agreements to support manufacturing needs for each other.
Effective September 30, 2021, Sanofi and Regeneron signed an amendment to their collaboration agreement in order to specify allocations of responsibilities and associated resources between the two parties in connection with the co-promotion of DUPIXENT in certain countries. The terms of the collaboration relating to REGN3500 (SAR440340 – itepekimab) are unchanged.
F-30 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Effective July 1, 2022, Sanofi and Regeneron signed an amendment to their collaboration agreement in order to increase the additional portion of Regeneron’s quarterly profit-share attributable to Sanofi from 10 % to 20 % with retroactive impact as of April 1, 2022.
Immuno-oncology (IO) collaboration agreements
On July 1, 2015, Sanofi and Regeneron signed two agreements – the IO Discovery and Development Agreement and the IO License and Collaboration Agreement (IO LCA) – relating to new antibody cancer treatments in the field of immuno-oncology.
The Amended IO Discovery Agreement, effective from December 31, 2018, was terminated through a Letter Amendment dated March 16, 2021 in which Sanofi formalized its opt-out from the BCMAxCD3 and MUC16xCD3 programs.
LIBTAYO (cemiplimab)
Under the 2015 IO LCA as amended in January 2018, Sanofi and Regeneron committed funding of no more than $1,640 million, split on a 50 /50 basis ($820 million per company), for the development of REGN2810 (cemiplimab, trademark LIBTAYO), a PD-1 inhibitor antibody. The funding was raised to $1,840 million by way of amendment effective on September 30, 2021. Regeneron was responsible for the commercialization of LIBTAYO in the United States, and Sanofi in all other territories. Sanofi has exercised its option to co-promote LIBTAYO in the United States. In 2021, Regeneron exercised its option to co-promote LIBTAYO in certain other countries.
The IO LCA also provided for a one-time milestone payment of $375 million by Sanofi to Regeneron in the event that sales of a PD-1 product were to exceed, in the aggregate, $2 billion in any consecutive 12-month period.
Under the IO LCA Sanofi and Regeneron shared equally in profits and losses generated by the commercialization of collaboration products, except that Sanofi was entitled to an additional portion of Regeneron’s profit-share (capped at 10 % of Regeneron’s share of quarterly profits) until Regeneron had paid 50 % of the cumulative development costs incurred by the parties under the IO Discovery Agreement, as amended.
LIBTAYO is approved in the United States and Europe for the treatment of two types of locally advanced or metastatic skin cancer (cutaneous squamous cell carcinoma and basal cell carcinoma) and non-small cell lung cancer (NSCLC). It is also approved in Brazil and Canada as a second line treatment for recurring or metastatic cervical cancer. In the fourth quarter of 2022, it was approved in the United States in association with chemotherapy for the treatment of NSCLC, and in Europe and Japan as a second line treatment for recurring or metastatic cervical cancer. LIBTAYO is currently approved in more than 30 countries.
In June 2022, Sanofi and Regeneron restructured their IO LCA. Under the terms of the Amended and Restated IO LCA, Regeneron holds exclusive worldwide licensing rights to LIBTAYO with effect from July 1, 2022.
In July 2022, Sanofi received as consideration an upfront payment of $900 million (€856 million), which was recognized within Other operating income on the date of receipt. The same line item also includes a regulatory milestone payment of $100 million (€96 million) following the US FDA approval in November 2022 of LIBTAYO in combination with chemotherapy as a first line treatment for NSCLC. In addition, Sanofi is entitled to royalties of 11 % and to milestone payments (€116 million in 2023, €111 million in 2022) linked to global net sales of LIBTAYO; those royalties are recognized within Other operating income in line with the pattern of sales. All of the cash inflows relating to the above items (€196 million in 2023, €952 million in 2022) are presented within Net cash provided by/(used in) operating activities in the consolidated statement of cash flows.
The amendment to the terms of the IO LCA resulted in Sanofi recognizing an accelerated amortization charge of €226 million in 2022; this was allocated to the LIBTAYO product rights included within the residual carrying amount of the intangible asset recognized in July 2015 to reflect rights to an antibody targeting the immune checkpoint receptor PD-1 (programmed cell death protein-1) under the Sanofi/Regeneron alliance.
The transaction also includes a time-limited transitional services agreement with Regeneron which includes manufacturing, distribution (for which Sanofi acts as agent), and promotion.
Investor agreement
In 2014 and 2020, Sanofi and Regeneron amended the investor agreement entered into by the two companies in 2007. Under the terms of the amendments, Sanofi accepted various restrictions, including “standstill” provisions that contractually prohibit Sanofi from seeking to directly or indirectly exert control of Regeneron or acquiring more than 30 % of Regeneron’s capital stock (consisting of the outstanding shares of common stock and the shares of Class A stock). This prohibition remains in place until the earlier of (i) the later of the fifth anniversaries of the expiration or earlier termination of the ZALTRAP collaboration agreement with Regeneron (related to the development and commercialization of ZALTRAP) or the collaboration agreement with Regeneron on monoclonal antibodies (see “Collaboration agreements on human therapeutic antibodies” above), each as amended or (ii) other specified events.
Starting in 2018 Sanofi began to sell shares of Regeneron stock and announced on May 29, 2020 the closing of its sale of 13 million shares of Regeneron common stock in a registered offering and a private sale to Regeneron (see Note D.2.).
Pursuant to subsequent sales in 2022, Sanofi no longer holds any shares of Regeneron stock, as of December 31, 2023.
SANOFI FORM 20-F 2023 | F-31 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
C.2. Agreements on the commercialization of BEYFORTUS (nirsevimab, previously MEDI8897) in the US
On March 1, 2017, Sanofi and AstraZeneca entered into an agreement to develop and commercialize a monoclonal antibody (MEDI8897, nirsevimab) for the prevention of Respiratory Syncytial Virus (RSV) associated illness in newborns and infants.
Under the terms of the agreement, Sanofi made an upfront payment of €120 million in March 2017, a development milestone payment of €30 million in the third quarter of 2019, a regulatory milestone payment of €25 million associated with the approval of BEYFORTUS (nirsevimab) by the EMA in Europe in November 2022, and a regulatory milestone payment of €65 million associated with the approval of BEYFORTUS (nirsevimab) by the US FDA in July 2023. In addition, Sanofi could pay up to €375 million if sales objectives are met. Those amounts are recognized as a component of the value of the intangible asset when payment becomes probable. During 2023, an amount of €25 million was recognized as an accrued expense further to a contractual threshold being passed.
The agreement also specifies that AstraZeneca is responsible for development and manufacturing, and Sanofi for commercialization. Sanofi recognizes the sales and cost of sales (purchases of finished products from AstraZeneca) and shares the Alliance’s commercial profits (i) 50 /50 in major territories and (ii) based on 25 % of net sales in other territories. The share of commercial profits and losses due to or from AstraZeneca is recognized as a component of operating income, within the line items Other operating income or Other operating expenses. In addition, Sanofi and AstraZeneca share development costs 50 /50, with Sanofi’s portion recognized within the income statement line item Research and development expenses.
On April 9, 2023, Sanofi and AstraZeneca simplified their contractual agreements for the development and commercialization of BEYFORTUS (nirsevimab) in the US. Sanofi thereby obtained control of all commercial rights to BEYFORTUS (nirsevimab) in the US, and ended the sharing of commercial profits between the two partners in that territory. In line with the terms of the revised agreements and in accordance with IAS 38, Sanofi recognized an intangible asset of €1.6 billion for the fair value of the additional US rights. On the same date, AstraZeneca and Sobi ended their participation agreement, signed in 2018, which transferred the economic rights for the US territory to Sobi.
Sanofi simultaneously entered into an agreement with Sobi relating to direct royalties on US net sales of BEYFORTUS (nirsevimab). In line with the terms of that agreement, on April 9, 2023 Sanofi recognized a financial liability amounting to €1.6 billion. That liability is classified as a financial liability at amortized cost under IFRS 9. Other than royalty payments, subsequent movements in the liability comprise (i) the unwinding of discount and (ii) changes in estimates of future cash outflows for royalty payments. Those movements will be recognized in the income statement within Net financial income/(expenses) in accordance with paragraph B.5.4.6 of IFRS 9.
As of December 31, 2023 the liability was remeasured by an amount of €541 million, reflecting the strong success of the US launch of BEYFORTUS (nirsevimab), which led to sales forecasts being revised upward from the initial estimate. The resulting adjustment was recognized within Financial expenses.
For territories other than the US (except for China, which is now considered a “major market,” with profits/losses shared 50 /50 with AstraZeneca), the existing agreement between AstraZeneca and Sanofi continues to govern the principal terms of the collaboration: Sanofi recognizes the sales and cost of sales and shares the Alliance’s commercial profits with AstraZeneca.
In May 2023, data from the HARMONIE Phase 3b study confirmed that nirsevimab prevents infant hospitalizations due to RSV with consistent and high efficacy.
D/ Presentation of the financial statements
D.1. Principal changes in the scope of consolidation in 2023
Acquisition of Provention Bio, Inc.
On March 13, 2023, Sanofi entered into a merger agreement with Provention Bio, Inc. (Provention), a US-based publicly traded biopharmaceutical company developing therapies to prevent and intercept immune-mediated diseases including type 1 diabetes. Under the terms of the agreement, Sanofi acquired the outstanding shares of Provention common stock for $25.00 per share in an all-cash transaction valued at approximately $2.8 billion.
The acquisition of Provention was completed on April 27, 2023, with Sanofi holding all of the shares of Provention on expiration of the tender offer.
Sanofi applied the optional test to identify concentration of fair value under paragraph B7A of IFRS 3. The transaction was accounted for as an acquisition of a group of assets, given that the principal asset (teplizumab-mzwv, commercialized in the United States under the name TZIELD) concentrates substantially all of the fair value of the acquired set of activities and assets.
Under the terms of a share purchase agreement entered into by Sanofi and Provention in February 2023, Sanofi already held an equity interest in Provention, representing approximately 3 % of Provention’s share capital. On the date Sanofi obtained control of Provention, that equity interest was remeasured at a price of $25.00 per share, representing a total amount of $68 million. The impact of the remeasurement was recognized in Other comprehensive income.
F-32 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The acquisition price for the shares not already held was $2,806 million. Out of the total price (including the fair value of the shares already held), $2,810 million was allocated to TZIELD and recognized within Other intangible assets. The difference between that amount and the acquisition price corresponds to the other assets acquired and liabilities assumed as part of the transaction, after taking account of the previously-held shares and acquisition-related costs.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of $2,722 million.
Acquisition of QRIB Intermediate Holdings, LLC
On July 28, 2023, Sanofi announced that it had acquired QRIB Intermediate Holdings, LLC (QRIB), the owner of QUNOL, a market-leading US-based health & wellness brand. The acquisition strengthened Sanofi's Consumer Healthcare (CHC) operations in the Vitamin, Mineral and Supplements (VMS) category.
The acquisition of QRIB by Sanofi was completed on September 29, 2023, at a purchase price of $1,419 million.
The provisional purchase price allocation led to the recognition of goodwill of €475 million, determined as follows:
| (€ million) | Fair value at acquisition date | ||||
Other intangible assets | |||||
Other current and non-current assets and liabilities | |||||
Cash and cash equivalents | |||||
Deferred taxes, net | ( | ||||
Net assets of QRIB Intermediate Holdings, LLC | |||||
Goodwill | |||||
Purchase price | |||||
The other acquired intangible assets identified consist of the QUNOL brand.
Goodwill mainly represents the expected future profits attributable to the development of the VMS platform in the United States as a result of the integration of QRIB into the Sanofi group.
The entire amount of goodwill is deductible for tax purposes over a period of 15 years.
Since the acquisition date, QRIB has generated net sales of €71 million, and has made an immaterial impact on consolidated net income.
The acquisition-related costs were recognized in profit or loss during 2023 within the line item Other operating expenses; the amount involved was immaterial .
The impact of this acquisition is reflected in Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows, and represents a net cash outflow of $1,410 million.
SANOFI FORM 20-F 2023 | F-33 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.2. Principal changes in the scope of consolidation in 2022 and 2021
D.2.1. Principal changes in the scope of consolidation in 2022
Acquisition of Amunix Pharmaceuticals, Inc.
On February 8, 2022, Sanofi acquired the entire share capital of the immuno-oncology company Amunix Pharmaceuticals, Inc. (Amunix), thereby gaining access to Amunix’s innovative PRO-XTEN technology and a promising pipeline of immunotherapies.
The acquisition price of Amunix comprises a fixed cash payment of €970 million, plus contingent consideration in the form of milestone payments based on attainment of certain future development objectives of up to $225 million, the fair value of which as of the acquisition date was €156 million. In accordance with IFRS 3, this contingent purchase consideration was recognized in Liabilities related to business combinations and non-controlling interests (see Note D.18.).
The final purchase price allocation led to the recognition of €609 million of goodwill, determined as follows:
| (€ million) | Fair value at acquisition date | ||||
Other intangible assets | |||||
Other current and non-current assets and liabilities | ( | ||||
Cash and cash equivalents | |||||
| Deferred taxes, net | ( | ||||
| Net assets of Amunix | |||||
| Goodwill | |||||
| Purchase price | |||||
“Other intangible assets” comprise PRO-XTEN, an innovative universal protease-releasable masking technology platform for the discovery and development of transformative cytokine therapies and T-cell engager (TCE) immunotherapies for patients with cancer. In 2023, an impairment loss was taken against the PRO-XTEN platform, in line with a strategic decision to de-prioritize certain R&D programs (see Note D.5., "Impairment of intangible assets and property, plant and equipment’).
Goodwill mainly represents the value of Amunix’s upstream research and development pipeline of immuno-oncology therapies based on next-generation conditionally activated biologics, especially when combined with Sanofi’s existing oncology portfolio.
The goodwill generated on this acquisition does not give rise to any deduction for income tax purposes.
Amunix has no commercial operations.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a cash outflow of €852 million.
EUROAPI - Loss of control and accounting implications
On March 17, 2022, the Sanofi Board of Directors approved a decision to put to a shareholder vote the proposed distribution in kind of approximately 58 % of the share capital of EUROAPI, thereby confirming Sanofi’s commitment (announced in February 2020) to discontinue its active pharmaceutical ingredient operations. As part of the same corporate action and on the same date, Sanofi entered into an investment agreement with EPIC Bpifrance, which undertook to acquire from Sanofi – via the French Tech Souveraineté fund – a 12 % equity interest in EUROAPI at a price not exceeding €150 million and to be determined on the basis of the volume weighted average price (VWAP) of EUROAPI shares on the Euronext Paris regulated market over the thirty-day period starting from the date of initial listing, i.e. May 6, 2022. On completion of those transactions, Sanofi holds an equity interest of 30.1 % in EUROAPI, which it has undertaken to retain for at least two years from the date of the distribution, subject to the customary exceptions. With effect from that date, Sanofi exercises significant influence over EUROAPI as a result of (i) its equity interest, and (ii) having one representative on the EUROAPI Board of Directors.
On May 3, 2022, the General Meeting of Sanofi shareholders approved the decision of the Board of Directors to distribute approximately 58 % of the share capital of EUROAPI in the form of an exceptional dividend in kind.
On May 10, 2022, the payment date of the dividend in kind in the days following the admission to listing of EUROAPI shares, those Sanofi shareholders who had retained their Sanofi shares received 1 EUROAPI share per 23 Sanofi shares, representing in total 57.88 % of the share capital of EUROAPI. As of that date, Sanofi lost control over the EUROAPI entities, based on an assessment of the criteria specified in IFRS 10 (Consolidated financial statements). The assets and liabilities of EUROAPI, which since March 17, 2022 had been presented as assets and liabilities held for sale within the Sanofi statement of financial position in accordance with IFRS 5 (Non-Current Assets Held for Sale), were deconsolidated. In addition, because EUROAPI operations do not constitute a discontinued operation under IFRS 5, the contribution from EUROAPI has not been presented within separate line items in the income statement and statement of cash flows or in information for prior comparative periods. The contribution of EUROAPI operations to the consolidated net sales of Sanofi in the year ended December 31, 2021 was €486 million.
F-34 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The principal consequences of the deconsolidation of EUROAPI are described below:
•the derecognition of the carrying amount of all the assets and liabilities of EUROAPI, representing a net amount of €1,227 million as of May 10, 2022. This includes goodwill of €164 million, determined in accordance with IAS 36 (“Impairment of Assets”), which was historically allocated to the Pharmaceuticals cash generating unit (CGU), and which for the purposes of the deconsolidation was allocated using an alternative method based on the relative values of goodwill as of the date of consolidation (the “notional goodwill method”). That method was considered more appropriate to the capital-intensive nature of EUROAPI operations than the method based on the relative values of EUROAPI operations and the retained portion of the CGU;
•a reduction in Equity attributable to equity holders of Sanofi reflecting the distribution in kind, measured at €793 million based on the weighted average price of €14.58 per share as of the date of delivery of the EUROAPI shares to Sanofi shareholders and corresponding to the fair value of the distribution in accordance with IFRIC 17 (Distribution of Non-Cash Assets to Owners);
•a cash inflow of €150 million from the divestment of 12 % of the share capital of EUROAPI to EPIC Bpifrance as of the settlement date of the shares, i.e. June 17, 2022;
•the recognition in the statement of financial position, within the line item Investments accounted for using the equity method, of the retained 30.1 % equity interest in EUROAPI at an amount of €413 million, determined on the basis of the weighted average price of €14.58 per share and representing the fair value of the equity interest in accordance with IFRS 10;
•the reclassification within the net gain/loss on deconsolidation of unrealized foreign exchange losses amounting to €35 million arising on EUROAPI subsidiaries, in accordance with IAS 21 (The Effects of Changes in Foreign Exchange Rates);
•the recognition of transaction-related costs and of the effects of undertakings made under agreements entered into with EUROAPI setting out the principles and terms of the legal reorganization carried out ahead of the date of deconsolidation. The principal undertakings made to EUROAPI relate to compensation for:
–environmental remediation obligations on non-operational chemical sites in France transferred to EUROAPI, amounting to €14 million, and
–regulatory compliance costs relating to certain state-of-the-art active pharmaceutical ingredients of EUROAPI, capped at €15 million.
These elements collectively resulted in a pre-tax loss on deconsolidation of €3 million, presented within the line item Other gains and losses, and litigation in the income statement. The tax effect of the deconsolidation was a net gain of €111 million, presented within the line item Income tax expense in the income statement.
The cash impact of the deconsolidation of EUROAPI, presented within the line item Disposals of consolidated undertakings and investments accounted for using the equity method in the statement of cash flows, was a net cash inflow of €101 million.
Sanofi has entered into an agreement with EUROAPI for the manufacture and supply of active pharmaceutical ingredients, intermediates and other substances, which took effect on October 1, 2021 and expires five years after the loss of control. Under the terms of the agreement, Sanofi committed to target annual net sales of approximately €300 million for a list of specified active ingredients until the agreement expires in 2026. As of December 31, 2022, that commitment amounted to €1.1 billion.
As of the date of deconsolidation, the 30.1 % equity interest in EUROAPI is accounted for using the equity method in accordance with IAS 28 (Investments in Associates and Joint Ventures), and the share of EUROAPI profits or losses arising from application of the equity method is excluded from “Business operating income”, the non-IFRS financial indicator used internally by Sanofi to measure the performance of its operating segments.
D.2.2. Principal changes in the scope of consolidation in 2021
Acquisition of Kymab
On April 8, 2021, Sanofi acquired the entire share capital of Kymab for an upfront payment of $1.1 billion (€973 million) and up to $350 million contingent upon reaching certain development milestones.
Sanofi applied the optional test to identify concentration of fair value under paragraph B7A of IFRS 3. The transaction was accounted for as an asset acquisition given that the principal asset (the KY1005 project, currently in Phase 2 clinical development, and relating to the human monoclonal antibody OX40L, an essential regulator of the immune system) concentrates substantially all of the fair value of the acquired set of activities and assets.
Of the total acquisition price paid, €965 million was allocated to Other intangible assets in accordance with IAS 38. The difference between that amount and the acquisition price corresponds to the other assets acquired and liabilities assumed as part of the transaction.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of €932 million.
SANOFI FORM 20-F 2023 | F-35 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Acquisition of Kiadis
On November 2, 2020, Sanofi and Kiadis, a biopharmaceutical company developing novel "off-the-shelf" natural killer (NK) cell therapies for patients with life-threatening diseases, entered into a definitive agreement whereby Sanofi was to make a public offer to acquire the entire share capital of Kiadis, i.e. 61 million shares, at a cash price of €5.45 per share.
The acquisition was approved unanimously by the Boards of Directors of Sanofi and Kiadis, and 95.03 % of the share capital of Kiadis had been tendered into the offer as of April 16, 2021. As of the end of the post-closing acceptance period on April 29, 2021, Sanofi held 97.39 % of the share capital of Kiadis, and proceeded to buy out the remaining non-controlling shareholders. As of December 31, 2022, Sanofi held 100.00 % of the share capital of Kiadis.
Sanofi applied the optional test to identify concentration of fair value under paragraph B7A of IFRS 3. The transaction was accounted for as an asset acquisition given that the principal asset (the K-NK technology platform) concentrates substantially all of the fair value of the acquired set of activities and assets. In 2023, an impairment loss was recognized against the K-NK technology platform to reflect the impact of the strategic decision to de-prioritize certain R&D programs (see Note D.5., "Impairment of intangible assets and property, plant and equipment").
Of the total acquisition price paid, €339 million was allocated to Other intangible assets in accordance with IAS 38. The difference between that amount and the acquisition price corresponds to the other assets acquired and liabilities assumed as part of the transaction.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of €326 million.
Acquisition of Tidal
On April 9, 2021, Sanofi acquired Tidal Therapeutics, a privately owned, pre-clinical stage biotech company with a unique mRNA-based approach for in vivo reprogramming of immune cells. The new technology platform will expand Sanofi’s research capabilities in immuno-oncology and inflammatory diseases, and may have applicability to other disease areas as well.
Tidal Therapeutics was acquired for an upfront payment of $160 million (€136 million), and up to $310 million contingent upon reaching certain development milestones.
Sanofi applied the optional test to identify concentration of fair value under paragraph B7A of IFRS 3. The transaction was accounted for as an asset acquisition given that the principal asset (the unique mRNA-based in vivo reprogramming technology) concentrates substantially all of the fair value of the acquired set of activities and assets.
Of the total acquisition price paid, €130 million was allocated to Other intangible assets in accordance with IAS 38. The difference between that amount and the acquisition price corresponds to the other assets acquired and liabilities assumed as part of the transaction.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of €135 million.
Acquisition of Translate Bio
On August 3, 2021, Sanofi entered into a definitive agreement with Translate Bio, a clinical-stage mRNA therapeutics company, under which Sanofi was to acquire all outstanding shares of Translate Bio for $38 per share. The Sanofi and Translate Bio Boards of Directors unanimously approved the transaction.
The acquisition of Translate Bio by Sanofi was completed on September 14, 2021, with Sanofi holding the entire share capital of Translate Bio upon expiration of the squeeze-out procedure.
The final purchase price allocation, as presented in the table below, led to the recognition of goodwill of €2,118 million:
| (€ million) | Fair value at acquisition date | ||||
| Other intangible assets | |||||
| Deferred tax liabilities | ( | ||||
| Other current and non-current assets and liabilities | |||||
| Cash and cash equivalents | |||||
| Shire contingent consideration liability (see Note D.18.) | ( | ||||
| Net assets of Translate Bio | |||||
| Goodwill | |||||
| Purchase price | |||||
The other intangible assets mainly comprise a messenger RNA technological platform applied to the development of vaccines and therapeutic agents.
Goodwill mainly represents the effects of expected future synergies and other benefits to be derived from the integration of Translate Bio into the Sanofi group, in particular by accelerating the delivery of development programs.
The goodwill generated on this acquisition did not give rise to any deduction for income tax purposes.
F-36 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Translate Bio has no commercial operations.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of €2,333 million.
Under the terms of the collaboration agreement between Sanofi and Translate Bio as announced on June 23, 2020, Sanofi held an equity interest of approximately 5 % in Translate Bio. As of the date when Sanofi obtained control of Translate Bio, that interest was remeasured at the purchase price of $38 per share. The change in fair value was recognized within Other comprehensive income, in accordance with paragraph 42 of IFRS 3 (see Note D.7.).
Acquisition of Kadmon
On September 8, 2021, Sanofi entered into a definitive merger agreement with Kadmon, a biopharmaceutical company that discovers, develops and markets transformative therapies for disease areas with significant unmet medical needs. Shareholders of Kadmon common stock received $9.50 per share in cash, representing a transaction valued at $1.9 billion on a fully-diluted basis. The Sanofi and Kadmon Boards of Directors unanimously approved the transaction.
The acquisition of Kadmon by Sanofi was completed on November 9, 2021, with Sanofi holding the entire share capital of Kadmon upon expiration of the squeeze-out procedure.
Sanofi applied the optional test to identify concentration of fair value under paragraph B7A of IFRS 3. The transaction was therefore accounted for as an asset acquisition given that the principal asset (belumosudil, commercialized in the United States under the brand name REZUROCK) concentrates substantially all of the fair value of the acquired set of activities and assets.
Of the total acquisition price paid, €1,739 million was allocated to Other intangible assets in accordance with IAS 38. The difference between that amount and the acquisition price corresponds to the other assets acquired and liabilities assumed as part of the transaction.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows is a net cash outflow of €1,575 million.
Acquisition of Origimm
On December 3, 2021, Sanofi acquired the entire share capital of Origimm Biotechnology GmbH, a privately owned Austrian biotechnology company specializing in the discovery of virulent skin microbiome components and antigens from bacteria that cause skin diseases such as acne, for an upfront payment of €55 million and up to €95 million contingent upon reaching certain development and regulatory milestones.
Sanofi applied the optional test to identify concentration of fair value under paragraph B7A of IFRS 3. The transaction was therefore accounted for as an asset acquisition given that the principal asset (the group of Propionibacterium acnes antigens) concentrates substantially all of the fair value of the acquired set of activities and assets.
Nearly €55 million of the acquisition price paid was allocated to Other intangible assets in accordance with IAS 38. The difference between that amount and the acquisition price corresponds to the other assets acquired and liabilities assumed as part of the transaction.
The impact of this acquisition as reflected within the line item Acquisitions of consolidated undertakings and investments accounted for using the equity method in the consolidated statement of cash flows for the year ended December 31, 2021 is a net cash outflow of €50 million.
SANOFI FORM 20-F 2023 | F-37 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.3. Property, plant and equipment
D.3.1. Property, plant and equipment owned
Property, plant and equipment owned by Sanofi is comprised of the following items:
| (€ million) | Land | Buildings | Machinery and equipment | Fixtures, fittings and other | Property, plant and equipment in process | Total | ||||||||||||||
| Gross value at January 1, 2021 | ||||||||||||||||||||
| Changes in scope of consolidation | ||||||||||||||||||||
| Acquisitions and other increases | ||||||||||||||||||||
| Disposals and other decreases | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Currency translation differences | ||||||||||||||||||||
Transfers(a) | ( | ( | ||||||||||||||||||
| Gross value at December 31, 2021 | ||||||||||||||||||||
| Changes in scope of consolidation | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Acquisitions and other increases | ||||||||||||||||||||
| Disposals and other decreases | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Currency translation differences | ||||||||||||||||||||
Transfers(a) | ( | ( | ( | |||||||||||||||||
| Gross value at December 31, 2022 | ||||||||||||||||||||
| Changes in scope of consolidation | ( | ( | ( | ( | ( | |||||||||||||||
| Acquisitions and other increases | ||||||||||||||||||||
| Disposals and other decreases | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ( | ( | ||||||||||||||
Transfers(a) | ( | ( | ( | |||||||||||||||||
| Gross value at December 31, 2023 | ||||||||||||||||||||
| Accumulated depreciation & impairment at January 1, 2021 | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Depreciation expense | ( | ( | ( | ( | ||||||||||||||||
| Impairment losses, net of reversals | ( | ( | ( | ( | ( | |||||||||||||||
| Disposals and other decreases | ||||||||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ||||||||||||||||
Transfers(a) | ( | |||||||||||||||||||
| Accumulated depreciation & impairment at December 31, 2021 | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Changes in scope of consolidation | ||||||||||||||||||||
| Depreciation expense | ( | ( | ( | ( | ||||||||||||||||
| Impairment losses, net of reversals | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Disposals and other decreases | ||||||||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ||||||||||||||||
Transfers(a) | ( | |||||||||||||||||||
| Accumulated depreciation & impairment at December 31, 2022 | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Changes in scope of consolidation | ||||||||||||||||||||
| Depreciation expense | ( | ( | ( | ( | ||||||||||||||||
| Impairment losses, net of reversals | ( | ( | ( | ( | ( | |||||||||||||||
| Disposals and other decreases | ||||||||||||||||||||
| Currency translation differences | ||||||||||||||||||||
Transfers(a) | ( | ( | ||||||||||||||||||
| Accumulated depreciation & impairment at December 31, 2023 | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Carrying amount at December 31, 2021 | ||||||||||||||||||||
| Carrying amount at December 31, 2022 | ||||||||||||||||||||
| Carrying amount at December 31, 2023 | ||||||||||||||||||||
(a) This line includes in particular property, plant and equipment in process brought into service during the period, but also the effect of the reclassification of assets to Assets held for sale or exchange.
F-38 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below sets forth acquisitions and capitalized interest by operating segment for the years ended December 31, 2023, 2022 and 2021:
| (€ million) | 2023 | 2022 | (a) | 2021 | (b) | ||||||||||||
| Acquisitions | |||||||||||||||||
Biopharma | |||||||||||||||||
| of which Manufacturing & Supply | |||||||||||||||||
Pharmaceuticals | |||||||||||||||||
Vaccines | |||||||||||||||||
| Consumer Healthcare | |||||||||||||||||
| of which Manufacturing & Supply | |||||||||||||||||
Of which capitalized interest | |||||||||||||||||
(a) 2022 figures have been adjusted to take into account of the two new operating segments, Biopharma and Consumer Healthcare, effective January 1, 2023.
(b) Due to a lack of available data and the complex adjustments that would be required (particularly for our reporting tools), the 2021 figures have not been restated to reflect changes arising from our new organizational structure.
Off balance sheet commitments relating to property, plant and equipment as of December 31, 2023, 2022 and 2021 are set forth below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Firm orders of property, plant and equipment | |||||||||||
| Property, plant and equipment pledged as security for liabilities | |||||||||||
The table below sets forth the net impairment losses recognized in each of the last three financial periods:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
Net impairment losses on property, plant and equipment(a) | |||||||||||
(a) These amounts mainly comprise impairment losses recognized as a result of decisions taken during the periods presented, relating primarily to shutdowns or changes in use of industrial sites.
SANOFI FORM 20-F 2023 | F-39 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.3.2. Property, plant and equipment leased – right-of-use assets
Right-of-use assets relating to property, plant and equipment leased by Sanofi are analyzed in the table below:
(€ million) | Right-of-use assets | ||||
| Gross value at January 1, 2021 | |||||
| Changes in scope of consolidation | |||||
Acquisitions and other increases(b) | |||||
| Disposals and other decreases | ( | ||||
| Currency translation differences | |||||
Transfers(a) | ( | ||||
| Gross value at December 31, 2021 | |||||
| Changes in scope of consolidation | ( | ||||
Acquisitions and other increases | |||||
| Disposals and other decreases | ( | ||||
| Currency translation differences | |||||
Transfers(a) | ( | ||||
| Gross value at December 31, 2022 | |||||
Acquisitions and other increases | |||||
| Disposals and other decreases | ( | ||||
| Currency translation differences | ( | ||||
Transfers(a) | ( | ||||
| Gross value at December 31, 2023 | |||||
| Accumulated depreciation & impairment at January 1, 2021 | ( | ||||
| Depreciation and impairment charged in the period | ( | ||||
| Disposals and other decreases | |||||
| Currency translation differences | ( | ||||
Transfers(a) | |||||
| Accumulated depreciation & impairment at December 31, 2021 | ( | ||||
| Changes in scope of consolidation | |||||
| Depreciation and impairment charged in the period | ( | ||||
| Disposals and other decreases | |||||
| Currency translation differences | ( | ||||
Transfers(a) | |||||
| Accumulated depreciation & impairment at December 31, 2022 | ( | ||||
| Depreciation and impairment charged in the period | ( | ||||
| Disposals and other decreases | |||||
| Currency translation differences | |||||
Transfers(a) | |||||
| Accumulated depreciation & impairment at December 31, 2023 | ( | ||||
| Carrying amount at December 31, 2021 | |||||
| Carrying amount at December 31, 2022 | |||||
| Carrying amount at December 31, 2023 | |||||
(a) This line also includes the effect of the reclassification of assets to Assets held for sale or exchange.
(b) In December 2018, Sanofi signed two leases on real estate assets in the United States (at Cambridge, Massachusetts) for an initial lease term of 15 years. The first lease, relating to office space, began in April 2021; Sanofi recognized a right-of-use asset of €320 424
Leased assets comprised offices and industrial premises (90 %) and the vehicle fleet (10 %) as of December 31, 2023.
Annual lease costs on short term leases and low value asset leases amounted to €19 million in the year ended December 31, 2023, €26 million in the year ended December 31, 2022, and €25 million in the year ended December 31, 2021. Variable lease payments, sub-leasing activities, and sale-and-leaseback transactions were immaterial.
Total cash outflows on leases (excluding annual lease costs on short term leases and low value asset leases) were €315 million in the year ended December 31, 2023, €389 million in the year ended December 31, 2022, and €302 million in the year ended December 31, 2021.
A maturity analysis of the lease liability is disclosed in Note D.17.2.
Commitments related to short-term leases and low value asset leases, including future payments for lease contracts committed but not yet commenced, are disclosed in Note D.21.
F-40 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.4. Goodwill and other intangible assets
Movements in goodwill comprise:
| (€ million) | Goodwill | ||||
| Balance at January 1, 2021 | |||||
| Acquisitions during the period | |||||
Other movements during the period(a) | ( | ||||
| Currency translation differences | |||||
| Balance at December 31, 2021 | |||||
| Acquisitions during the period | |||||
Other movements during the period(a) | ( | ||||
| Currency translation differences | |||||
| Balance at December 31, 2022 | |||||
| Acquisitions during the period | |||||
Other movements during the period(a) | ( | ||||
| Currency translation differences | ( | ||||
| Balance at December 31, 2023 | |||||
(a) This line mainly comprises the amount of goodwill allocated to divested operations in accordance with paragraph 86 of IAS 36, and in 2022 the loss of control of EUROAPI (see note D.2.1.).
In accordance with IAS 36, goodwill is allocated to groups of Cash Generating Units (CGUs) at a level corresponding to the Biopharma and Consumer Healthcare segments. When testing goodwill annually for impairment, the recoverable amount is determined for each of the
The allocation of goodwill by segment as of December 31, 2023 is as follows:
(€ million) | Biopharma | Consumer Healthcare | Total | ||||||||
Goodwill | |||||||||||
Acquisition of QRIB Intermediate Holdings, LLC (2023)
The provisional purchase price allocation for QRIB Intermediate Holdings, LLC resulted in the recognition of intangible assets (other than goodwill) of €774 million as of the acquisition date (September 29, 2023), and of goodwill provisionally measured at €475 million as of the acquisition date (see Note D.1.).
Acquisition of Amunix Pharmaceuticals, Inc. (2022)
The final purchase price allocation for Amunix Pharmaceuticals, Inc. resulted in the recognition of intangible assets (other than goodwill) of €493 million as of the acquisition date (February 8, 2022), and of goodwill measured at €609 million as of the acquisition date (see Note D.2.1.).
Acquisition of Translate Bio (2021)
The final purchase price allocation for Translate Bio resulted in the recognition of intangible assets (other than goodwill) of €396 million as of the acquisition date (September 14, 2021), and of goodwill measured at €2,118 million as of the acquisition date (see Note D.2.2.).
SANOFI FORM 20-F 2023 | F-41 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Movements in other intangible assets comprise:
| (€ million) | Acquired R&D | Products, trademarks and other rights | Software | Total other intangible assets | ||||||||||
Gross value at January 1, 2021(a) | ||||||||||||||
Changes in scope of consolidation(c) | ||||||||||||||
Acquisitions and other increases | ||||||||||||||
| Disposals and other decreases | ( | ( | ( | ( | ||||||||||
Currency translation differences | ||||||||||||||
Transfers(b) | ( | ( | ||||||||||||
Gross value at December 31, 2021 | ||||||||||||||
Changes in scope of consolidation(c) | ( | |||||||||||||
| Acquisitions and other increases | ||||||||||||||
| Disposals and other decreases | ( | ( | ( | ( | ||||||||||
| Currency translation differences | ||||||||||||||
Transfers(b) | ( | ( | ( | |||||||||||
Gross value at December 31, 2022 | ||||||||||||||
Changes in scope of consolidation(c) | ||||||||||||||
Acquisitions and other increases(f) | ||||||||||||||
| Disposals and other decreases | ( | ( | ( | ( | ||||||||||
| Currency translation differences | ( | ( | ( | ( | ||||||||||
Transfers(b) | ( | ( | ( | |||||||||||
| Gross value at December 31, 2023 | ||||||||||||||
Accumulated amortization & impairment at January 1, 2021(a) | ( | ( | ( | ( | ||||||||||
Amortization expense | ( | ( | ( | |||||||||||
Impairment losses, net of reversals(d) | ( | ( | ( | |||||||||||
| Disposals and other decreases | ||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ||||||||||
Accumulated amortization & impairment at December 31, 2021 | ( | ( | ( | ( | ||||||||||
Changes in scope of consolidation(c) | ||||||||||||||
Amortization expense(e) | ( | ( | ( | |||||||||||
Impairment losses, net of reversals(d) | ( | |||||||||||||
| Disposals and other decreases | ||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ||||||||||
Transfers(b) | ( | |||||||||||||
Accumulated amortization & impairment at December 31, 2022 | ( | ( | ( | ( | ||||||||||
Changes in scope of consolidation(c) | ||||||||||||||
Amortization expense | ( | ( | ( | |||||||||||
Impairment losses, net of reversals(d) | ( | ( | ( | |||||||||||
| Disposals and other decreases | ||||||||||||||
| Currency translation differences | ||||||||||||||
Transfers(b) | ||||||||||||||
| Accumulated amortization & impairment at December 31, 2023 | ( | ( | ( | ( | ||||||||||
| Carrying amount at December 31, 2021 | ||||||||||||||
| Carrying amount at December 31, 2022 | ||||||||||||||
| Carrying amount at December 31, 2023 | ||||||||||||||
(a) Includes the impact of the IFRIC agenda decision of March 2021 on the costs of configuring or customising application software used in a Software as a Service (SaaS) arrangement (see Note A.2.1. to our 2021 consolidated financial statements, included in our annual report on Form 20-F for that year).
(b) The “Transfers” line mainly comprises (i) acquired R&D that came into commercial use during the period and (ii) reclassifications of assets as Assets held for sale or exchange.
(c) The “Changes in scope of consolidation” line mainly comprises the fair value of intangible assets recognized in connection with acquisitions made during the period (see Notes D.1. and D.2.).
(d) See Note D.5.
(e) The amendment to the terms of the IO License and Collaboration Agreement resulted in the recognition of an amortization charge of €226 million in 2022 (see Note C.1.).
(f) This line mainly comprises:
–the rights acquired as a result of the simplification agreed between Sanofi and AstraZeneca in April 2023 in respect of the agreements on BEYFORTUS (nirsevimab) (see Note C.2.);
–an upfront payment of $500 million relating to the rights acquired under the agreement with Teva Pharmaceuticals on the co-development and co-commercialization of TEV’574; and
–an upfront payment of $175 million for the rights acquired under the agreement with Janssen Pharmaceuticals, Inc. relating to a vaccine against extra-intestinal pathogenic strains of E-Coli.
F-42 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
“Products, trademarks and other rights” mainly comprise:
•“marketed products”, with a carrying amount of €16.6 billion as of December 31, 2023 (versus €12.7 billion as of December 31, 2022 and €11.7 billion as of December 31, 2021) and a weighted average amortization period of approximately 11 years; and
•“technology platforms”, with a carrying amount of €1.2 billion as of December 31, 2023 (versus €2.2 billion as of December 31, 2022 and €1.2 billion as of December 31, 2021) and a weighted average amortization period of approximately 18 years.
The table below provides information about the principal “marketed products”, which were recognized in connection with major acquisitions made by Sanofi and represented 96 % of the carrying amount of that item as of December 31, 2023:
| (€ million) | Gross value | Accumulated amortization & impairment | December 31, 2023 | Amortization period (years)(a) | Residual amortization period (years)(b) | Carrying amount at December 31, 2022 | Carrying amount at December 31, 2021 | ||||||||||||||||
Genzyme(c) | ( | ||||||||||||||||||||||
Boehringer Ingelheim Consumer Healthcare(c) | ( | ||||||||||||||||||||||
Aventis(c) | ( | ||||||||||||||||||||||
Chattem(c) | ( | ||||||||||||||||||||||
Protein Sciences(c) | ( | ||||||||||||||||||||||
Ablynx(c) | ( | ||||||||||||||||||||||
Bioverativ(c) | ( | ||||||||||||||||||||||
| REZUROCK | ( | ||||||||||||||||||||||
TZIELD | ( | ||||||||||||||||||||||
| BEYFORTUS | ( | ||||||||||||||||||||||
| QUNOL | ( | ||||||||||||||||||||||
| Total: principal marketed products | ( | ||||||||||||||||||||||
(a) Weighted averages. The amortization periods for these products vary between 1 and 25 years.
(b) Weighted averages.
(c) Commercialized products derived from the acquisition of these companies.
The principal intangible assets brought into service during 2023 were:
ALTUVIIIO (efanesoctocog alfa), which extends protection from bleeds and treats acute hemorrhages in people with hemophilia A. The asset came into service on the date of marketing approval (February 23, 2023), and has a gross value of €1,110 million.
During 2022, some of the acquired research and development came into commercial use, and started being amortized from the date of marketing approval; the main item involved was ENJAYMO (sutimlimab-jome), a treatment for cold agglutinin disease.
The main asset brought into service during 2021 was the Translate Bio mRNA technology platform.
Amortization of other intangible assets is recognized in the income statement within the line item Amortization of intangible assets, except for amortization of software and other rights of an industrial or operational nature which is recognized in the relevant classification of expense by function. An analysis of amortization of software is shown in the table below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Cost of sales | |||||||||||
| Research and development expenses | |||||||||||
| Selling and general expenses | |||||||||||
| Other operating expenses | |||||||||||
| Total | |||||||||||
SANOFI FORM 20-F 2023 | F-43 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.5. Impairment of intangible assets and property, plant and equipment
Goodwill
When testing goodwill annually for impairment, the recoverable amount is determined for each of the two segments (Biopharma and Consumer Healthcare) on the basis of value in use, as derived from discounted estimates of the future cash flows in accordance with the policies described in Note B.6.1.
The value in use of each segment was determined by applying an after-tax discount rate to estimated future after-tax cash flows.
A separate discount rate is used for each segment to reflect the specific economic conditions of that segment.
The rates used for impairment testing in 2023 were 7.00 % for the Consumer Healthcare segment and 7.25 % for the Biopharma segment; an identical value in use for Sanofi as a whole would be obtained by applying a uniform 7.2 % rate to two segments.
The pre-tax discount rates applied to estimated pre-tax cash flows are calculated by iteration from the previously-determined value in use. Those pre-tax discount rates were 9.3 % for the Consumer Healthcare segment and 10.0 % for the Biopharma segment, and equate to a uniform rate of 9.9 % for Sanofi as a whole.
The assumptions used in testing goodwill for impairment are reviewed annually. Apart from the discount rate, the principal assumptions used in 2023 were as follows:
•the perpetual growth rates applied to future cash flows were zero for the Biopharma segment, and 1 % for the Consumer Healthcare segment;
•Sanofi also applies assumptions on the probability of success of current research and development projects, and more generally on its ability to renew the product portfolio in the longer term.
Value in use (determined as described above) is compared with the carrying amount, and this comparison is then subjected to sensitivity analyses by reference to the principal parameters, including:
•changes in the discount rate;
•changes in the perpetual growth rate; and
•fluctuations in operating margin.
No impairment of goodwill would need to be recognized in the event of a reasonably possible change in the assumptions used in 2023.
A value in use calculation for each of the segments would not result in an impairment loss using:
•a discount rate up to 2.8 percentage points above the rates actually used; or
•a perpetual growth rate up to 4.9 percentage points below the rates actually used; or
•an operating margin up to 7.2 percentage points below the rates actually used.
Other intangible assets
When there is evidence that an asset may have become impaired, the asset’s value in use is calculated by applying an after-tax discount rate to the estimated future after-tax cash flows from that asset. For the purposes of impairment testing, the tax cash flows relating to the asset are determined using a notional tax rate incorporating the notional tax benefit that would result from amortizing the asset if its value in use were regarded as its depreciable amount for tax purposes. Applying after-tax discount rates to after-tax cash flows gives the same values in use as would be obtained by applying pre-tax discount rates to pre-tax cash flows.
The after-tax discount rates used in 2023 for impairment testing of other intangible assets in the Biopharma and Consumer Healthcare segments were obtained by adjusting Sanofi’s weighted average cost of capital to reflect specific country and business risks, giving after-tax discount rates in a range from 7.25 % to 8.25 %.
In most instances, there are no market data that would enable fair value less costs to sell to be determined other than by means of developing a similar estimate based on future cash flows. Consequently, recoverable amount is in substance equal to value in use. The estimates used to determine value in use are sensitive to assumptions specific to the nature of the asset and to Sanofi's activities. Apart from the discount rate, the principal assumptions used in 2023 were as follows:
•mid-term and long-term forecasts;
•perpetual growth or attrition rates, when applicable; and
•probability of success of current research and development projects.
The assumptions used in testing intangible assets for impairment are reviewed at least annually.
F-44 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
In 2023, 2022 and 2021, impairment testing of other intangible assets (excluding software) resulted in the recognition of net impairment losses as shown below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
Impairment of other intangible assets, net of reversals (excluding software) | ( | ||||||||||
| Marketed products | ( | ||||||||||
Biopharma(b) | ( | ||||||||||
| Consumer Healthcare | ( | ||||||||||
Research and development projects and technology platforms(a)(b)(c) | |||||||||||
Others | |||||||||||
(a) For 2023, this amount mainly comprises an impairment loss of €833 million, reflecting the impact of the strategic decision to de-prioritize certain R&D programs, in particular those related to the NK Cell and PRO-XTEN technology platforms.
(b) For 2022, this amount mainly comprises a reversal of €2,154 million of impairment losses taken against ELOCTATE and BIVV001 (assets belonging to the ELOCTATE franchise), consisting of €1,554 million for marketed products and €600 million for research and development projects respectively. In 2019, the launch of competing products for ELOCTATE led Sanofi to update its sales forecasts for products belonging to the franchise, as a result of which impairment losses of €2.8 billion were recognized against the assets in question. The reversal reflects the approval by the FDA on February 22, 2023 of ALTUVIIIO (the commercial name of efanesoctocog alpha, corresponding to the BIVV001 project), which was submitted in 2022.
(c) For 2022, this amount mainly comprises:
–an impairment loss of €1,586 million taken against the development project for SAR444245 (non-alpha interleukin-2), recognized following revised cash flow projections reflecting unfavorable developments in the launch schedule;
–the €600 million reversal relating to the BIVV001 project (see above).
For 2021, this line relates to the discontinuation of the development of sutimlimab in the treatment of Immune Thrombocytopenic Purpura (ITP), and to the termination of various research projects in Vaccines.
Property, plant and equipment
Impairment losses taken against property, plant and equipment are disclosed in Note D.3.
Risks and opportunities related to climate change
Sanofi has identified specific plausible scenarios to assess climate risks and opportunities liable to impact its activities in the medium and longer term.
These include:
•an Aggressive Mitigation scenario, based on global collaboration to start reducing emissions immediately to meet Paris Agreement goals (limit temperature increase to 1.5°C above pre-industrial levels), generating risks related to transitioning to a lower carbon economy and entailing extensive policy, legal, technology, and market changes to address mitigation and adaptation requirements;
•a No Climate Action scenario (leading to global warming of 4°C above pre-industrial levels by 2100), with event-driven physical risks resulting from climate change or longer term shifts in climate patterns leading to potential financial implications such as direct damage to assets and indirect impacts from supply chain disruption; changes in water availability, and in the sourcing or quality of resources; food security; and extreme temperature changes affecting premises, operations, supply chain, transport needs, and employee safety; and
•a Most Likely scenario, encompassing fragmented regional efforts to start reducing emissions but not at a sufficient level to meet Paris Agreement goals (emissions continue to increase but at a slowed rate, leading to a 2.8°C temperature increase).
The importance and likelihood of such risks have been assessed and have not led Sanofi to identify any material impact that could generate a risk of impairment of the assets of Sanofi’s CGUs.
D.6. Investments accounted for using the equity method
Investments accounted for using the equity method comprise associates and joint ventures (see Note B.1.), and are set forth below.
| (€ million) | % interest | 2023 | 2022 | 2021 | ||||||||||
EUROAPI(a) | ||||||||||||||
Infraserv GmbH & Co. Höchst KG(b) | ||||||||||||||
MSP Vaccine Company(c) | ||||||||||||||
| Other investments | ||||||||||||||
| Total | ||||||||||||||
(a) Following the distribution in kind and the acquisition of an equity interest by EPIC Bpifrance, Sanofi holds an equity interest in EUROAPI accounted for using the equity method in accordance with IAS 28 (see Note D.2.). As of December 31, 2023, an impairment loss of €231 million was recognized on the equity-accounted investment in EUROAPI in view of the fall in the share price since March 2023. The amount of the loss was determined on the basis of the share price as of December 31, 2023 (€5.73 ).
(b) Joint venture.
(c) Joint venture. MSP Vaccine Company owns 100 % of MCM Vaccine BV.
SANOFI FORM 20-F 2023 | F-45 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below shows Sanofi’s overall share of (i) profit or loss and (ii) other comprehensive income from investments accounted for using the equity method, showing the split between associates and joint ventures in accordance with IFRS 12 (the amounts for each individual associate or joint venture are not material):
| 2023 | 2022 | 2021 | ||||||||||||||||||
| (€ million) | Joint ventures | Associates | Joint ventures | Associates | Joint ventures | Associates | ||||||||||||||
Share of profit/(loss) from investments accounted for using the equity method(a) | ( | ( | ||||||||||||||||||
| Share of other comprehensive income from investments accounted for using the equity method | ( | ( | ( | ( | ||||||||||||||||
| Total | ( | ( | ||||||||||||||||||
(a) An impairment loss of €231 million was recognized on the equity-accounted interest in EUROAPI as of December 31, 2023, to reflect the fall in the share price since March 2023. The amount of the loss was determined on the basis of the quoted market price as of December 31, 2023 (€5.73 ).
The financial statements include arm’s length transactions between Sanofi and some equity-accounted investments that are classified as related parties. The principal transactions and balances with related parties are summarized below:
| (€ million) | 2023 | 2022 | (a) | 2021 | |||||||||||||
| Sales | |||||||||||||||||
Royalties and other income | |||||||||||||||||
Accounts receivable and other receivables(b) | |||||||||||||||||
Purchases and other expenses (including research expenses) | |||||||||||||||||
Accounts payable and other payables | |||||||||||||||||
(a) In 2022, these items include Sanofi's transactions with EUROAPI from May 10, 2022 (see Note D.2.).
(b) Includes loans to joint ventures and associates.
There were no
For off balance sheet commitments of an operational nature involving joint ventures, see Note D.21.1.
D.7. Other non-current assets
Other non-current assets comprise:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Equity instruments at fair value through other comprehensive income (D.7.1.) | |||||||||||
| Debt instruments at fair value through other comprehensive income (D.7.2.) | |||||||||||
| Other financial assets at fair value through profit or loss (D.7.3.) | |||||||||||
| Pre-funded pension obligations (Note D.19.1.) | |||||||||||
Long-term prepaid expenses | |||||||||||
Long-term loans and advances and other non-current receivables(a) | |||||||||||
| Derivative financial instruments (Note D.20.) | |||||||||||
| Total | |||||||||||
(a) As of December 31, 2023, this line includes:
- the renewal of a loan of €157 million to the BioAtrium joint venture which matures on December 1, 2031, of which €156 million was recognized in "Other current assets" as of December 31, 2022; and
- a receivable under a sub-lease amounting to €132 million (€170 million before discounting), versus €164 million (or €201 million before discounting) as of December 31, 2022.
F-46 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.7.1. Equity instruments at fair value through other comprehensive income
Quoted equity investments
The line “Equity instruments at fair value through other comprehensive income” includes equity investments quoted in an active market with a carrying amount of €470 million as of December 31, 2023, €387 million as of December 31, 2022 and €396 million as of December 31, 2021.
There were no material movements in quoted equity investments during the year ended December 31, 2023.
The main changes during previous years in quoted equity investments included in the “Equity instruments at fair value through other comprehensive income” category are described below:
•in 2022:
–the sale in June 2022 of the residual equity interest in Regeneron (see Note C.1.) for $174 million, the entire loss on which was recorded within Other comprehensive income, and
–the acquisition of an equity interest in Innovent Biologics, in connection with a strategic collaboration agreement to intensify development in oncology medicines signed in August 2022, which had a fair value of €250 million as of that date and €228 million as of December 31, 2022;
•in 2021:
–following completion of the acquisition of Translate Bio on September 14, 2021 (see Note D.2.2), the equity interest of approximately 5 % in Translate Bio previously held by Sanofi ceased to be accounted for in the “Equity instruments at fair value through other comprehensive income” category.
A 10% decline in stock prices of the quoted equity investments included within “Equity instruments at fair value through other comprehensive income” would have had a pre-tax impact of €47 million on Other comprehensive income as of December 31, 2023.
Unquoted equity investments
The line item “Equity instruments at fair value through other comprehensive income” also includes equity investments not quoted in an active market with a carrying amount of €618 million as of December 31, 2023, €549 million as of December 31, 2022 and €427 million as of December 31, 2021.
The change in unquoted equity investments included in the “Equity instruments at fair value through other comprehensive income” category during the year ended December 31, 2023 was mainly due to various equity stakes acquired through the Sanofi Ventures fund.
In addition, commitments relating to equity investments classified in this asset category amount to €65 million as of December 31, 2023 (compared to €60 million as of December 31, 2022).
D.7.2. Debt instruments at fair value through other comprehensive income
The “Debt instruments at fair value through other comprehensive income” category includes quoted euro-denominated senior bonds amounting to €346 million as of December 31, 2023, including €107 million of securities obtained in exchange for financial assets held to meet obligations to employees under post-employment benefit plans.
Sanofi held €329 million of quoted senior bonds as of December 31, 2022 and €447 million as of December 31, 2021.
As regards debt instruments held to meet obligations to employees under post-employment benefit plans, an increase of 10 basis points in market interest rates as of December 31, 2023 would have had a pre-tax impact of €1 million on Other comprehensive income.
As regards other quoted debt instruments, an increase of 10 basis points in market interest rates as of December 31, 2023 would have had a pre-tax impact of €1 million on Other comprehensive income.
Other comprehensive income recognized in respect of “Equity instruments at fair value through other comprehensive income” and “Debt instruments at fair value through other comprehensive income” represented unrealized after-tax gains of €349 million for the year ended December 31, 2023, versus unrealized after-tax gains of €256 million for the year ended December 31, 2022 and of €322 million for the year ended December 31, 2021.
An analysis of the change in gains and losses recognized in Other comprehensive income, and of items reclassified to profit or loss, is presented in Note D.15.7.
D.7.3. Other financial assets at fair value through profit or loss
The “Other financial assets at fair value through profit or loss” category includes:
•contingent consideration receivable by Sanofi following the dissolution of the Sanofi Pasteur MSD (SPMSD) joint venture, based on a percentage of MSD’s future sales during the 2017-2024 period of specified products previously distributed by SPMSD (see Note D.12.).
The fair value of the MSD contingent consideration was determined by applying the royalty percentage stipulated in the contract to discounted sales projections. A reduction of one percentage point in the discount rate would increase the fair value of the MSD contingent consideration by approximately 1 %.
SANOFI FORM 20-F 2023 | F-47 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Changes in the fair value of this contingent consideration are recognized in the income statement within the line item Fair value remeasurement of contingent consideration (see Note B.18.). As of December 31, 2023, the contingent consideration asset amounted to €214 million (including a non-current portion of €104 million), versus €303 million (non-current portion: €196 million) as of December 31, 2022 and €378 million (non current portion: €275 million) as of December 31, 2021;
•a portfolio of financial investments (amounting to €572 million as of December 31, 2023) held to fund a deferred compensation plan provided to certain employees (versus €512 million as of December 31, 2022 and €549 million as of December 31, 2021);
•unquoted securities not meeting the definition of equity instruments amounting to €132 million as of December 31, 2023 (versus €115 million as of December 31, 2022 and €78 million as of December 31, 2021). In addition, commitments relating to unquoted securities classified in this asset category amount to €159 million as of December 31, 2023 (compared to €192 million as of December 31, 2022).
D.8. Assets held for sale or exchange and liabilities related to assets held for sale or exchange
Assets held for sale or exchange, and liabilities related to assets held for sale or exchange, comprise:
(€ million) | December 31, 2023 | December 31, 2022 | December 31, 2021 | ||||||||
| Assets held for sale or exchange | |||||||||||
| Liabilities related to assets held for sale or exchange | |||||||||||
D.9. Inventories
Inventories comprise the following:
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||
| (€ million) | Gross value | Allowances | Carrying amount | Gross value | Allowances | Carrying amount | Gross value | Allowances | Carrying amount | ||||||||||||||||||||
| Raw materials | ( | ( | ( | ||||||||||||||||||||||||||
| Work in process | ( | ( | ( | ||||||||||||||||||||||||||
| Finished goods | ( | ( | ( | ||||||||||||||||||||||||||
| Total | ( | ( | ( | ||||||||||||||||||||||||||
Allowances include write-downs of products on hand pending marketing approval, except in specific circumstances where it is possible to estimate that recovery of the value of inventories as of the end of the reporting period is highly probable.
D.10. Accounts receivable
Accounts receivable break down as follows:
| (€ million) | December 31, 2023 | December 31, 2022 | December 31, 2021 | ||||||||
| Gross value | |||||||||||
| Allowances | ( | ( | ( | ||||||||
| Carrying amount | |||||||||||
The impact of allowances against accounts receivable in 2023 was a net expense of €8 million (versus a net amount of less than €1 million in 2022 and a net expense of €12 million in 2021).
The gross value of overdue receivables was €689 million as of December 31, 2023, versus €452 million as of December 31, 2022 and €455 million as of December 31, 2021.
| Overdue accounts | Overdue by | Overdue by | Overdue by | Overdue by | Overdue by | |||||||||||||||
| (€ million) | gross value | <1 month | 1 to 3 months | 3 to 6 months | 6 to 12 months | > 12 months | ||||||||||||||
| December 31, 2023 | ||||||||||||||||||||
| December 31, 2022 | ||||||||||||||||||||
| December 31, 2021 | ||||||||||||||||||||
Amounts overdue by more than one month relate mainly to public-sector customers.
Some Sanofi subsidiaries have assigned receivables to factoring companies or banks without recourse. The amount of receivables derecognized was €761 million as of December 31, 2023 (€131 million as of December 31, 2022 and €3 million as of December 31, 2021). The residual guarantees relating to such transfers were immaterial as of December 31, 2023.
F-48 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.11. Other current assets
An analysis of Other current assets is set forth below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Tax receivables, other than corporate income taxes | |||||||||||
| Prepaid expenses | |||||||||||
Other receivables(a) | |||||||||||
| Interest rate derivatives measured at fair value (see Note D.20.) | |||||||||||
| Currency derivatives measured at fair value (see Note D.20.) | |||||||||||
Other current financial assets(b) | |||||||||||
| Total | |||||||||||
(a) This line mainly comprises advance payments to suppliers, and receivables relating to Sanofi's activities as agent under a transitional services agreement.
(b) This item mainly comprises bank loans and receivables maturing in less than one year with high-grade counterparties. For 2021, this item also includes debt instruments derived from the acquisitions of Translate Bio and Kadmon (carried out in 2021) with maturities of more than three months at inception and less than 12 months at December 31, 2021.
D.12. Financial assets and liabilities measured at fair value
Under IFRS 7 (Financial Instruments: Disclosures), fair value measurements must be classified using a fair value hierarchy with the following levels:
•level 1: quoted prices in active markets for identical assets or liabilities (without modification or repackaging);
•level 2: quoted prices in active markets for similar assets and liabilities, or valuation techniques in which all important inputs are derived from observable market data;
•level 3: valuation techniques in which not all important inputs are derived from observable market data.
The valuation techniques used are described in Note B.8.5.
The table below shows the balance sheet amounts of assets and liabilities measured at fair value.
| 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||
Level in the fair value hierarchy | Level in the fair value hierarchy | Level in the fair value hierarchy | ||||||||||||||||||||||||||||||
(€ million) | Note | Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | ||||||||||||||||||||||
| Financial assets measured at fair value | ||||||||||||||||||||||||||||||||
| Quoted equity investments | D.7.1. | |||||||||||||||||||||||||||||||
| Unquoted equity investments | D.7.1. | |||||||||||||||||||||||||||||||
| Quoted debt securities | D.7.2. | |||||||||||||||||||||||||||||||
Unquoted debt securities not meeting the definition of equity instruments | D.7.3. | |||||||||||||||||||||||||||||||
| Contingent consideration relating to divestments | D.7.3. | |||||||||||||||||||||||||||||||
| Financial assets held to meet obligations under deferred compensation plans | D.7.3. & D.11. | |||||||||||||||||||||||||||||||
| Non-current derivatives | D.7. | |||||||||||||||||||||||||||||||
| Current derivatives | D.11. | |||||||||||||||||||||||||||||||
| Mutual fund investments | D.13. | |||||||||||||||||||||||||||||||
| Total financial assets measured at fair value | ||||||||||||||||||||||||||||||||
| Financial liabilities measured at fair value | ||||||||||||||||||||||||||||||||
| Bayer contingent purchase consideration arising from the acquisition of Genzyme | D.18. | |||||||||||||||||||||||||||||||
| MSD contingent consideration (European vaccines business) | D.18. | |||||||||||||||||||||||||||||||
Shire contingent consideration arising from the acquisition of Translate Bio | D.18. | |||||||||||||||||||||||||||||||
Contingent consideration arising from acquisition of Amunix | D.18. | |||||||||||||||||||||||||||||||
Other contingent consideration arising from business combinations and acquisitions | D.18. | |||||||||||||||||||||||||||||||
| Non-current derivatives | D.20. | |||||||||||||||||||||||||||||||
| Current derivatives | D.19.5 | |||||||||||||||||||||||||||||||
| Total financial liabilities measured at fair value | ||||||||||||||||||||||||||||||||
No transfers between the different levels of the fair value hierarchy occurred during 2023.
SANOFI FORM 20-F 2023 | F-49 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.13. Cash and cash equivalents
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Cash | |||||||||||
Cash equivalents(a) | |||||||||||
| Cash and cash equivalents | |||||||||||
(a) As of December 31, 2023, cash equivalents mainly comprised the following: (i) €5,349 million invested in euro and US dollar denominated money-market mutual funds (December 31, 2022: €9,537 million; December 31, 2021: €5,057 million); (ii) €1,191 million of term deposits (December 31, 2022: €1,167 million; December 31, 2021: €2,768 million) and (iii) zero commercial paper (December 31, 2022: zero ; December 31, 2021: €179 million). Cash equivalents also include €476 million held by captive insurance and reinsurance companies in accordance with insurance regulations (December 31, 2022: €439 million; December 31, 2021: €427 million).
D.14. Net deferred tax position
An analysis of the net deferred tax position is set forth below:
| (€ million) | 2023 | 2022 | 2021 | |||||||||||
| Deferred taxes on: | ||||||||||||||
| Consolidation adjustments (intragroup margin in inventory) | ||||||||||||||
| Provision for pensions and other employee benefits | ||||||||||||||
Remeasurement of other acquired intangible assets | ( | (a) | ( | ( | ||||||||||
| Recognition of acquired property, plant and equipment at fair value | ( | ( | ( | |||||||||||
Equity interests in subsidiaries and investments in other entities(b) | ( | ( | ( | |||||||||||
| Tax losses available for carry-forward | ||||||||||||||
| Stock options and other share-based payments | ||||||||||||||
Accrued expenses and provisions deductible at the time of payment(c) | ||||||||||||||
Other(d) | ||||||||||||||
| Net deferred tax asset/(liability) | ||||||||||||||
(a) As of December 31, 2023, includes remeasurements of the acquired intangible assets of Bioverativ (€1,241 million), Principia (€610 million), Ablynx (€204 million), Genzyme (€50 million) and Amunix (€109 million).
(b) In some countries, Sanofi is liable for withholding taxes and other tax charges when dividends are distributed. Consequently, Sanofi recognizes a deferred tax liability on the reserves of French and foreign subsidiaries (approximately €60.4 billion) which it regards as likely to be distributed in the foreseeable future. In determining the amount of the deferred tax liability as of December 31, 2023, Sanofi took into account changes in the ownership structure of certain subsidiaries, and the effects of changes in the taxation of dividends in France, following the ruling of the Court of Justice of the European Union in the Steria case and the resulting amendments to the 2015 Finance Act. As of December 31, 2023, this line includes a deferred tax liability arising from temporary differences on investments in subsidiaries which Sanofi expects will reverse in connection with the proposed separation of the Consumer Healthcare business, as announced in October 2023 (see Note D.30.).
(c) Includes deferred tax assets related to restructuring provisions, amounting to €286 million as of December 31, 2023, €256 million as of December 31, 2022, and €226 million as of December 31, 2021.
(d) Includes deferred taxes arising on the spread tax deduction of R&D expenses, amounting to €1,331 million as of December 31, 2023 and €742 million as of December 31, 2022.
The reserves of Sanofi subsidiaries that would be taxable if distributed but for which no distribution is planned, and for which no deferred tax liability has therefore been recognized, totaled €10.0 billion as of December 31, 2023, compared with €10.6 billion as of December 31, 2022 and €10.0 billion as of December 31, 2021.
Most of Sanofi’s tax loss carry-forwards are available indefinitely. For a description of policies on the recognition of deferred tax assets, refer to Note B.22. For each tax consolidation, the recognition of deferred tax assets is determined on the basis of profit forecasts that are consistent with Sanofi’s medium-term strategic plan, and taking into consideration the tax consequences of the strategic opportunities available to Sanofi within the period of availability of tax loss carry-forwards and the specific circumstances of each tax consolidation. Deferred tax assets relating to tax loss carry-forwards as of December 31, 2023 amounted to €2,729 million, of which €1,203 million were not recognized (including €473 million in respect of capital losses). This compares with €2,650 million as of December 31, 2022 (of which €1,144 million were not recognized) and €2,391 million as of December 31, 2021 (of which €875 million were not recognized).
F-50 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below shows when tax losses available for carry-forward are due to expire:
| (€ million) | Tax losses available for carry-forward(a) | ||||
| 2024 | |||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| 2028 | |||||
| 2029 and later | |||||
| Total as of December 31, 2023 | |||||
| Total as of December 31, 2022 | |||||
| Total as of December 31, 2021 | |||||
(a) Excluding tax loss carry-forwards on asset disposals. Such carry-forwards amounted to €5 million as of December 31, 2023, €5 million as of December 31, 2022 and €5 million as of December 31, 2021.
Use of tax loss carry-forwards is limited to the entity in which they arose. In jurisdictions where tax consolidations are in place, tax losses can be netted against taxable income generated by entities in the same tax consolidation.
Deferred tax assets not recognized (primarily because their future recovery was not regarded as probable given the expected results of the entities in question) amounted to €1,062 million in 2023, €995 million in 2022 and €615 million in 2021.
D.15. Consolidated shareholders’ equity
D.15.1. Share capital
As of December 31, 2023, the share capital was €2,529,599,938 , consisting of 1,264,799,969 shares with a par value of €2 . Treasury shares held by Sanofi are as follows:
Number of shares (million) | % of share capital for the period | |||||||
| December 31, 2023 | % | |||||||
| December 31, 2022 | % | |||||||
| December 31, 2021 | % | |||||||
| January 1, 2021 | % | |||||||
Treasury shares are deducted from shareholders’ equity. Gains and losses on disposals of treasury shares are recorded directly in equity and are not recognized in net income for the period.
Movements in the share capital of the Sanofi parent company over the last three years are set forth below:
| Date | Transaction | Number of shares | ||||||
| December 31, 2020 | ||||||||
| During 2021 | Capital increase by exercise of stock subscription options(a) | |||||||
| During 2021 | Capital increase by issuance of restricted shares(b) | |||||||
Board meeting of July 28, 2021 | Capital increase reserved for employees | |||||||
| December 31, 2021 | ||||||||
| During 2022 | Capital increase by exercise of stock subscription options(a) | |||||||
| During 2022 | Capital increase by issuance of restricted shares(b) | |||||||
Board meeting of July 27, 2022 | Capital increase reserved for employees | |||||||
Board meeting of December 14, 2022 | Reduction in share capital by cancellation of treasury shares | ( | ||||||
| December 31, 2022 | ||||||||
| During 2023 | Capital increase by exercise of stock subscription options(a) | |||||||
| During 2023 | Capital increase by issuance of restricted shares(b) | |||||||
Board meeting of July 27, 2023 | Capital increase reserved for employees | |||||||
| December 31, 2023 | ||||||||
(a) Shares issued on exercise of Sanofi stock subscription options.
(b) Shares vesting under restricted share plans and issued in the period.
For the disclosures about the management of capital required under IFRS 7, refer to Note B.27.
SANOFI FORM 20-F 2023 | F-51 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.15.2. Restricted share plans
Restricted share plans are accounted for in accordance with the policies described in Note B.24.3. The principal characteristics of those plans are as follows:
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Type of plan | Performance share plans | Performance share plans | Performance share plans | Performance share plans | Performance share plans | Performance share plans | ||||||||||||||
| Date of Board meeting approving the plan | May 25, 2023 | December 13, 2023 | May 3, 2022 | December 14, 2022 | April 30, 2021 | October 27, 2021 | ||||||||||||||
| Service period | ||||||||||||||||||||
| Total number of shares awarded | ||||||||||||||||||||
| Of which with no market condition | ||||||||||||||||||||
Fair value per share awarded(a) | € | € | € | € | € | |||||||||||||||
| Of which with market condition | ||||||||||||||||||||
Fair value per share awarded other than to the Chief Executive Officer(b) | € | € | € | € | € | € | ||||||||||||||
Fair value per share awarded other than to the Chief Executive Officer - additional shares(c) | € | € | € | € | ||||||||||||||||
Fair value per share awarded to the Chief Executive Officer(b) | € | € | € | |||||||||||||||||
Fair value of plan at the date of grant (€ million) | ||||||||||||||||||||
(a) Market price of Sanofi shares at the date of grant, adjusted for dividends expected during the vesting period.
(b) Weighting between (i) fair value determined using the Monte Carlo model and (ii) market price of Sanofi shares at the date of grant, adjusted for dividends expected during the vesting period.
(c) Additional tranche subject to a higher level of market conditions: 121,097 additional shares were awarded in May 2023, and 5,838 additional shares were awarded in December 2023 (versus 114,874 additional shares awarded in May 2022, and 9,066 additional shares awarded in December 2022).
The total expense recognized for all restricted share plans, and the number of restricted shares not yet fully vested, are shown in the table below:
| 2023 (a) | 2022 (a) | 2021 | |||||||||
Total expense for restricted share plans (€ million) | |||||||||||
Number of shares not yet fully vested as of December 31 | |||||||||||
Under 2023 plans | |||||||||||
Under 2022 plans | |||||||||||
Under 2021 plans | |||||||||||
Under 2020 plans | |||||||||||
Under 2019 plans | |||||||||||
(a) Additional tranche subject to a higher level of market conditions: 126,935 additional shares were awarded in 2023, versus 123,940 additional shares awarded in 2022.
D.15.3. Capital increases
The characteristics of the employee share ownership plans awarded in the form of a capital increase reserved for employees in 2023, 2022 and 2021 are summarized in the table below:
| 2023 | 2022 | 2021 | |||||||||
| Date of Board meeting approving the plan | February 2, 2023 | February 3, 2022 | February 4, 2021 | ||||||||
Subscription price (€)(a) | |||||||||||
| Subscription period | June 5-23, 2023 | June 9-29, 2022 | June 7-25, 2021 | ||||||||
| Number of shares subscribed | |||||||||||
| Number of shares issued immediately as employer’s contribution | |||||||||||
(a) Subscription price representing 80 20
The table below sets forth the expense recognized for each plan:
| (€ million) | 2023 | 2022 | 2020 | ||||||||
| Expense recognized | |||||||||||
| of which employer’s contribution | |||||||||||
F-52 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.15.4. Repurchase of Sanofi shares
The Annual General Meetings of Sanofi shareholders held on May 25, 2023, May 3, 2022 and April 30, 2021 each authorized a share repurchase program for a period of 18 The following repurchases have been made under those programs:
(in number of shares and € million) Year of authorization | 2023 | 2022 | 2021 | |||||||||||||||||
| Number of shares | Value | Number of shares | Value | Number of shares | Value | |||||||||||||||
| 2023 program | — | — | — | — | ||||||||||||||||
| 2022 program | — | — | ||||||||||||||||||
| 2021 program | — | — | ||||||||||||||||||
| 2020 program | — | — | — | — | ||||||||||||||||
D.15.5. Reductions in share capital
Reductions in share capital for the accounting periods presented are described in the table included at Note D.15.1. above.
Those reductions have no impact on shareholders’ equity.
D.15.6. Currency translation differences
Currency translation differences comprise the following:
(€ million) | 2023 | 2022 | 2021 | ||||||||
| Attributable to equity holders of Sanofi | ( | ( | |||||||||
| Attributable to non-controlling interests | ( | ( | ( | ||||||||
| Total | ( | ( | |||||||||
The balance as of December 31, 2023 includes an after-tax amount of €(574 ) million relating to hedges of net investments in foreign operations (refer to Note B.8.3. for a description of the relevant accounting policy), compared with €(580 ) million as of December 31, 2022 and €(317 ) million as of December 31, 2021.
The movement in Currency translation differences is mainly attributable to the US dollar.
SANOFI FORM 20-F 2023 | F-53 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.15.7. Other comprehensive income
Movements within other comprehensive income are shown below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Actuarial gains/(losses): | |||||||||||
•Actuarial gains/(losses) excluding investments accounted for using the equity method (see Note D.19.1.) | ( | ||||||||||
•Actuarial gains/(losses) of investments accounted for using the equity method, net of taxes | |||||||||||
•Tax effects | ( | ( | |||||||||
Equity instruments included in financial assets and financial liabilities: | — | — | — | ||||||||
•Change in fair value (excluding investments accounted for using the equity method) | ( | ||||||||||
•Change in fair value (investments accounted for using the equity method, net of taxes) | |||||||||||
•Equity risk hedging instruments designated as fair value hedges | |||||||||||
•Tax effects | ( | ( | ( | ||||||||
| Items not subsequently reclassifiable to profit or loss | ( | ||||||||||
Debt instruments included in financial assets: | |||||||||||
•Change in fair value (excluding investments accounted for using the equity method)(a) | ( | ( | |||||||||
•Tax effects | ( | ||||||||||
| Cash flow and fair value hedges: | |||||||||||
•Change in fair value (excluding investments accounted for using the equity method)(b) | ( | ||||||||||
•Change in fair value (investments accounted for using the equity method, net of taxes) | ( | ||||||||||
▪Tax effects | ( | ||||||||||
| Change in currency translation differences: | |||||||||||
•Currency translation differences on foreign subsidiaries (excluding investments accounted for using the equity method)(c) | ( | ||||||||||
•Currency translation differences (investments accounted for using the equity method)(c) | ( | ( | |||||||||
•Hedges of net investments in foreign operations(c) | ( | ( | |||||||||
•Tax effects | ( | ||||||||||
| Items subsequently reclassifiable to profit or loss | ( | ||||||||||
(a) Amounts reclassified to profit or loss: immaterial amount in 2023, immaterial amount in 2022 and €4 million in 2021.
(b) Amounts reclassified to profit or loss: €1 million in 2023, €2 million in 2022 and €12 million in 2021.
(c) Amounts reclassified to profit or loss: €(56 ) million in 2023, and €(40 ) million in 2022 (including €(35 ) million relating to the deconsolidation of EUROAPI). The amounts reclassified to profit and loss were immaterial in 2021. Currency translation differences arise from the translation into euros of the financial statements of foreign subsidiaries, and are mainly due to the appreciation of the dollar against the euro.
F-54 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.15.8. Stock options
Stock option plans awarded and measurement of stock option plans
No stock options were awarded during 2023, 2022 or 2021.
Stock subscription option plans
Details of the terms of exercise of stock subscription options granted under the various plans are presented below in Sanofi share equivalents. These plans were awarded to certain corporate officers and employees of Sanofi companies.
The table shows all Sanofi stock subscription option plans still outstanding or under which options were exercised in the year ended December 31, 2023:
| Source | Date of grant | Number of options granted | Start date of exercise period | Expiry date | Exercise price (€) | Number of options outstanding as of 12/31/2023 | ||||||||||||||
| Sanofi | 03/05/2013 | 03/06/2017 | 03/05/2023 | |||||||||||||||||
| Sanofi | 03/05/2014 | 03/06/2018 | 03/05/2024 | |||||||||||||||||
| Sanofi | 06/24/2015 | 06/25/2019 | 06/24/2025 | |||||||||||||||||
| Sanofi | 05/04/2016 | 05/05/2020 | 05/04/2026 | |||||||||||||||||
| Sanofi | 05/10/2017 | 05/11/2021 | 05/10/2027 | |||||||||||||||||
| Sanofi | 05/02/2018 | 05/03/2022 | 05/02/2028 | |||||||||||||||||
| Sanofi | 04/30/2019 | 05/02/2023 | 04/30/2029 | |||||||||||||||||
| Total | ||||||||||||||||||||
The exercise of all outstanding stock subscription options would increase shareholders’ equity by approximately €107 million. The exercise of each option results in the issuance of one share.
Summary of stock option plans
A summary of stock options outstanding at each balance sheet date, and of movements during the relevant periods, is presented below:
| Number of options | Weighted average exercise price per share (€) | Total (€ million) | |||||||||
| Options outstanding at January 1, 2021 | |||||||||||
| Options exercisable | |||||||||||
| Options exercised | ( | ( | |||||||||
Options cancelled(a) | ( | ( | |||||||||
| Options forfeited | ( | ||||||||||
| Options outstanding at December 31, 2021 | |||||||||||
| Options exercisable | |||||||||||
| Options exercised | ( | ( | |||||||||
Options cancelled(a) | ( | ( | |||||||||
| Options outstanding at December 31, 2022 | |||||||||||
| Options exercisable | |||||||||||
| Options exercised | ( | ( | |||||||||
| Options outstanding at December 31, 2023 | |||||||||||
| Options exercisable | |||||||||||
(a) Mainly due to the grantees leaving Sanofi.
The table below provides summary information about options outstanding and exercisable as of December 31, 2023:
| Outstanding | Exercisable | ||||||||||||||||
| Range of exercise prices per share | Number of options | Weighted average residual life (years) | Weighted average exercise price per share (€) | Number of options | Weighted average exercise price per share (€) | ||||||||||||
From € | |||||||||||||||||
From € | |||||||||||||||||
From € | |||||||||||||||||
| Total | |||||||||||||||||
SANOFI FORM 20-F 2023 | F-55 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.15.9. Number of shares used to compute diluted earnings per share
Diluted earnings per share is computed using the number of shares outstanding plus stock options with dilutive effect and restricted shares.
| (million) | 2023 | 2022 | 2021 | ||||||||
| Average number of shares outstanding | |||||||||||
| Adjustment for stock options with dilutive effect | |||||||||||
| Adjustment for restricted shares | |||||||||||
| Average number of shares used to compute diluted earnings per share | |||||||||||
In 2023 and 2022, all stock options were taken into account in computing diluted earnings per share because they all had a dilutive effect. In 2021, 0.6 million stock options were not taken into account in computing diluted earnings per share because they had no dilutive effect.
D.16. Non-controlling interests
D.17. Debt, cash and cash equivalents and lease liabilities
D.17.1. Debt, cash and cash equivalents
Changes in Sanofi's financial position during the period were as follows:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Long-term debt | |||||||||||
| Short-term debt and current portion of long-term debt | |||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ||||||||||
| Total debt | |||||||||||
| Cash and cash equivalents | ( | ( | ( | ||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | ( | ( | ( | ||||||||
Net debt(a) | |||||||||||
(a) Net debt does not include lease liabilities, which amounted to €2,030 million as of December 31, 2023, €2,181 million as of December 31, 2022, and €2,108 million as of December 31, 2021 (see the maturity analysis at Note D.17.2.).
“Net debt” is a non-IFRS financial measure used by management and investors to measure Sanofi’s overall net indebtedness.
Reconciliation of carrying amount to value on redemption
| Value on redemption | ||||||||||||||||||||
| (€ million) | Carrying amount at December 31, 2023 | Amortized cost | Adjustment to debt measured at fair value | December 31, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||||||
| Long-term debt | ||||||||||||||||||||
| Short-term debt and current portion of long-term debt | ||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | ( | ( | ||||||||||||||||
| Total debt | ||||||||||||||||||||
| Cash and cash equivalents | ( | ( | ( | ( | ||||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | ( | ( | ( | ( | ||||||||||||||||
| Net debt | ||||||||||||||||||||
F-56 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
a) Principal financing transactions during the year
The table below shows the movement in total debt during the period:
| Cash flows from financing activities | Non-cash items | |||||||||||||||||||||||||
| (€ million) | December 31, 2022 | Repayments | New borrowings | Other cash flows (a) | Currency translation differences(b) | Reclassification from non-current to current | Other items(c) | December 31, 2023 | ||||||||||||||||||
| Long-term debt | ( | ( | ( | |||||||||||||||||||||||
| Short-term debt and current portion of long-term debt | ( | ( | ||||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | ||||||||||||||||||||||||
| Total debt | ( | ( | ||||||||||||||||||||||||
(a) These amounts mainly comprise €946 million related to the US commercial paper program.
(b) These amounts include gains and losses, and the impact of foreign currency translation of the financial statements of subsidiaries outside the Euro zone.
(c) These amounts mainly comprise changes in accrued interest balances, and fair value adjustments.
Sanofi did not carry out any bond issues in 2023.
i.€1,750 million issued in March 2018, redeemed at maturity on March 21, 2023;
ii.$1,000 million issued in June 2018, redeemed at maturity on June 20, 2023; and
iii.€1,000 million issued in November 2013, redeemed at maturity on November 14, 2023.
Sanofi entered into the following transactions relating to its syndicated credit facilities during 2023:
•a new €4 billion revolving credit facility, linked to sustainable development indicators, was put in place on March 8, 2023. This syndicated facility was initially due to mature in March 2028, and included two extension options of one year each; it replaced an existing €4 billion facility that was canceled on the same day; and
•the maturity of this credit facility was extended to March 7, 2029, following the exercise of an extension option in December 2023.
Consequently, as of December 31, 2023, Sanofi had two syndicated credit facilities to provide liquidity for the purposes of current operations, each of them linked to environmental and social indicators:
•a €4 billion facility maturing December 6, 2027, with no further extension option available; and
•a €4 billion facility maturing March 7, 2029, with a one-year extension option.
In line with Sanofi’s commitment to embed sustainable development in the “Play to Win” strategy, those two revolving credit facilities build in an adjustment mechanism that links the credit spread to the attainment of two sustainable development performance indicators:
•for the facility maturing in December 2027: (i) Sanofi’s contribution to polio eradication, and (ii) the reduction in Sanofi’s carbon footprint; and
•for the facility maturing in March 2029: (i) Sanofi’s contribution to improving access to essential medicines in low-income and intermediate-income countries via its Sanofi Global Health non-profit unit, and (ii) the reduction in Sanofi’s carbon footprint.
The table below shows the movement in total debt during prior periods:
| Cash flows from financing activities | Non-cash items | |||||||||||||||||||||||||
| (€ million) | December 31, 2021 | Repayments | New borrowings | Other cash flows | Currency translation differences(a) | Reclassification from non-current to current | Other items(b) | December 31, 2022 | ||||||||||||||||||
| Long-term debt | ( | ( | ( | |||||||||||||||||||||||
| Short-term debt and current portion of long-term debt | ( | |||||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | ||||||||||||||||||||||||
| Total debt | ( | ( | ||||||||||||||||||||||||
(a) These amounts include gains and losses, and the impact of foreign currency translation of the financial statements of subsidiaries outside the Euro zone.
(b) These amounts include movements in accrued interest and fair value remeasurements.
SANOFI FORM 20-F 2023 | F-57 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
| Cash flows from financing activities | Non-cash items | |||||||||||||||||||||||||
| (€ million) | December 31, 2020 | Repayments | New borrowings | Other cash flows | Currency translation differences (a) | Reclassification from non-current to current | Other items(b) | December 31, 2021 | ||||||||||||||||||
| Long-term debt | ( | ( | ( | |||||||||||||||||||||||
| Short-term debt and current portion of long-term debt | ( | ( | ||||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | ||||||||||||||||||||||||
| Total debt | ( | ( | ||||||||||||||||||||||||
(a) These amounts include gains and losses, and the impact of foreign currency translation of the financial statements of subsidiaries outside the Euro zone.
(b) These amounts include (i) effects of changes in the scope of consolidation, amounting to €299 million; (ii) movements in accrued interest; and (iii) fair value remeasurements.
b) Net debt by type, at value on redemption
| 2023 | 2022 | 2021 | |||||||||||||||||||||||||||
| (€ million) | Non- current | Current | Total | Non- current | Current | Total | Non- current | Current | Total | ||||||||||||||||||||
| Bond issues | |||||||||||||||||||||||||||||
| Other bank borrowings | |||||||||||||||||||||||||||||
| Other borrowings | |||||||||||||||||||||||||||||
| Bank credit balances | |||||||||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||
| Total debt | |||||||||||||||||||||||||||||
| Cash and cash equivalents | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||
Net debt(a) | ( | ( | ( | ||||||||||||||||||||||||||
(a) Net debt does not include lease liabilities (see the maturity schedule in Note D.17.2.).
Bond issues carried out by Sanofi under the Euro Medium Term Note (EMTN) program are as follows:
| Issuer | ISIN code | Issue date | Maturity | Annual interest rate | Amount (€ million) | ||||||||||||
| Sanofi | FR0013143997 | April 2016 | April 2024 | ||||||||||||||
| Sanofi | FR0013505104 | March 2020 | April 2025 | ||||||||||||||
| Sanofi | FR0014009KS6 | April 2022 | April 2025 | ||||||||||||||
| Sanofi | FR0012969038 | September 2015 | September 2025 | ||||||||||||||
| Sanofi | FR0013324340 | March 2018 | March 2026 | ||||||||||||||
| Sanofi | FR0012146801 | September 2014 | September 2026 | ||||||||||||||
| Sanofi | FR0013201639 | September 2016 | January 2027 | ||||||||||||||
| Sanofi | FR0013144003 | April 2016 | April 2028 | ||||||||||||||
| Sanofi | FR0013409844 | March 2019 | March 2029 | ||||||||||||||
| Sanofi | FR0014009KQ0 | April 2022 | April 2029 | ||||||||||||||
| Sanofi | FR0013324357 | March 2018 | March 2030 | ||||||||||||||
| Sanofi | FR0013505112 | March 2020 | April 2030 | ||||||||||||||
| Sanofi | FR0013409851 | March 2019 | March 2034 | ||||||||||||||
| Sanofi | FR0013324373 | March 2018 | March 2038 | ||||||||||||||
Bond issues carried out by Sanofi under the public bond issue program (shelf registration statement) registered with the US Securities and Exchange Commission (SEC) comprise:
| Issuer | ISIN code | Issue date | Maturity | Annual interest rate | Amount ($ million) | ||||||||||||
| Sanofi | US801060AD60 | June 2018 | June 2028 | % | |||||||||||||
F-58 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The “Other borrowings” line mainly comprises participating shares issued between 1983 and 1987, of which 58,115 remain outstanding, with a nominal amount of €9 million.
In order to manage its liquidity needs for current operations, as of December 31, 2023 Sanofi had:
•a syndicated credit facility of €4 billion, drawable in euros and in US dollars, maturing December 6, 2027; and
•a syndicated credit facility of €4 billion, drawable in euros and in US dollars, maturing March 7, 2029.
Sanofi also has two commercial paper programs:
•a €6 billion Negotiable European Commercial Paper program in France, with an average drawdown of €0.2 billion and a maximum drawdown of €0.8 billion during 2023, and an amount of €40 million drawn down as of December 31, 2023; and
•a $10 billion Commercial Paper program in the United States, with an average drawdown of $3.7 billion and a maximum drawdown of $7.3 billion during 2023, and an amount of $1.0 billion drawn down as of December 31, 2023.
The financing in place as of December 31, 2023 at the level of the holding company (which manages most of Sanofi’s financing needs centrally) is not subject to any financial covenants, and contains no clauses linking spreads or fees to the credit rating.
c) Debt by maturity, at value on redemption
| December 31, 2023 | Current | Non-current | |||||||||||||||||||||
| (€ million) | Total | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 and later | ||||||||||||||||
| Bond issues | |||||||||||||||||||||||
| Other bank borrowings | |||||||||||||||||||||||
| Other borrowings | |||||||||||||||||||||||
| Bank credit balances | |||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | |||||||||||||||||||||
| Total debt | |||||||||||||||||||||||
| Cash and cash equivalents | ( | ( | |||||||||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | ( | ( | |||||||||||||||||||||
Net debt(a) | ( | ||||||||||||||||||||||
(a) Net debt does not include lease liabilities, which amounted to €2,030 million as of December 31, 2023; €2,181 million as of December 31, 2022; and €2,108 million as of December 31, 2021 (see the maturity analysis at Note D.17.2.).
As of December 31, 2023, the main undrawn confirmed general-purpose credit facilities at holding company level amounted to €8 billion, half of which expired in 2027 and the other half of which expires in 2029.
As of December 31, 2023, no single counterparty represented more than 6% of Sanofi’s undrawn confirmed credit facilities.
| December 31, 2022 | Current | Non-current | |||||||||||||||||||||
| (€ million) | Total | 2023 | 2024 | 2025 | 2026 | 2027 | 2028 and later | ||||||||||||||||
| Bond issues | |||||||||||||||||||||||
| Other bank borrowings | |||||||||||||||||||||||
| Other borrowings | |||||||||||||||||||||||
| Bank credit balances | |||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | |||||||||||||||||||||
| Total debt | |||||||||||||||||||||||
| Cash and cash equivalents | ( | ( | |||||||||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | ( | ( | |||||||||||||||||||||
| Net debt | ( | ||||||||||||||||||||||
SANOFI FORM 20-F 2023 | F-59 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
| December 31, 2021 | Current | Non-current | |||||||||||||||||||||
| (€ million) | Total | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 and later | ||||||||||||||||
| Bond issues | |||||||||||||||||||||||
| Other bank borrowings | |||||||||||||||||||||||
| Other borrowings | |||||||||||||||||||||||
| Bank credit balances | |||||||||||||||||||||||
| Interest rate and currency derivatives used to manage debt | ( | ( | |||||||||||||||||||||
| Total debt | |||||||||||||||||||||||
| Cash and cash equivalents | ( | ( | |||||||||||||||||||||
| Interest rate and currency derivatives used to manage cash and cash equivalents | ( | ( | |||||||||||||||||||||
| Net debt | ( | ||||||||||||||||||||||
d) Debt by interest rate, at value on redemption
The table below splits net debt between fixed and floating rate, and by maturity, as of December 31, 2023. The figures shown are values on redemption, before the effects of derivative instruments:
| (€ million) | Total | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 and later | ||||||||||||||||
| Fixed-rate debt | |||||||||||||||||||||||
| of which euro | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| % fixed-rate | % | ||||||||||||||||||||||
| Floating-rate debt | |||||||||||||||||||||||
| of which euro | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| % floating-rate | % | ||||||||||||||||||||||
| Debt | |||||||||||||||||||||||
| Cash and cash equivalents | ( | ( | |||||||||||||||||||||
| of which euro | ( | ||||||||||||||||||||||
| of which US dollar | ( | ||||||||||||||||||||||
| % floating-rate | % | ||||||||||||||||||||||
| Net debt | ( | ||||||||||||||||||||||
Sanofi issues debt in two currencies, the euro and the US dollar, and also invests its cash and cash equivalents in those currencies. Sanofi also operates cash pooling arrangements to manage the surplus cash and short-term liquidity needs of foreign subsidiaries located outside the euro zone.
F-60 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
To optimize the cost of debt or reduce the volatility of debt and manage its exposure to financial foreign exchange risk, Sanofi uses derivative instruments (interest rate swaps, currency swaps, foreign exchange swaps and forward contracts) that alter the fixed/floating rate split and the currency split of its net debt:
| (€ million) | Total | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 and later | ||||||||||||||||
| Fixed-rate debt | ( | ||||||||||||||||||||||
| of which euro | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| % fixed-rate | % | ||||||||||||||||||||||
| Floating-rate debt | |||||||||||||||||||||||
| of which euro | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| % floating-rate | % | ||||||||||||||||||||||
| Debt | |||||||||||||||||||||||
| Cash and cash equivalents | ( | ||||||||||||||||||||||
| of which euro | ( | ||||||||||||||||||||||
| of which US dollar | ( | ||||||||||||||||||||||
| of which Singapore dollar | ( | ||||||||||||||||||||||
| % floating-rate | % | ||||||||||||||||||||||
| Net debt | ( | ||||||||||||||||||||||
The table below shows the fixed/floating rate split of net debt at value on redemption after taking account of derivative instruments as of December 31, 2022 and December 31, 2021:
| (€ million) | 2022 | % | 2021 | % | ||||||||||
| Fixed-rate debt | % | % | ||||||||||||
| Floating-rate debt | % | % | ||||||||||||
| Debt | % | % | ||||||||||||
| Cash and cash equivalents | ( | ( | ||||||||||||
| Net debt | ||||||||||||||
The weighted average interest rate on debt as of December 31, 2023 was 1.6 % before derivative instruments and 2.1 % after derivative instruments. Cash and cash equivalents were invested as of December 31, 2023 at an average rate of 3.9 % before derivative instruments and 3.7 % after derivative instruments.
The projected full-year sensitivity of net debt to interest rate fluctuations for 2024 is as follows:
| Change in short-term interest rates | Impact on pre-tax net income (€ million) | Impact on pre-tax income/(expense) recognized directly in equity (€ million) | ||||||
| +100 bp | ||||||||
| +25 bp | ||||||||
| -25 bp | ( | |||||||
| -100 bp | ( | |||||||
e) Debt by currency, at value on redemption
The table below shows net debt by currency at December 31, 2023, before and after derivative instruments contracted to convert the foreign-currency net debt of exposed entities into their functional currency:
| (€ million) | Before derivative instruments | After derivative instruments | ||||||
| Euro | ||||||||
| US dollar | ( | |||||||
| Singapore dollar | ( | ( | ||||||
| Pound sterling | ||||||||
Chinese yuan renminbi | ( | |||||||
| Other currencies | ||||||||
| Net debt | ||||||||
SANOFI FORM 20-F 2023 | F-61 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below shows net debt by currency at December 31, 2022 and 2021, after derivative instruments contracted to convert the foreign currency net debt of exposed entities into their functional currency:
| (€ million) | 2022 | 2021 | ||||||
| Euro | ||||||||
| US dollar | ( | ( | ||||||
| Other currencies | ( | ( | ||||||
| Net debt | ||||||||
f) Market value of net debt
The market value of Sanofi’s debt, net of cash and cash equivalents and derivatives and excluding accrued interest, is as follows:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Market value | |||||||||||
| Value on redemption | |||||||||||
The fair value of debt is determined by reference to quoted market prices at the balance sheet date in the case of quoted instruments (level 1 in the IFRS 7 hierarchy, see Note D.12.), and by reference to the fair value of interest rate and currency derivatives used to manage net debt (level 2 in the IFRS 7 hierarchy, see Note D.12.).
g) Future contractual cash flows relating to debt and related derivatives
The table below shows the amount of future undiscounted contractual cash flows (principal and interest) relating to debt and to derivative instruments designated as hedges of debt:
| December 31, 2023 | Payments due by period | ||||||||||||||||||||||
| (€ million) | Total | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 and later | ||||||||||||||||
| Debt | |||||||||||||||||||||||
| Principal | |||||||||||||||||||||||
Interest(a) | |||||||||||||||||||||||
| Net cash flows related to derivative instruments | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
(a) Interest flows are estimated on the basis of forward interest rates applicable as of December 31, 2023.
Future contractual cash flows are shown on the basis of the carrying amount in the balance sheet at the reporting date, without reference to any subsequent management decision that might materially alter the structure of Sanofi’s debt or its hedging policy.
The tables below show the amount of future undiscounted contractual cash flows (principal and interest) relating to debt and to derivative instruments designated as hedges of debt as of December 31, 2022 and 2021:
| December 31, 2022 | Payments due by period | ||||||||||||||||||||||
| (€ million) | Total | 2023 | 2024 | 2025 | 2026 | 2027 | 2028 and later | ||||||||||||||||
| Debt | |||||||||||||||||||||||
| Principal | |||||||||||||||||||||||
Interest(a) | |||||||||||||||||||||||
| Net cash flows related to derivative instruments | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
(a) Interest flows are estimated on the basis of forward interest rates applicable as of December 31, 2022.
| December 31, 2021 | Payments due by period | ||||||||||||||||||||||
| (€ million) | Total | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 and later | ||||||||||||||||
| Debt | |||||||||||||||||||||||
| Principal | |||||||||||||||||||||||
Interest(a) | |||||||||||||||||||||||
| Net cash flows related to derivative instruments | ( | ( | ( | ||||||||||||||||||||
| Total | |||||||||||||||||||||||
(a) Interest flows are estimated on the basis of forward interest rates applicable as of December 31, 2021.
F-62 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.17.2. Lease liabilities
A maturity analysis of lease liabilities as of December 31, 2023, 2022 and 2021 is set forth below:
| Total | Undiscounted future minimum lease payments | |||||||||||||||||||
| (€ million) | Less than 1 year | From 1 to 3 years | From 3 to 5 years | More than 5 years | Discounting effect | |||||||||||||||
Total lease liabilities as of December 31, 2023 | ( | |||||||||||||||||||
Total lease liabilities as of December 31, 2022 | ( | |||||||||||||||||||
Total lease liabilities as of December 31, 2021 | ( | |||||||||||||||||||
Lease liabilities include leases relating to real estate assets located at Cambridge, MA (United States), as described in Note D.3., which have a lease term of 15 years.
D.18. Liabilities related to business combinations and to non-controlling interests
For a description of the nature of the liabilities reported in the line item Liabilities related to business combinations and to non-controlling interests, refer to Note B.8.5. The principal acquisitions are described in Notes D.1. and D.2.
The liabilities related to business combinations and to non-controlling interests shown in the table below are level 3 instruments under the IFRS 7 fair value hierarchy (see Note D.12.).
Movements in liabilities related to business combinations and to non-controlling interests are shown below:
| (€ million) | Bayer contingent consideration arising from the acquisition of Genzyme | MSD contingent consideration (European Vaccines business) | Shire contingent consideration arising from the acquisition of Translate Bio | Contingent consideration arising from acquisition of Amunix | Other | Total(a) | ||||||||||||||
| Balance at January 1, 2021 | ||||||||||||||||||||
New transactions(c) | ||||||||||||||||||||
Payments made(d) | ( | ( | ( | ( | ||||||||||||||||
Fair value remeasurements through profit or loss: (gain)/loss (including unwinding of discount)(b) | ( | ( | ( | |||||||||||||||||
| Other movements | ( | ( | ||||||||||||||||||
| Currency translation differences | ||||||||||||||||||||
| Balance at December 31, 2021 | ||||||||||||||||||||
New transactions | ||||||||||||||||||||
Payments made | ( | ( | ( | ( | ||||||||||||||||
Fair value remeasurements through profit or loss: (gain)/loss (including unwinding of discount)(b) | ( | ( | ||||||||||||||||||
| Other movements | ||||||||||||||||||||
| Currency translation differences | ||||||||||||||||||||
| Balance at December 31, 2022 | ||||||||||||||||||||
| New transactions | ||||||||||||||||||||
| Payments made | ( | ( | ( | ( | ||||||||||||||||
Fair value remeasurements through profit or loss: (gain)/loss (including unwinding of discount)(b) | ( | |||||||||||||||||||
| Other movements | ||||||||||||||||||||
| Currency translation differences | ( | ( | ( | |||||||||||||||||
| Balance at December 31, 2023 | ||||||||||||||||||||
(a) Portion due after more than one year: €501 million as of December 31, 2023 (€674 million as of December 31, 2022 and €577 million as of December 31, 2021); portion due within less than one year: €208 million as of December 31, 2023 (€105 million as of December 31, 2022 and €137 million as of December 31, 2021).
(b) Amounts reported within the income statement line item Fair value remeasurement of contingent consideration, and mainly comprising unrealized gains and losses.
(c) Mainly corresponds to the recognition of the Shire Human Genetic Therapies Inc. (Shire) contingent consideration liability of $382 million resulting from the acquisition of Translate Bio in September 2021.
(d) The “Other” column mainly relates to the contingent consideration liability due to True North Therapeutics as a result of Sanofi's acquisition of Bioverativ which was settled in the first half of 2021.
SANOFI FORM 20-F 2023 | F-63 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
As of December 31, 2023, Liabilities related to business combinations and to non-controlling interests mainly comprised:
•the MSD contingent consideration liability arising from the 2016 acquisition of the Sanofi Pasteur activities carried on within the former Sanofi Pasteur MSD joint venture, which amounted to €127 million as of December 31, 2023, €204 million as of December 31, 2022 and €269 million as of December 31, 2021 (see Note D.12.). The fair value of this contingent consideration is determined by applying the royalty percentage stipulated in the contract to discounted sales projections. If the discount rate were to fall by one percentage point, the fair value of the MSD contingent consideration liability would increase by approximately 1 %;
•a contingent consideration liability towards Shire Human Genetic Therapies Inc. (Shire) arising from Sanofi’s acquisition of Translate Bio in September 2021. In a business combination carried out in December 2016 and predating the acquisition of control by Sanofi, Translate Bio (then called Rana Therapeutics, Inc.) acquired from Shire the intellectual property rights relating to the latter’s Messenger RNA Therapeutics (MRT) program. As of December 31, 2023, Shire was entitled to receive the following potential payments:
–milestone payments contingent on the launch of products based on MRT technology, and on the attainment of a specified level of sales of those products, and
–a percentage of sales of those products.
The fair value of the Shire liability was measured at €441 million as of December 31, 2023, compared with €380 million as of December 31, 2022 and €354 million as of December 31, 2021; it was determined by applying the contractual terms to development and sales projections which were weighted to reflect the probability of success, and discounted. If the discount rate were to fall by one percentage point, the fair value of the Shire liability would increase by approximately 12 %;
•the contingent consideration liability arising from the 2022 acquisition of Amunix. The fair value of the liability is determined on the basis of the nominal value of payments due subject to the attainment of specified development milestones. The liability was measured at €137 million as of December 31, 2023 compared with €165 million as of December 31, 2022. If the discount rate were to fall by one percentage point, the fair value of the liability would increase by approximately 1 %.
The Bayer contingent consideration liability arising from Sanofi’s acquisition of Genzyme in 2011 was extinguished during 2023 in accordance with the contractual terms.
The table below sets forth the maximum amount of contingent consideration payable in respect of already-marketed products:
| December 31, 2023 | Total | Payments due by period | |||||||||||||||
| (€ million) | Less than 1 year | From 1 to 3 years | From 3 to 5 years | More than 5 years | |||||||||||||
Commitments relating to contingent consideration in connection with business combinations | |||||||||||||||||
D.19. Provisions, income tax liabilities and other liabilities
The line item Non current provisions and other non-current liabilities comprises the following:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Provisions | |||||||||||
Other non-current liabilities(a) | |||||||||||
| Total | |||||||||||
(a) Includes derivative financial instruments: €164 million as of December 31, 2023, €232 million as of December 31, 2022, €6 million as of December 31, 2021.
The figure as of December 31, 2023 includes €1,960 million for the liability in respect of royalties payable to Sobi on net sales of BEYFORTUS (nirsevimab) in the United States (see Note C.2.). Given the method used to calculate royalties payable, an increase or decrease in sales forecasts would lead to a proportionate change in the amount of the liability. The nominal value of payments estimated to be due within more than one year but less than five years is €1,112 million; the nominal value of payments estimated to be due after more than five years is €2,860 million.
The figure as of December 31, 2023 includes €
Non-current income tax liabilities are described in Note D.19.4., and other current liabilities in Note D.19.5.
F-64 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below sets forth movements in non-current provisions for the reporting periods presented:
| (€ million) | Provisions for pensions and other post-employment benefits (D.19.1.) | Provisions for other long-term benefits | Restructuring provisions (D.19.2.) | Other provisions (D.19.3.) | Total | ||||||||||||||||||
| Balance at January 1, 2021 | (b) | ||||||||||||||||||||||
| Changes in scope of consolidation | ( | ||||||||||||||||||||||
| Increases in provisions | (a) | ||||||||||||||||||||||
| Provisions utilized | ( | (a) | ( | ( | ( | ( | |||||||||||||||||
| Reversals of unutilized provisions | ( | (a) | ( | ( | ( | ( | |||||||||||||||||
| Transfers | ( | ( | ( | ( | ( | ||||||||||||||||||
| Net interest related to employee benefits, and unwinding of discount | |||||||||||||||||||||||
| Currency translation differences | |||||||||||||||||||||||
Actuarial gains and losses on defined-benefit plans | ( | ( | |||||||||||||||||||||
| Balance at December 31, 2021 | |||||||||||||||||||||||
| Changes in scope of consolidation | ( | ( | ( | ( | |||||||||||||||||||
| Increases in provisions | (a) | ||||||||||||||||||||||
| Provisions utilized | ( | (a) | ( | ( | ( | ( | |||||||||||||||||
| Reversals of unutilized provisions | ( | (a) | ( | ( | ( | ( | |||||||||||||||||
| Transfers | ( | ( | ( | ||||||||||||||||||||
| Net interest related to employee benefits, and unwinding of discount | |||||||||||||||||||||||
| Currency translation differences | ( | ||||||||||||||||||||||
Actuarial gains and losses on defined-benefit plans | ( | ( | |||||||||||||||||||||
| Balance at December 31, 2022 | |||||||||||||||||||||||
| Changes in scope of consolidation | |||||||||||||||||||||||
| Increases in provisions | (a) | ||||||||||||||||||||||
| Provisions utilized | ( | (a) | ( | ( | ( | ( | |||||||||||||||||
| Reversals of unutilized provisions | ( | (a) | ( | ( | ( | ( | |||||||||||||||||
| Transfers | ( | ( | ( | ( | |||||||||||||||||||
| Net interest related to employee benefits, and unwinding of discount | |||||||||||||||||||||||
| Currency translation differences | ( | ( | ( | ( | |||||||||||||||||||
Actuarial gains and losses on defined-benefit plans | |||||||||||||||||||||||
| Balance at December 31, 2023 | |||||||||||||||||||||||
(a) In the case of “Provisions for pensions and other post-employment benefits”, the “Increases in provisions” line corresponds to rights vesting in employees during the period, and past service cost; the “Provisions utilized” line corresponds to contributions paid into pension funds and to beneficiaries; and the “Reversals of unutilized provisions” line corresponds to plan curtailments, settlements and amendments.
(b) Includes the impact of the April 2021 IFRIC agenda decision on the allocation of benefits to service periods, as described in Note A.2.1. to the consolidated financial statements for the year ended December 31, 2021.
D.19.1. Provisions for pensions and other post-employment benefits
Sanofi offers its employees pension plans and other post-employment benefit plans. The specific features of the plans (benefit formulas, fund investment policy and fund assets held) vary depending on the applicable laws and regulations in each country where the employees work. These employee benefits are accounted for in accordance with IAS 19 (see Note B.23.).
Sanofi’s pension obligations in four major countries represented approximately 89 % of the total value of the defined-benefit obligation and approximately 88 % of the total value of plan assets as of December 31, 2023. The features of the principal defined-benefit plans in each of those four countries are described below.
France
Lump-sum retirement benefit plans
All employees working for Sanofi in France are entitled on retirement to a lump-sum payment, the amount of which depends both on their length of service and on the rights guaranteed by collective and internal agreements. The employee’s final salary is used in calculating the amount of these lump-sum retirement benefits. These plans represent approximately 39 % of Sanofi’s total obligation in France.
SANOFI FORM 20-F 2023 | F-65 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Defined-benefit pension plans
These plans provide benefits from the date of retirement. Employees must fulfil a number of criteria to be eligible for these benefits. All of these plans are now closed. These plans represent approximately 61 % of Sanofi’s total obligation in France.
Germany
Top-up defined-benefit pension plan
The benefits offered under this pension plan are wholly funded by the employer (there are no employee contributions) via a Contractual Trust Agreement (CTA), under which benefits are estimated on the basis of a career average salary. Employees are entitled to receive an annuity under this plan if their salary exceeds the social security ceiling. The amount of the pension is calculated by reference to a range of vesting rates corresponding to salary bands. The plan also includes disability and death benefits. This plan represents approximately 60 % of Sanofi’s total obligation in Germany.
Sanofi-Aventis plus (SAV plus)
A top-up pension plan (SAV plus) replaced a previous top-up defined-benefit plan. New entrants joining the plan after April 1, 2015 contribute to a defined-contribution plan that is partially funded via the company’s CTA.
All employees whose salary exceeds the social security ceiling are automatically covered by the plan. The employer’s contribution is 14 % of the amount by which the employee’s salary exceeds the social security ceiling.
Multi-employer plan (Pensionskasse)
This is a defined-benefit plan treated as a defined-contribution plan, in accordance with the accounting policies described in Note B.23. Currently, contributions cover the level of annuities. Only the portion relating to the future revaluation of the annuities is included in the defined-benefit pension obligation. The obligation relating to this revaluation amounted to €744 million as of December 31, 2023, versus €652 million as of December 31, 2022 and €877 million as of December 31, 2021. This plan represents approximately 26 % of Sanofi’s total defined-benefit obligation in Germany.
United States
Defined-benefit pension plans
In the United States, there are two types of defined-benefit plan:
•“qualified” plans within the meaning of the Employee Retirement Income Security Act of 1974 (ERISA), which provide guaranteed benefits to eligible employees during retirement, and in the event of death or disability. Employees can elect to receive a reduced annuity, in exchange for an annuity to be paid in the event of their death to a person designated by them. An annuity is also granted under the plan if the employee dies before retirement age. Eligible employees do not pay any contributions. These plans are closed to new entrants, and the vesting of rights for future service periods is partially frozen. These plans represent approximately 59 % of Sanofi’s total obligation in the United States;
•“non-qualified” plans within the meaning of ERISA provide top-up retirement benefits to some eligible employees depending on the employee’s level of responsibility and subject to a salary cap. These plans represent approximately 14 % of Sanofi’s total obligation in the United States.
Healthcare cover and life insurance
Sanofi companies provide some eligible employees with healthcare cover and life insurance during the retirement period (the company’s contributions are capped at a specified level). These plans represent approximately 27 % (or €412 million) of Sanofi’s total obligation and 3 % (or €23 million) of total plan assets in the United States.
United Kingdom
Defined-benefit pension plans
Sanofi operates a number of pension plans in the United Kingdom that reflect past acquisitions. The most significant arrangements are defined-benefit plans that have been closed since October 1, 2015. With effect from that date, employees can no longer pay into these plans.
Under these defined-benefit plans, an annuity is paid from the retirement date. This annuity is calculated on the basis of the employee’s length of service as of September 30, 2015, and of the employee’s final salary (or salary on the date he or she leaves Sanofi).
The rates used for the vesting of rights vary from member to member. For most members, rights vest at the rate of 1.25 % or 1.50 % of final salary for each qualifying year of service giving entitlement. The notional retirement age varies according to the category to which the member belongs, but in most cases retirement is at age 65 . Members may choose to retire before or after the notional retirement age (60 years), in which case the amount of the annual pension is adjusted to reflect the revised estimate of the length of the retirement phase. Pensions are usually indexed to the Retail Price Index (RPI). Members paid a fixed-percentage contribution into their pension plan (the percentage varied according to the employee category), and the employer topped up the contribution to the required amount. These plans represent approximately 100 % of Sanofi’s total obligation in the United Kingdom.
For service periods subsequent to October 1, 2015, employees belong to a new defined-contribution plan.
F-66 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Actuarial assumptions used to measure Sanofi’s obligations
Actuarial valuations of Sanofi’s benefit obligations were computed by management with assistance from external actuaries as of December 31, 2023, 2022 and 2021.
Those calculations were based on the following financial and demographic assumptions:
| 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||||||||
| France | Germany | US | UK | France | Germany | US | UK | France | Germany | US | UK | |||||||||||||||||||||||||||
Discount rate(a)(b) | to | to | ||||||||||||||||||||||||||||||||||||
General inflation rate(c) | — | — | — | |||||||||||||||||||||||||||||||||||
| Pension benefit indexation | — | — | — | |||||||||||||||||||||||||||||||||||
Healthcare cost inflation rate(d) | — | — | to | — | — | — | to | — | — | — | — | |||||||||||||||||||||||||||
| Retirement age | to | to | to | to | to | to | to | to | to | |||||||||||||||||||||||||||||
| Mortality table | TGH/ TGF 05 | Heubeck RT 2018 G | RP2012 Proj. MP2021 White Collar | SAPS S3 | TGH/ TGF 05 | Heubeck RT 2018 G | RP2012 Proj. MP2021 White Collar | SAPS S3 | TGH/ TGF 05 | Heubeck RT 2018 G | RP2012 Proj. G. Scale MP2020 White Collar | SAPS S3 | ||||||||||||||||||||||||||
(a) The discount rates used were based on market rates for high quality corporate bonds with a duration close to that of the expected benefit payments under the plans. The benchmarks used to determine discount rates were the same for all periods presented.
(b) The rate depends on the duration of the plan (0 to 7 years, 7 to 10 years, or more than 10 years).
(c) Inflation for the euro zone is determined using a multi-criterion method.
(d) No post-employment healthcare benefits are provided in France since 2020, Germany and UK.
Weighted average duration of obligation for pensions and other long-term benefits in principal countries
The table below shows the duration of Sanofi’s obligations in the principal countries:
| 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||||||||
| (years) | France | Germany | US | UK | France | Germany | US | UK | France | Germany | US | UK | ||||||||||||||||||||||||||
| Weighted average duration | ||||||||||||||||||||||||||||||||||||||
Sensitivity analysis
The table below shows the sensitivity of Sanofi’s obligations for pensions and other post-employment benefits to changes in key actuarial assumptions:
(€ million) | Pensions and other post-employment benefits, by principal country | ||||||||||||||||
| Measurement of defined-benefit obligation | Change in assumption | France | Germany | US | UK | ||||||||||||
| Discount rate | - | % | + | + | + | + | |||||||||||
| General inflation rate | + | % | + | + | + | ||||||||||||
| Pension benefit indexation | + | % | + | + | + | ||||||||||||
| Healthcare cost inflation rate | + | % | + | + | + | ||||||||||||
| Mortality table | +1 year | + | + | + | + | ||||||||||||
SANOFI FORM 20-F 2023 | F-67 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below reconciles the net obligation in respect of Sanofi’s pension and other post-employment benefit plans with the amounts recognized in the consolidated financial statements:
| Pensions and other post-employment benefits | ||||||||||||||
| (€ million) | 2023 | 2022 | 2021 | (a) | ||||||||||
| Measurement of the obligation: | ||||||||||||||
| Beginning of period | ||||||||||||||
| Current service cost | ||||||||||||||
| Interest cost | ||||||||||||||
| Actuarial losses/(gains) due to changes in demographic assumptions | ( | ( | ( | |||||||||||
| Actuarial losses/(gains) due to changes in financial assumptions | ( | ( | ||||||||||||
| Actuarial losses/(gains) due to experience adjustments | ( | |||||||||||||
Plan amendments, curtailments or settlements not specified in the terms of the plan(b) | ( | ( | ( | |||||||||||
| Plan settlements specified in the terms of the plan | ( | ( | ( | |||||||||||
| Benefits paid | ( | ( | ( | |||||||||||
| Changes in scope of consolidation and transfers | ( | ( | ( | |||||||||||
| Currency translation differences | ( | |||||||||||||
| Obligation at end of period | ||||||||||||||
| Fair value of plan assets: | ||||||||||||||
| Beginning of period | ||||||||||||||
| Interest income on plan assets | ||||||||||||||
| Difference between actual return and interest income on plan assets | ( | |||||||||||||
| Administration costs | ( | ( | ( | |||||||||||
| Plan settlements specified in the terms of the plan | ( | ( | ( | |||||||||||
| Plan settlements not specified in the terms of the plan | ( | ( | ( | |||||||||||
| Contributions from plan members | ||||||||||||||
| Employer’s contributions | ||||||||||||||
| Benefits paid | ( | ( | ( | |||||||||||
| Changes in scope of consolidation and transfers | ( | ( | ( | |||||||||||
| Currency translation differences | ( | |||||||||||||
| Fair value of plan assets at end of period | ||||||||||||||
| Net amount shown in the balance sheet: | ||||||||||||||
| Net obligation | ||||||||||||||
| Effect of asset ceiling | ||||||||||||||
| Net amount shown in the balance sheet at end of period | ||||||||||||||
| Amounts recognized in the balance sheet: | ||||||||||||||
Pre-funded obligations (see Note D.7.)(b) | ( | ( | ( | |||||||||||
| Obligations provided for | ||||||||||||||
| Net amount recognized at end of period | ||||||||||||||
| Benefit cost for the period: | ||||||||||||||
| Current service cost | ||||||||||||||
(Gains)/losses related to plan amendments, curtailments or settlements not specified in the terms of the plan | ( | ( | ||||||||||||
| Net interest (income)/cost | ||||||||||||||
| Contributions from plan members | ( | ( | ( | |||||||||||
| Administration costs and taxes paid during the period | ||||||||||||||
| Expense recognized directly in profit or loss | ||||||||||||||
Remeasurement of net defined-benefit (asset)/liability (actuarial gains and losses)(c) | ( | ( | ||||||||||||
| Expense/(gain) for the period | ( | ( | ||||||||||||
(a) These amounts include the impact of applying the April 2021 IFRIC agenda decision on the attribution of benefits to periods of service.
(b) For 2023, this line includes €66 million of assets in the United Kingdom (versus €99 million for 2022 and €220 million for 2021); those amounts are not subject to any asset ceiling, in accordance with IFRIC 14.
(c) Amounts recognized in Other comprehensive income (see Note D.15.7.).
F-68 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The tables below show Sanofi’s net liability in respect of pension plans and other post-employment benefits by geographical region:
| (€ million) | Pensions and other post-employment benefits by geographical region | |||||||||||||||||||
| December 31, 2023 | France | Germany | US | UK | Other | Total | ||||||||||||||
| Measurement of obligation | ||||||||||||||||||||
| Fair value of plan assets | ||||||||||||||||||||
| Effect of asset ceiling | ( | ( | ||||||||||||||||||
| Net amount shown in the balance sheet at end of period | ( | |||||||||||||||||||
| (€ million) | Pensions and other post-employment benefits by geographical region | |||||||||||||||||||
| December 31, 2022 | France | Germany | US | UK | Other | Total | ||||||||||||||
| Measurement of obligation | ||||||||||||||||||||
| Fair value of plan assets | ||||||||||||||||||||
| Effect of asset ceiling | ( | ( | ||||||||||||||||||
| Net amount shown in the balance sheet at end of period | ( | |||||||||||||||||||
| (€ million) | Pensions and other post-employment benefits by geographical region | |||||||||||||||||||
| December 31, 2021 | France | Germany | US | UK | Other | Total | ||||||||||||||
| Measurement of obligation | ||||||||||||||||||||
| Fair value of plan assets | ||||||||||||||||||||
| Effect of asset ceiling | ( | ( | ||||||||||||||||||
| Net amount shown in the balance sheet at end of period | ( | |||||||||||||||||||
The adoption in April 2023 of pension reforms in France (including the raising of the retirement age from 62 to 64 years) qualifies as a plan amendment within the meaning of IAS 19, and resulted in the recognition of an immaterial amount in the income statement and the balance sheet for the year ended December 31, 2023.
The table below shows the fair value of plan assets relating to Sanofi’s pension and other post-employment plans, split by asset category:
| 2023 | 2022 | 2021 | |||||||||
| Securities quoted in an active market | % | % | % | ||||||||
| Cash and cash equivalents | % | % | % | ||||||||
| Equity instruments | % | % | % | ||||||||
| Bonds and similar instruments | % | % | % | ||||||||
| Real estate | % | % | % | ||||||||
| Derivatives | % | % | % | ||||||||
| Commodities | % | % | % | ||||||||
| Other | % | % | % | ||||||||
| Other securities | % | % | % | ||||||||
| Hedge funds | % | % | % | ||||||||
| Insurance policies | % | % | % | ||||||||
| Total | % | % | % | ||||||||
Sanofi has a long-term objective of maintaining or increasing the extent to which its pension obligations are covered by assets. To this end, Sanofi uses an asset-liability management strategy, matching plan assets to its pension obligations. This policy aims to ensure the best fit between the assets held on the one hand, and the associated liabilities and expected future payments to plan members on the other. To meet this aim, Sanofi operates a risk monitoring and management strategy (mainly focused on interest rate risk and inflation risk), while investing a growing proportion of assets in high-quality bonds with comparable maturities to those of the underlying obligations and in contracts entered into with leading insurance companies to fund certain post-employment benefit obligations.
SANOFI FORM 20-F 2023 | F-69 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The tables below show the service cost for Sanofi’s pension and other post-employment benefit plans, by geographical region:
| (€ million) | Pensions and other post-employment benefits by geographical region | |||||||||||||||||||
| Service cost for 2023 | France | Germany | US | UK | Other | Total | ||||||||||||||
| Current service cost | ||||||||||||||||||||
| (Gains)/losses related to plan amendments, curtailments or settlements not specified in the terms of the plan | ( | ( | ( | |||||||||||||||||
| Net interest cost/(income) including administration costs and taxes paid during the period | ( | |||||||||||||||||||
| Contributions from plan members | ( | ( | ||||||||||||||||||
| Expense/(gain) recognized directly in profit or loss | ( | |||||||||||||||||||
| Remeasurement of net defined-benefit (asset)/ liability (actuarial gains and losses) | ||||||||||||||||||||
| Expense/(gain) for the period | ||||||||||||||||||||
| (€ million) | Pensions and other post-employment benefits by geographical region | |||||||||||||||||||
| Service cost for 2022 | France | Germany | US | UK | Other | Total | ||||||||||||||
| Current service cost | ||||||||||||||||||||
| (Gains)/losses related to plan amendments, curtailments or settlements not specified in the terms of the plan | ( | ( | ( | ( | ||||||||||||||||
| Net interest cost/(income) including administration costs and taxes paid during the period | ( | |||||||||||||||||||
| Contributions from plan members | ( | ( | ||||||||||||||||||
| Expense/(gain) recognized directly in profit or loss | ( | |||||||||||||||||||
| Remeasurement of net defined-benefit (asset)/ liability (actuarial gains and losses) | ( | ( | ( | ( | ( | |||||||||||||||
| Expense/(gain) for the period | ( | ( | ( | ( | ( | |||||||||||||||
| (€ million) | Pensions and other post-employment benefits by geographical region | |||||||||||||||||||
| Service cost for 2021 | France | Germany | US | UK | Other | Total | ||||||||||||||
| Current service cost | ||||||||||||||||||||
| (Gains)/losses related to plan amendments, curtailments or settlements not specified in the terms of the plan | ||||||||||||||||||||
| Net interest cost/(income) including administration costs and taxes paid during the period | ||||||||||||||||||||
| Contributions from plan members | ( | ( | ||||||||||||||||||
| Expense/(gain) recognized directly in profit or loss | ||||||||||||||||||||
| Remeasurement of net defined-benefit (asset)/liability (actuarial gains and losses) | ( | ( | ( | ( | ( | ( | ||||||||||||||
| Expense/(gain) for the period | ( | ( | ( | ( | ( | ( | ||||||||||||||
An analysis of the “Remeasurement of net defined-benefit (asset)/liability (actuarial gains and losses)” line in the preceding tables is set forth below:
| (€ million) | 2023 | 2022 | 2021 | |||||||||||||||||||||||||||||||||||
| France | Germany | US | UK | France | Germany | US | UK | France | Germany | US | UK | |||||||||||||||||||||||||||
Actuarial gains/(losses) arising during the period | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||
| Comprising: | ||||||||||||||||||||||||||||||||||||||
Gains/(losses) on experience adjustments(a) | ( | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||
| Gains/(losses) on demographic assumptions | ||||||||||||||||||||||||||||||||||||||
| Gains/(losses) on financial assumptions | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||||||
(a) Experience adjustments are mainly due to the effect on plan assets of trends in the financial markets.
The net pre-tax actuarial loss (excluding investments accounted for using the equity method) recognized directly in equity is presented below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Net pre-tax actuarial loss | ( | ( | ( | ||||||||
F-70 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The present value of Sanofi’s obligations in respect of pension and other post-employment benefit plans at the end of each reporting period is shown below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Present value of wholly or partially funded obligations in respect of pension and other post-employment benefit plans | |||||||||||
| Present value of unfunded obligations | |||||||||||
| Total | |||||||||||
The total expense for pensions and other post-employment benefits (€190 million in 2023) is allocated between income statement line items as follows:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Cost of sales | |||||||||||
| Research and development expenses | |||||||||||
| Selling and general expenses | |||||||||||
| Other operating (income)/expenses, net | ( | ( | |||||||||
| Restructuring costs | ( | ( | |||||||||
| Financial expenses | |||||||||||
| Total | |||||||||||
The estimated amounts of employer’s contributions to plan assets in 2024 are as follows:
| (€ million) | France | Germany | US | UK | Other | Total | ||||||||||||||
Employer’s contributions in 2024 (estimate): | ||||||||||||||||||||
| 2024 | ||||||||||||||||||||
The table below shows the expected timing of benefit payments under pension and other post-employment benefit plans for future years:
| (€ million) | France | Germany | US | UK | Other | Total | ||||||||||||||
| Estimated future benefit payments | ||||||||||||||||||||
| 2024 | ||||||||||||||||||||
| 2025 | ||||||||||||||||||||
| 2026 | ||||||||||||||||||||
| 2027 | ||||||||||||||||||||
| 2028 | ||||||||||||||||||||
2029 to 2033 | ||||||||||||||||||||
The table below shows estimates as of December 31, 2023 for the timing of future payments in respect of unfunded pension and other post-employment benefit plans:
| Total | Payments due by period | ||||||||||||||||
| (€ million) | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | |||||||||||||
| Estimated payments | |||||||||||||||||
SANOFI FORM 20-F 2023 | F-71 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.19.2. Restructuring provisions
The table below shows movements in restructuring provisions classified in non-current and current liabilities:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Balance, beginning of period | |||||||||||
| Of which: | |||||||||||
•Classified in non-current liabilities | |||||||||||
•Classified in current liabilities | |||||||||||
| Change in provisions recognized in profit or loss for the period | |||||||||||
Provisions utilized(a) | ( | ( | ( | ||||||||
| Transfers | |||||||||||
| Unwinding of discount | |||||||||||
| Currency translation differences | ( | ( | |||||||||
| Balance, end of period | |||||||||||
| Of which: | |||||||||||
•Classified in non-current liabilities | |||||||||||
•Classified in current liabilities | |||||||||||
(a) Provisions utilized mainly correspond to payments related to employees affected by separation programs.
Provisions for employee termination benefits as of December 31, 2023 amounted to €968 million (compared with €1,039 million as of December 31, 2022 and €943 million as of December 31, 2021).
The provisions apply mainly to France, and relate to various voluntary redundancy programs:
•agreement under the Job Management and Career Paths (GEPP) scheme affecting several French legal entities, signed on February 28, 2022 and announced in April 2022 as part of the “Play to Win” strategy. The agreement provides internal transfer and outplacement opportunities for employees whose jobs are undergoing transformation, and also includes an end-of-career paid leave program and an external retraining program. Most of the provisions charged in 2022 relate to this plan, which began to be implemented in 2022. The provisions charged in 2023 reflect adjustments to the job profiles deemed to be "sensitive"; the reversals recognized during 2023 are due mainly to the Borne Law, which raises the retirement age to 64 and hence disqualifies some participants eligible under previous legislation (in light of the maximum period for portage workers);
•collectively-agreed separation programs involving a number of legal entities announced at the end of June 2020 as part of the rollout of the “Play to Win” strategy; these include an end-of-career paid leave plan and an external retraining program, and were still ongoing during 2023. In addition, Sanofi-Aventis Recherche & Développement (i) announced a voluntary redundancy program in 2020 in connection with the reorganization of R&D operations in France, which was implemented in 2021, and (ii) signed a collectively-agreed termination program in 2021 as part of the rollout of the “Play to Win” strategy; these programs, which cover support functions, include an end-of-career paid leave plan and an end-of-career transition plan;
•programs announced in 2019 relating to (i) R&D (Sanofi-Aventis Recherche & Développement) and (ii) sales forces (the “SAF 2019” plan implemented by Sanofi-Aventis France);
•collectively-agreed separation programs announced in 2018 relating to the reorganization of support functions (“Horizon 2020” plan).
The remainder of the provision for France comprises termination benefits associated with previously-announced programs (early retirement plans and end-of-career transition plans).
The provision includes the present values of:
•gross annuities for self-funded plans;
•employer’s social security charges on early retirement annuities for all plans (outsourced and self-funded); and
•the levy charged on those annuities under the “Fillon” law (only for plans with termination of employment contracts).
The average residual portage periods under these plans were 2.22 years, 2.60 years and 1.94 years as of December 31, 2023, 2022 and 2021, respectively.
The main other countries covered by restructuring provisions are Germany, Japan and the United States.
F-72 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The timing of future termination benefit payments is as follows:
| December 31, 2023 | Total | Benefit payments by period | |||||||||||||||
| (€ million) | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | |||||||||||||
| Employee termination benefits | |||||||||||||||||
•France | |||||||||||||||||
•Other countries | |||||||||||||||||
| Total | |||||||||||||||||
| December 31, 2022 | Benefit payments by period | ||||||||||||||||
| (€ million) | Total | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | ||||||||||||
| Employee termination benefits | |||||||||||||||||
•France | |||||||||||||||||
•Other countries | |||||||||||||||||
| Total | |||||||||||||||||
| December 31, 2021 | Benefit payments by period | ||||||||||||||||
| (€ million) | Total | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | ||||||||||||
| Employee termination benefits | |||||||||||||||||
•France | |||||||||||||||||
•Other countries | |||||||||||||||||
| Total | |||||||||||||||||
D.19.3. Other provisions
Other provisions include provisions for risks and litigation relating to environmental, tax, commercial and product liability matters.
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Environmental risks | |||||||||||
| Product liability risks, litigation and other | |||||||||||
| Total | |||||||||||
Provisions for environmental risks relate primarily to contingencies arising from business divestitures, and include remediation costs relating to such environmental risks.
Identified environmental risks are covered by provisions estimated on the basis of the costs Sanofi believes it will be obliged to meet over a period not exceeding (other than in exceptional cases) 30 years. Sanofi expects that €86 million of those provisions will be utilized in 2024, and €194 million over the period from 2025 through 2028.
As regards greenhouse gas emission quotas, which relate to Sanofi production facilities in France and Ireland, in the absence of specific IFRS pronouncements Sanofi has adopted the "net liability approach". That involves recognizing a liability at the balance sheet date if actual emissions exceed the quotas held, in accordance with IAS 37 and French GAAP (Plan Comptable Général, Article 615-1s). Quotas are managed as a production cost, and as such are recognized in inventory at a zero value (if received free of charge) and at acquisition cost (if bought on the market). As of December 31, 2023, no liability was recognized, and quotas valued at €3 million were held in inventory.
“Product liability risks, litigation and other” mainly comprises provisions for risks relating to product liability (including IBNR provisions as described in Note B.12.), government investigations, regulatory or antitrust law claims, contingencies arising from business divestitures (other than environmental risks), and remediation costs related to leases.
The main pending legal and arbitral proceedings and government investigations are described in Note D.22.
A full risk and litigation assessment is performed with the assistance of Sanofi’s legal advisers, and provisions are recorded as required by circumstances in accordance with the principles described in Note B.12.
SANOFI FORM 20-F 2023 | F-73 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.19.4. Non-current income tax liabilities
Non-current income tax liabilities amounted to €1,842 million as of December 31, 2023 (versus €1,979 million as of December 31, 2022 and €2,039 million as of December 31, 2021).
This line item includes the residual liability due after more than one year arising from the estimated tax charge on deemed repatriation attributable to the accumulated earnings of non-US operations (€247 million as of December 31, 2023, €459 million as of December 31, 2022 and €576 million as of December 31, 2021. The expense was initially recognized in 2018 at an amount of $1,092 million, and payment is being made over eight years through 2025.
Non-current income tax liabilities include uncertainties over income tax treatments totaling €1,595 million as of December 31, 2023, versus €1,520 million as of December 31, 2022 and €1,463 million as of December 31, 2021.
A US legal restructuring resulted in a capital loss of €3 billion recognized in the 2020 final tax filing. One-third of the capital loss has been used against 2020 capital gains and the remaining balance will be eligible to carry back for three years . Due to management’s judgement about potential alternative interpretations of the prevailing tax law, no tax benefit has been recognized on this transaction in accordance with IFRIC 23.
D.19.5. Current provisions and other current liabilities
Current provisions and other current liabilities comprise the following:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Taxes payable, other than corporate income taxes | |||||||||||
| Employee-related liabilities | |||||||||||
| Restructuring provisions (see Note D.19.2.) | |||||||||||
| Interest rate derivatives (see Note D.20.) | |||||||||||
| Currency derivatives (see Note D.20.) | |||||||||||
| Equity derivatives (see Note D.20.) | |||||||||||
| Amounts payable for acquisitions of non-current assets | |||||||||||
Customer contract liabilities(a) | |||||||||||
Other current liabilities(b)(c) | |||||||||||
| Total | |||||||||||
(a) See Note A.5., “Agreements relating to the recombinant COVID-19 vaccine candidate developed by Sanofi in collaboration with GSK”. The year-on-year change in this item between 2022 and 2023 includes revenue of €269 million recognized in profit or loss during 2023 previously included in “Customer contract liabilities” as of December 31, 2022, compared with revenue of €85 million recognized in profit or loss during 2022 previously included in “Customer contract liabilities” as of December 31, 2021 . No such revenue was recognized in 2021.
(b) “Other current liabilities” mainly comprises provisions and liabilities for customer rebates and returns; provisions for discounts and rebates granted to healthcare authorities and governmental programs (see Note D.23.); and the liability payable at each reporting date under the Monoclonal Antibody Alliance with Regeneron.
(c) As of December 31, 2023 includes €131 million (nominal value: €137 million) for the current liability relating to royalties payable to Sobi on net sales of BEYFORTUS (nirsevimab) in the United States (see Note C.2.)
D.20. Derivative financial instruments and market risks
The table below shows the fair value of derivative instruments as of December 31, 2023, 2022 and 2021:
| (€ million) | Non-current assets | Current assets | Total assets | Non-current liabilities | Current liabilities | Total liabilities | Market value at December 31, 2023 (net) | Market value at December 31, 2022 (net) | Market value at December 31, 2021 (net) | ||||||||||||||||||||
| Currency derivatives | ( | ( | |||||||||||||||||||||||||||
| operating | ( | ( | |||||||||||||||||||||||||||
| financial | ( | ( | |||||||||||||||||||||||||||
| Interest rate derivatives | ( | ( | ( | ( | ( | ||||||||||||||||||||||||
| Equity derivatives | ( | ||||||||||||||||||||||||||||
| Total | ( | ( | ( | ( | ( | ||||||||||||||||||||||||
F-74 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Objectives of the use of derivative financial instruments
Sanofi uses derivative instruments to manage operating exposure to movements in exchange rates, and financial exposure to movements in interest rates and exchange rates (where the debt or receivable is not contracted in the functional currency of the borrower or lender entity). On occasion, Sanofi uses equity derivatives in connection with the management of its portfolio of equity investments.
Sanofi performs periodic reviews of its transactions and contractual agreements in order to identify any embedded derivatives, which are accounted for separately from the host contract in accordance with IFRS 9. Sanofi had no material embedded derivatives as of December 31, 2023, 2022 or 2021.
Counterparty risk
For a description of counterparty risk, refer to “Item 11. — Quantitative and Qualitative Disclosures about Market Risk”.
a) Currency derivatives used to manage operating risk exposures
For a description of Sanofi’s objectives, policies and procedures for the management of operating foreign exchange risk, refer to “Item 11. — Quantitative and Qualitative Disclosures about Market Risk”.
The table below shows operating currency hedging instruments in place as of December 31, 2023, with the notional amount translated into euros at the relevant closing exchange rate:
| December 31, 2023 | Of which derivatives designated as cash flow hedges | Of which derivatives not eligible for hedge accounting | |||||||||||||||||||||
| (€ million) | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | Notional amount | Fair value | ||||||||||||||||
| Forward currency sales | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| of which Chinese yuan renminbi | |||||||||||||||||||||||
| of which Singapore dollar | ( | ( | |||||||||||||||||||||
of which Japanese yen | ( | ( | |||||||||||||||||||||
| of which Korean won | ( | ( | |||||||||||||||||||||
| Forward currency purchases | ( | ( | |||||||||||||||||||||
| of which US dollar | ( | ( | |||||||||||||||||||||
| of which Singapore dollar | |||||||||||||||||||||||
| of which Chinese yuan renminbi | ( | ( | |||||||||||||||||||||
| of which Korean won | |||||||||||||||||||||||
of which Japanese yen | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
The table below shows operating currency hedging instruments in place as of December 31, 2022, with the notional amount translated into euros at the relevant closing exchange rate:
| December 31, 2022 | Of which derivatives designated as cash flow hedges | Of which derivatives not eligible for hedge accounting | |||||||||||||||||||||
| (€ million) | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | Notional amount | Fair value | ||||||||||||||||
| Forward currency sales | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| of which Chinese yuan renminbi | |||||||||||||||||||||||
| of which Japanese yen | ( | ( | |||||||||||||||||||||
| of which Singapore dollar | |||||||||||||||||||||||
| of which Korean won | ( | ( | |||||||||||||||||||||
| Forward currency purchases | ( | ( | |||||||||||||||||||||
| of which US dollar | ( | ( | |||||||||||||||||||||
| of which Singapore dollar | ( | ( | |||||||||||||||||||||
| of which Chinese yuan renminbi | |||||||||||||||||||||||
| of which Korean won | |||||||||||||||||||||||
| of which Taiwan dollar | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
SANOFI FORM 20-F 2023 | F-75 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below shows operating currency hedging instruments in place as of December 31, 2021, with the notional amount translated into euros at the relevant closing exchange rate:
| December 31, 2021 | Of which derivatives designated as cash flow hedges | Of which derivatives not eligible for hedge accounting | |||||||||||||||||||||
(€ million) | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | Notional amount | Fair value | ||||||||||||||||
| Forward currency sales | |||||||||||||||||||||||
| of which US dollar | |||||||||||||||||||||||
| of which Chinese yuan renminbi | ( | ( | |||||||||||||||||||||
| of which Singapore dollar | ( | ( | |||||||||||||||||||||
| of which Japanese yen | |||||||||||||||||||||||
| of which Taiwan dollar | ( | ( | |||||||||||||||||||||
| Forward currency purchases | |||||||||||||||||||||||
| of which US dollar | ( | ( | |||||||||||||||||||||
| of which Singapore dollar | |||||||||||||||||||||||
| of which Chinese yuan renminbi | |||||||||||||||||||||||
| of which Hungarian forint | |||||||||||||||||||||||
| of which Russian rouble | ( | ( | |||||||||||||||||||||
| Total | |||||||||||||||||||||||
b) Currency and interest rate derivatives used to manage financial exposure
For a description of Sanofi’s objectives, policies and procedures for the management of financial foreign exchange risk and interest rate risk, refer to “Item 11. — Quantitative and Qualitative Disclosures about Market Risk”.
The table below shows financial currency hedging instruments in place, with the notional amount translated into euros at the relevant closing exchange rate:
| 2023 | 2022 | 2021 | ||||||||||||||||||||||||||||||
| (€ million) | Notional amount | Fair value | Expiry | Notional amount | Fair value | Expiry | Notional amount | Fair value | Expiry | |||||||||||||||||||||||
| Forward currency sales | ||||||||||||||||||||||||||||||||
| of which US dollar | (a) | 2024 | 2023 | 2022 | ||||||||||||||||||||||||||||
of which Singapore dollar | 2024 | |||||||||||||||||||||||||||||||
| of which Chinese yuan renminbi | 2024 | 2023 | ( | 2022 | ||||||||||||||||||||||||||||
| Forward currency purchases | ( | |||||||||||||||||||||||||||||||
| of which US dollar | (b) (c) | ( | 2024 | ( | 2023 | 2022 | ||||||||||||||||||||||||||
| of which Singapore dollar | ( | 2024 | 2023 | 2022 | ||||||||||||||||||||||||||||
| of which Japanese yen | 2024 | 2023 | ( | 2022 | ||||||||||||||||||||||||||||
| Total | ||||||||||||||||||||||||||||||||
(a) Includes forward sales with a notional amount of $3,615 million expiring in 2024, designated as a hedge of Sanofi’s net investment in Bioverativ. As of December 31, 2023, the fair value of these forward contracts represented an asset of €54 million; the opposite entry was recognized in "Other comprehensive income", with the impact on financial income and expense being immaterial.
(b) Includes forward purchases with a notional amount of $1,000 million expiring in 2024, designated as a fair value hedge of the exposure of $1,000 million of bond issues to fluctuations in the EUR/USD spot rate. As of December 31, 2023, the fair value of the contracts was a liability of €31 million, the opposite entry for €2.7 million of which was credited to “Other comprehensive income” under the cost of hedging accounting treatment.
(c) Includes forward purchases with a notional amount of $1,044 million expiring in 2024, designated as a fair value hedge of the exposure of an equivalent amount of commercial paper. As of December 31, 2023, the fair value of the swaps was a liability of €3 million, the opposite entry for €0.7 million of which was credited to “Other comprehensive income” under the cost of hedging accounting treatment.
F-76 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below shows interest rate hedging instruments in place as of December 31, 2023:
| Notional amounts by expiry date as of December 31, 2023 | Of which designated as fair value hedges | Of which designated as cash flow hedges | |||||||||||||||||||||||||||||||||||||||
| (€ million) | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 and later | Total | Fair value | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | ||||||||||||||||||||||||||||
| Interest rate swaps | |||||||||||||||||||||||||||||||||||||||||
pay capitalized SOFR USD/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized SOFR USD/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized Ester/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized Ester/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized Ester / receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
| Total | ( | ( | |||||||||||||||||||||||||||||||||||||||
The table below shows interest rate hedging instruments in place as of December 31, 2022:
| Notional amounts by expiry date as of December 31, 2022 | Of which designated as fair value hedges | Of which designated as cash flow hedges | |||||||||||||||||||||||||||||||||||||||
| (€ million) | 2023 | 2024 | 2025 | 2026 | 2027 | 2028 and later | Total | Fair value | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | ||||||||||||||||||||||||||||
Interest rate swaps | |||||||||||||||||||||||||||||||||||||||||
pay capitalized SOFR USD/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized SOFR USD/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized Ester/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized Ester/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
| Total | ( | ( | |||||||||||||||||||||||||||||||||||||||
The table below shows interest rate hedging instruments in place as of December 31, 2021:
| Notional amounts by expiry date as of December 31, 2021 | Of which designated as fair value hedges | Of which designated as cash flow hedges | |||||||||||||||||||||||||||||||||||||||
| (€ million) | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 and later | Total | Fair value | Notional amount | Fair value | Notional amount | Fair value | Of which recognized in equity | ||||||||||||||||||||||||||||
| Interest rate swaps | |||||||||||||||||||||||||||||||||||||||||
pay capitalized EONIA/receive | |||||||||||||||||||||||||||||||||||||||||
pay - | |||||||||||||||||||||||||||||||||||||||||
pay capitalized USD SOFR/receive | ( | ( | |||||||||||||||||||||||||||||||||||||||
pay capitalized USD SOFR/receive | |||||||||||||||||||||||||||||||||||||||||
receive capitalized Eonia/pay | ( | ( | |||||||||||||||||||||||||||||||||||||||
| Total | |||||||||||||||||||||||||||||||||||||||||
(a) These interest rate swaps hedge fixed-rate bonds with a nominal of €99 million held in a Professional Specialized Investment Fund dedicated to Sanofi and recognized within “Long-term loans and advances and other non-current receivables” (see Note D.7.).
SANOFI FORM 20-F 2023 | F-77 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
c) Actual or potential effects of netting arrangements
The table below is prepared in accordance with the accounting policies described in Note B.8.3.:
| (€ million) | 2023 | 2022 | 2021 | |||||||||||||||||
| Derivative financial assets | Derivative financial liabilities | Derivative financial assets | Derivative financial liabilities | Derivative financial assets | Derivative financial liabilities | |||||||||||||||
| Gross carrying amounts before offset (a) | ( | ( | ( | |||||||||||||||||
| Gross amounts offset (in accordance with IAS 32) (b) | ||||||||||||||||||||
| Net amounts as reported in the balance sheet (a) - (b) = (c) | ( | ( | ( | |||||||||||||||||
| Effects of other netting arrangements (not fulfilling the IAS 32 criteria for offsetting) (d) | ||||||||||||||||||||
| Financial instruments | ( | ( | ( | |||||||||||||||||
| Fair value of financial collateral | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||||||
| Net exposure (c) + (d) | ( | ( | ( | |||||||||||||||||
D.21. Off balance sheet commitments
The off balance sheet commitments presented below are shown at their nominal value.
D.21.1. Off balance sheet commitments relating to operating activities
Off balance sheet commitments relating to Sanofi’s operating activities comprise the following:
| December 31, 2023 | Payments due by period | ||||||||||||||||
| (€ million) | Total | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | ||||||||||||
Leases with a term of less than 12 months, low value asset leases and lease contracts committed but not yet commenced(a) | |||||||||||||||||
Irrevocable purchase commitments(b) | |||||||||||||||||
•given(c) | |||||||||||||||||
•received | ( | ( | ( | ( | |||||||||||||
| Research and development license agreements - commitments given | |||||||||||||||||
•commitments related to R&D and other commitments(d) | |||||||||||||||||
•contingent milestone payments in connection with development programs in progress(e) | |||||||||||||||||
Total - net commitments given | |||||||||||||||||
(a) Includes the variable portion of future lease payments not recognized as lease liabilities as of December 31, 2023; the equivalent amount of these commitments as of December 31, 2022 was €38 million.
During 2023, Sanofi signed a15-year lease which will take effect in 2025 and for which Sanofi is committed for a minimum period of 12 years, corresponding to a minimum commitment of $0.2 billion. The lease includes two extension options of five years each.
During 2023, Sanofi signed a
(b) These comprise irrevocable commitments to suppliers of (i) property, plant and equipment, net of down-payments (see Note D.3.) and (ii) goods and services. As of December 31, 2022, irrevocable commitments amounted to €6,194 million given and €574 million received.
(c) Irrevocable purchase commitments given as of December 31, 2023 include €926 million of commitments to joint ventures, and the commitment to EUROAPI as described in Note D.2. and amounting to €804 million as of December 31, 2023.
(d) Commitments related to R&D, and other commitments, amounted to €259 million as of December 31, 2022.
(e) This line includes only contingent milestone payments on development projects in progress. The equivalent amount as of December 31, 2022 was €2,919 million.
In pursuance of its strategy, Sanofi may acquire technologies and rights to products. Such acquisitions may be made in various contractual forms: acquisitions of shares, loans, license agreements, joint development, and co-marketing. These arrangements generally involve upfront payments on signature of the agreement, development milestone payments, and royalties. Some of these complex agreements include undertakings to fund research programs in future years and payments contingent upon achieving specified development milestones, the granting of approvals or licenses, or the attainment of sales targets once a product is commercialized.
The “Research and development license agreements” line comprises future service commitments to fund research and development or technology, and contingent milestone payments regarded as reasonably achievable (i.e. all potential milestone payments relating to projects in the development phase, for which the future financial consequences are known or probable and for which there is a sufficiently reliable estimate). This line excludes:
•commitments given relating to (i) projects in the research phase, amounting to €16.8 billion as of December 31, 2023 and €18.0 billion as of December 31, 2022 and (ii) payments contingent upon the attainment of sales targets once a product is commercialized, amounting to €17.9 billion as of December 31, 2023 and €18.5 billion as of December 31, 2022);
F-78 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
•commitments received amounting to €10.0 billion as of December 31, 2023 (€8.8 billion as of December 31, 2022), mainly comprising research, development and commercialization agreements with partners further to the acquisitions of (i) Ablynx (€0.9 billion as of December 31, 2023, versus €1.0 billion as of December 31, 2022); (ii) Kymab (€0.2 billion as of December 31, 2023, versus €0.2 billion as of December 31, 2022) and (iii) Provention Bio (€0.3 billion as of December 31, 2023), plus contingent consideration receivable based on attainment of regulatory and sales milestones for commercialized products under the terms of licenses or rights assignment agreements amounting to €8.5 billion as of December 31, 2023 (€7.6 billion as of December 31, 2022).
The major agreements entered into by Sanofi in 2023 are described below:
•on June 19, 2023, Sanofi expanded its collaboration with Scribe Therapeutics signed in September 2022 and entered into an exclusive license agreement on CasX-Editor(XE) genome editing technology associated with guide RNAs for multiple targets including sickle cell disease and other genomic diseases. Under the terms of the agreement, Scribe Therapeutics received an upfront payment of $40 million and may receive more than $1.2 billion based on the achievement of certain milestones;
•on October 3, 2023, Sanofi entered into an agreement with Janssen Pharmaceuticals, Inc. (Janssen), to develop and commercialize the vaccine candidate against extra intestinal pathogenic strains of E. coli developed by Janssen, currently in Phase 3. Under the terms of the agreement, both parties will co-fund current and future research and development costs. Sanofi paid $175 million upfront to Janssen and may pay to Janssen milestone payments contingent on the attainment of certain development and commercial objectives;
•on October 4, 2023, Sanofi entered into a collaboration with Teva Pharmaceuticals to co-develop and co-commercialize asset TEV’574, currently in Phase 2b clinical trials for the treatment of Ulcerative Colitis and Crohn's Disease, two types of inflammatory bowel disease. Under the terms of the new collaboration agreement, Teva received an upfront payment of €469 million ($500 million) and may receive up to €940 million ($1 billion) in milestone payments, depending on the achievement of development and commercialization goals.
In addition, by acquiring all of the outstanding shares of Provention Bio, Inc. on April 27, 2023 (see Note D.1.), Sanofi assumed commitments amounting to €946 million made by that company to various partners under collaboration agreements previously entered into.
The amount of commitments as of December 31, 2023 also includes commitments under agreements entered into by Sanofi in prior years, the principal ones of which are described below. For a full description of each agreement, refer to the Annual Report on Form 20-F for the year in which the agreement was entered into.
The major agreements entered into by Sanofi in 2022 are described below:
•with Exscentia: an innovative license agreement and research collaboration to develop up to 15 novel small molecule candidates across oncology and immunology, leveraging Exscientia’s end-to-end AI-driven platform utilizing actual patient samples;
•with ABL Bio: a licensing and collaboration agreement for the development of ABL301, a bispecific antibody intended as a treatment for alpha-synucleinopathies;
•a collaboration and exclusive license agreement with Adagene Inc., a company specializing in the discovery and development of antibody-based therapies;
•a strategic risk-sharing collaboration with Blackstone under which funds managed by Blackstone Life Sciences (BXLS) will contribute up to €300 million to accelerate the global pivotal studies and clinical development program for the subcutaneous formulation and delivery of the anti-CD38 antibody SARCLISA, to treat patients with multiple myeloma. That amount will be paid to Sanofi on the basis of development expenses incurred. In addition, Sanofi may pay royalties on future sales of this solution;
•an exclusive collaboration agreement with IGM Biosciences, Inc. to create, develop, manufacture and commercialize IgM antibody agonists against three oncology targets and three immunology/inflammation targets;
•a collaboration agreement with Skyhawk Therapeutics, Inc. to discover and develop novel small molecules that modulate RNA splicing to address challenging oncology and immunology targets;
•a collaboration agreement with Atomwise that will leverage its ATOMNET platform to identify and synthesize up to five drug targets;
•a research collaboration with Scribe Therapeutics to leverage its CRISPR by Design platform and to obtain a non-exclusive license to genome editing CasX-Editor(XE) technology for multiple oncology targets;
•a strategic research collaboration with Insilico Medicine to leverage Insilico Medicine’s AI platform, Pharma.AI, to advance drug development candidates for up to six new therapeutic targets;
•Sanofi and Innate Pharma SA announced an expansion of their collaboration, with Sanofi licensing a natural killer (NK) cell engager program targeting B7-H3 from Innate’s ANKET (Antibody-based NK Cell Engager Therapeutics) platform.
The principal agreements entered into by Sanofi in previous years are listed below:
•Biond Biologics (2021): license agreement for the development and commercialization of BND-22 (a humanized IgG4 antagonist antibody targeting the Ig-like transcript 2 (ILT2) receptor, in development for the treatment of solid tumors);
•Kymera (2020): agreement to develop and commercialize protein degrader therapies targeting IRAK4 in patients with immune-inflammatory diseases;
•Nurix Therapeutics (2020): collaboration to develop novel targeted protein degradation therapies;
•Denali Therapeutics Inc. (2018): collaboration agreement on the development of multiple molecules with the potential to treat a range of neurological and systemic inflammatory diseases. The two lead molecules are DNL747 in multiple sclerosis and amyotrophic lateral sclerosis, and DNL758 in systemic inflammatory diseases such as rheumatoid arthritis and psoriasis;
SANOFI FORM 20-F 2023 | F-79 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Sanofi and one of its alliance partners have decided to terminate the following agreement (the related commitments are no longer included in Sanofi’s off balance sheet disclosures as of December 31, 2023):
•on January 6, 2023, Sanofi and Regulus Therapeutics Inc. terminated their collaboration and license agreement on the discovery, development and commercialization of new micro-RNA derived molecules in fibrosis. The termination took effect on February 5 ,2023.
In addition, under the collaboration agreement with Regeneron on monoclonal antibodies (see Note C.1.), Sanofi is entitled to receive an additional share of quarterly profits (capped at 10 % of Regeneron’s share of quarterly profits until March 31, 2022, and thereafter at 20 %), until Regeneron has paid 50 % of the cumulative development costs incurred by the parties to the alliance. As of December 31, 2023 this represented total commitments received of €2.1 billion (versus €2.7 billion as of December 31, 2022), against cumulative development costs of €8.6 billion.
Sanofi entered into an agreement with Royalty Pharma in December 2014 relating to development programs, under which Royalty Pharma bore a portion of the remaining development costs of the project on a quarterly basis in return for royalties on future sales. The products in development under that agreement have been launched in territories including the United States and Europe, marking the end of the joint development programs.
On February 27, 2017, Sanofi and Lonza announced a strategic partnership in the form of a joint venture (BioAtrium AG) to build and operate a large-scale mammalian cell culture facility for monoclonal antibody production in Visp, Switzerland. An initial investment of approximately €0.3 billion to finance construction of the facility, split 50 /50 between the two partners, has now been made in full. In addition, Sanofi could pay BioAtrium AG in the region of €0.6 billion over the 2023-2031 period as its share of operating expenses and the cost of producing future batches.
In February 2014, pursuant to the “Pandemic Influenza Preparedness Framework for the sharing of influenza viruses and access to vaccines and other benefits” (still effective as of December 31, 2023), Sanofi Pasteur and the World Health Organization (WHO) signed a bilateral “Standard Material Transfer Agreement” (SMTA 2). This agreement stipulates that Sanofi Pasteur will, during declared pandemic periods, (i) donate 7.5 % of its real-time production of pandemic vaccines against any strain with potential to cause a pandemic, and (ii) reserve a further 7.5 % of such production on affordable terms. The agreement cancels and replaces all preceding commitments to donate pandemic vaccines to the WHO.
D.21.2. Off balance sheet commitments relating to financing activities
Credit facilities
Undrawn credit facilities are as follows:
| December 31, 2023 | Total | Expiry | |||||||||||||||
| (€ million) | Less than 1 year | 1 to 3 years | 3 to 5 years | More than 5 years | |||||||||||||
| General-purpose credit facilities | |||||||||||||||||
As of December 31, 2023, total credit facilities amounted to €8,000 million (versus €8,000 million as of December 31, 2022 and €8,000 million as of December 31, 2021).
Guarantees
The table below shows the amount of guarantees given and received:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Guarantees given: | |||||||||||
•Guarantees provided to banks in connection with credit facilities | |||||||||||
•Other guarantees given | |||||||||||
| Guarantees received | ( | ( | ( | ||||||||
D.21.3. Off balance sheet commitments relating to asset acquisitions and divestments, and to changes in the scope of consolidation
As of December 31, 2023, Sanofi had received commitments amounting in aggregate to €0.3 billion in respect of (i) divestments of assets relating to transactions not yet finalized as of that date and (ii) contingent consideration arising under past agreements.
Off balance sheet commitments of a financing nature with associates and joint ventures are disclosed in Note D.6.
Off-balance sheet commitments relating to securities classified in the categories Equity instruments at fair value through other comprehensive income and Unquoted debt securities not meeting the definition of equity instruments are respectively disclosed in Notes D .7.1. and D.7.3..
The maximum amount of contingent consideration relating to business combinations is disclosed in Note D.18.
F-80 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.22. Legal and arbitral proceedings
Sanofi and its affiliates are involved in litigation, arbitration and other legal proceedings. These proceedings typically are related to product liability claims, intellectual property rights (particularly claims against generic companies seeking to limit the patent protection of Sanofi products), competition law and trade practices, commercial claims, employment and wrongful discharge claims, tax assessment claims, waste disposal and pollution claims, and claims under warranties or indemnification arrangements relating to business divestitures. Provisions related to legal and arbitral proceedings are recorded in accordance with the principles described in Note B.12.
Most of the issues raised by these claims are highly complex and subject to substantial uncertainties; therefore, the probability of loss and an estimation of damages are difficult to ascertain. Contingent liabilities are cases for which either we are unable to make a reasonable estimate of the expected financial effect that will result from ultimate resolution of the proceeding, or a cash outflow is not probable. In either case, a brief description of the nature of the contingent liability is disclosed and, where practicable, an estimate of its financial effect, an indication of the uncertainties relating to the amount and timing of any outflow, and the possibility of any reimbursement are provided in application of paragraph 86 of IAS 37.
In the cases that have been settled or adjudicated, or where quantifiable fines and penalties have been assessed, we have indicated our losses or the amount of provision accrued that is the estimate of the probable loss.
In a limited number of ongoing cases, while we are able to make a reasonable estimate of the expected loss or range of the possible loss and have accrued a provision for such loss, we believe that publication of this information on a case-by-case basis or by class would seriously prejudice the Company’s position in the ongoing legal proceedings or in any related settlement discussions. Accordingly, in those cases, we have disclosed information with respect to the nature of the contingency but have not disclosed our estimate of the range of potential loss, in accordance with paragraph 92 of IAS 37.
These assessments can involve a series of complex judgments about future events and can rely heavily on estimates and assumptions. Our assessments are based on estimates and assumptions that have been deemed reasonable by management. We believe that the aggregate provisions recorded for the above matters are adequate based upon currently available information. However, given the inherent uncertainties related to these cases and involved in estimating contingent liabilities, we could in the future incur judgments that could have a material adverse effect on our net income in any particular period.
Long term provisions are disclosed in Note D.19. They include:
•provisions for product liability risks, litigation and other amount to €1,283 million in 2023. These provisions are mainly related to product liabilities, government investigations, competition law, regulatory claims, warranties in connection with certain contingent liabilities arising from business divestitures other than environmental matters and other claims;
•provisions for environmental risks and remediation amount to €493 million in 2023, the majority of which are related to contingencies that have arisen from business divestitures.
a) Products
Sanofi Pasteur Hepatitis B Vaccine Product Litigation
Since 1996, more than 180 lawsuits have been filed in various French civil courts against Sanofi Pasteur and/or Sanofi Pasteur MSD S.N.C., the former French subsidiary of Sanofi, and the latter a joint venture company with Merck & Co., Inc. now terminated, for which past ongoing litigation is now managed by the originating party. In such lawsuits, the plaintiffs allege that they suffer from a variety of neurological disorders and autoimmune diseases, including multiple sclerosis and Guillain-Barré syndrome as a result of receiving the hepatitis B vaccine.
In January 2018, the Appeal Court of Bordeaux found a causal link between hepatitis B vaccine and multiple sclerosis. In July 2019, the French Supreme Court (Cour de cassation) cancelled the judgment of the Appeal Court of Bordeaux and referred the case back to the Appeal Court of Toulouse. On March 30, 2022, the Appeal Court of Toulouse dismissed all the plaintiff’s claims.
As of December 31, 2023, there were six ongoing lawsuits related to Sanofi Pasteur hepatitis B vaccine.
TAXOTERE Product Litigation in the US
As of December 31, 2023, there were approximately 6,770 ingesting plaintiffs cases remaining in courts across the country.
Lawsuits have been filed against affiliates of Sanofi under US state law for personal injuries allegedly sustained in connection with the use of TAXOTERE. The actions are held in several jurisdictions around the country. In 2021, there were two bellwether trials as part of a federal multi-district litigation in the Eastern District of Louisiana both resulting in jury verdicts in Sanofi's favor. In 2023, Sanofi settled approximately 100 individual cases.
It is not possible, at this stage, to assess with certainty the outcome of these lawsuits.
TAXOTERE – Mississippi Attorney General Litigation in the US
In October 2018, the Attorney General for the State of Mississippi filed a civil action in Hinds County, Mississippi, Chancery Court against various Sanofi Defendants related to TAXOTERE. The State asserts one cause of action based on the Mississippi Consumer Protection Act (MCPA) and seeks a permanent injunction prohibiting Defendants’ conduct and civil penalties of up to $10,000 for each violation. Sanofi filed a motion to dismiss the entire action in Hinds County, Mississippi, Chancery Court, which is currently pending.
It is not possible, at this stage, to assess with certainty the outcome of these lawsuits.
SANOFI FORM 20-F 2023 | F-81 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
ZANTAC Litigation in the US
In September 2019, the US Food and Drug Administration (FDA) announced it was investigating the claims of an online pharmacy’s Citizen Petition that the medication ZANTAC (the brand name for ranitidine) used for stomach heartburn contains or can generate the chemical N-Nitrosodimethylamine (NDM), an alleged human carcinogen. As a precautionary measure, Sanofi initiated a voluntary recall of branded over-the-counter ZANTAC in October 2019. Concurrent with FDA’s investigation, multiple personal injury lawsuits and class actions alleging that ZANTAC causes various cancers and seeking damages for either alleged personal injuries or alleged economic injuries were filed. Federal court cases were coordinated into a Multi-District Litigation (MDL) in the Southern District of Florida in February 2020.
On December 6, 2022, the MDL Court granted Sanofi and other defendants’ Daubert and summary judgment motions. As a result, the Court entered final judgment in all cases involving plaintiffs’ five designated cancers and dismissed the class action cases. Based on the preliminary estimates, more than 12,000 plaintiffs have filed notices to appeal the Daubert ruling in the Eleventh Circuit. The MDL Court subsequently dismissed all pending cases alleging a non-designated cancer for failure to serve expert reports.
Other cases are pending in various state courts. The majority of the state court plaintiffs have cases pending in Delaware, where a hearing on defendants’ Daubert motions to exclude plaintiffs’ experts was scheduled for January 2024. Trials are scheduled in 2024 in single-plaintiff cases in several states. No ZANTAC case has gone to trial to date.
Overall, there are currently around 3,280 product liability “complaints” filed. These complaints encompass 26,984 individual product liability “plaintiffs” who have all filed against Sanofi. The vast majority of these plaintiffs participated in the MDL Court’s census registry program, allege cancers that the plaintiffs’ leadership decided not to designate and pursue in the MDL, and have since filed their complaints in state courts. Additional cases may be filed.
In addition, in November 2019, Sanofi received a Civil Investigative Demand (CID) related to this issue from the Arizona Attorney General. Sanofi provided responses in December 2019 and July 2020 and has not received any follow-up requests.
In June 2020, the New Mexico Attorney General filed a complaint against Sanofi, the previous marketing authorization holders for branded ZANTAC, a dozen generic manufacturers, and several retailers. The complaint brings claims for alleged violations of the New Mexico Unfair Practices Act, violations of the New Mexico False Advertising Act, violations of the New Mexico Public Nuisance Statute, common law public nuisance, and negligence. Trial in the case is scheduled for September 2025.
In June 2020, Sanofi received a notice from the US Department of Justice Civil Division and US Attorney’s Office for the Eastern District of Pennsylvania of an investigation into allegations that pharmaceutical manufacturers violated the False Claims Act, 31 U.S.C. § 3729, in relation to the drug ZANTAC and ranitidine hydrochloride through alleged failure to disclose to the federal government information about the potential presence of NDMA. In response to the notice, Sanofi provided information and documents including applications and communications with FDA, in August 2020. Sanofi has not received any subsequent requests from the federal government.
In November 2020, the Mayor and City Council of Baltimore filed a complaint against Sanofi, the previous marketing authorization holders for branded ZANTAC, generic manufacturers, and several retailers. The complaint alleges violations of the Maryland Consumer Protection statute, public nuisance, and negligence. Trial in the case is scheduled for June 2025.
In January 2021, Sanofi was served with the Center for Environmental Health’s Second Amended Complaint alleging Proposition 65 violations. The case is pending in California Superior Court in Alameda County, and no trial date has been set.
It is not possible, at this stage, to assess with certainty the outcome of these lawsuits.
ZANTAC Litigation in Canada
Between 2019 and 2022, seven proposed class actions naming some or all of Sanofi Consumer Health Inc., Sanofi-Aventis Canada Inc., Chattem (Canada) Inc., Sanofi and Sanofi Pasteur Limited as Defendants, relating to ranitidine were filed in courts in various Canadian provinces. The cases allege that proposed class members suffered personal injury from the ingestion of ranitidine, and seek damages in unspecified amounts, disgorgement of profits, restitution in the amount of the purchase price of ZANTAC and subrogated damages on behalf of provincial health insurers for health care costs related to ranitidine use.
In May 2023, in the proceedings pending before the Supreme Court of British Columbia, the Court dismissed the action, ruling that there is no scientific support for plaintiff’s claims. After this ruling, the Superior Court of Quebec stayed the corresponding proposed ZANTAC class proceedings in Quebec until the result of the US Multi-District Litigation (MDL) appeal is announced or October 15, 2024 (whichever comes first).
It is not possible, at this stage, to assess with certainty the outcome of the remaining lawsuits.
GOLD BOND Product Litigation in the US
Over the last few years, Sanofi affiliates have been named in product liability actions in the United States regarding the alleged presence of asbestos in talc products originating from past acquisitions. A certain number of these claims were also dismissed during that time. As of December 31, 2023, there were approximately 500 product liability ongoing actions. To date, no cases have proceeded to trial.
It is not possible, at this stage, to assess with certainty the outcome of these lawsuits.
F-82 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
DEPAKINE Product Litigation in France
Civil proceedings
As of December 31, 2023, 79 families brought a civil claim involving 133 people exposed in utero to sodium valproate against a French affiliate of Sanofi seeking indemnification under French law for personal injuries allegedly suffered by children in connection with the use of sodium valproate by their mothers during pregnancy to treat their epilepsy (DEPAKINE). These actions are held in several jurisdictions in France.
Concerning three other cases relating to births that occurred between 2005 and 2009, the Court held, on the basis of a non-fault liability, that Sanofi was liable in light of the wording of the patient information leaflet. Provisional compensation amounts were set in the range of €0.1 million to €0.5 million.
All the judgments have been appealed and are still pending.
In the class action lawsuit filed in May 2017 by the APESAC (Association des Parents d’Enfants souffrant du Syndrome de l’Anti-Convulsivant) against the French affiliate, the Judicial Tribunal of Paris ruled on January 5, 2022 that a class is admissible, retaining Sanofi’s liability between 1984 and January 2006 for malformations and between 2001 and January 2006 for neuro-developmental disorders (NDD). This decision is based on the conclusions of a criminal expert report within the frame of ongoing criminal proceeding, for which the Chambre de l’Instruction of the Appeal Court of Paris had ordered a counter-expertise (see below). The APESAC, Sanofi and its insurers appealed the Judicial Tribunal of Paris’ ruling related to the class action.
On July 21, 2021, the Judicial Tribunal of Créteil (France) dismissed a claim for damages brought against Sanofi regarding a child born in 1995. The Judicial Tribunal considered that the risk of occurrence of NDD in children born to mother exposed to sodium valproate during pregnancy was not demonstrated by the state of scientific knowledge at the time of her pregnancy. This decision was appealed and the proceeding is now pending before the Appeal Court of Paris, which had ordered a stay in the proceeding until the end of the criminal investigation.
In July 2020 and December 2022, a collective redress against the French affiliate was filed by 64 families which represents 273 claimants including 100 people exposed in utero, seeking indemnification for a prejudice of anxiety. In September 2023, 27 people exposed to sodium valproate in utero and 56 indirect victims requested the withdrawal of their claims. The trial hearing is set for June 6, 2024.
Criminal investigation
A criminal investigation was initiated in May 2015 before the Paris Civil Court. In January 2020, the French affiliate of Sanofi was indicted for aggravated deception and involuntary injuries and in July 2020 for involuntary manslaughter. In July 2020, a judicial supervision of the affiliate was ordered, together with the implementation of financial guarantees. In November 2020, the Health Authority (ANSM) was similarly indicted for involuntary injuries and involuntary manslaughters.
On March 9, 2022, the Chambre de l’Instruction of the Appeal Court of Paris (Cour d’appel) ruled that certain complaints for involuntary manslaughter and several others for aggravated deception and involuntary injuries were time-barred. The Public Prosecutor, as well as the civil parties, have brought the matter before the Chambre Criminelle of the Supreme Court (Cour de cassation). In September 2022, the investigating judges appointed two experts for a counter-expertise following the Chambre de l’Instruction’s ruling handed down end of 2021. Since 2022, several individual medical assessments have been ordered by the investigating judge.
In June 2023, the Chambre Criminelle of the French Supreme Court (Cour de cassation) confirmed the Paris Court of Appeal’s decision (Chambre de l’Instruction) dated March 2022 which had ruled that certain complaints for involuntary manslaughter and several others for aggravated deception and involuntary injuries were time-barred. In August 2023, Sanofi received the counter expertise report and sent its comments in November 2023.
Public compensation scheme
In 2017, the French government set up a public compensation scheme to indemnify patients for damages suffered in connection with the prescription of sodium valproate and its derivatives. The scheme was further amended through the 2020 Finance Law, with notably the introduction of presumptions of default for lack of information of the mother since 1982 for malformations and since 1984 for NDD. The scheme was amended again through the 2021 Finance Law in order to increase the maximum premium applicable in case of refusal to make an offer (or insufficient offer) which would be deemed unjustified by a court ruling.
The committee of the compensation scheme has issued several final opinions holding the French affiliate liable for damages either in full or in part along with the French State, and, in some cases, healthcare practitioners. The French affiliate disagreed with the committee’s conclusions and has accordingly not offered indemnification to the claimants who have received compensation from the ONIAM (Office National d’Indemnisation des Accidents Médicaux). The ONIAM is now seeking reimbursement from Sanofi who has filed legal actions to oppose ONIAM’s payment orders.
SANOFI FORM 20-F 2023 | F-83 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
Administrative Actions
In July 2020, March and June 2021, the Montreuil Administrative Court had held the French State liable in five administrative proceedings initiated by families against the State. In March 2021, the Administrative Court did not retain any lack of information of the mother regarding the risk of neurodevelopmental disorders for births in 1999 and in 2002, based on the state of scientific knowledge at the time. However, regarding the risk of malformations, liabilities were retained against the State, the healthcare professionals and Sanofi, notably for discrepancy between the SmPC (Summary of the Product Characteristics) and the patient leaflet. In other cases involving births in 2005-2008, the liability of the State was retained for both malformations and neurodevelopmental disorders, and partially exonerated, taking into account the roles of healthcare practitioners and Sanofi. Given that the French affiliate was not a party to these administrative proceedings, its arguments (i.e. notably several requests from the French affiliate to the Health Authorities to reinforce warnings to healthcare professionals and patients in relation to DEPAKINE) were not considered. All rulings were appealed by the claimants. Sanofi has filed requests for voluntary intervention in these proceedings to present its arguments before the Administrative Court of Appeal. In one proceeding, the claimants decided to withdraw their claims.
It is not possible, at this stage, to make a reliable assessment of the outcome of these cases.
DEPAKINE Product Litigation in other EU countries and in the UK
In Switzerland, eleven families have filed a civil claim for damages concerning seventeen people exposed in utero. Some of them also involve the claimants’ physicians. In November 2022, one action was declared time-barred by the judge. The claimant appealed this court decision on the merit. The appeal is on-going.
In Spain, there are six ongoing actions relating to thirteen children. In March 2022, in one trial, the Court condemned Sanofi to indemnify four patients. Sanofi appealed this decision. In January 2023, in another trial filed by one patient, the Appeal Court confirmed the first instance's decision and dismissed the claim. The other actions are still at a preliminary stage.
In Belgium, there are two civil proceedings (currently on hold) and a criminal complaint against X and against Sanofi.
In Ireland, there are two cases in Pre-Action stage and two civil claims on-going.
In the United Kingdom, there are three cases in the Pre-Action stage in Great Britain and one civil claim ongoing in Northern Ireland.
It is not possible, at this stage, to assess reliably the outcome of these cases.
DENGVAXIA (Philippines)
Since early 2018 up to present date, several claims were filed in the Philippines by parents of deceased children whose deaths were allegedly due to vaccination with DENGVAXIA. Early March 2019, 2020 and 2022, the Philippine Department of Justice (DOJ) prosecution panel announced it had found probable cause to indict several Sanofi employees/former employees and former Government officials for “reckless imprudence” resulting in homicides. Since then, several criminal actions have been filed in court as a result of this finding and are pending at various stages of the legal procedure. Petitions for Review to the DOJ Secretary have been filed and the said petitions remain pending. Meanwhile, the majority of the respondents have challenged the jurisdiction of the lower court where the first eight cases had been assigned and this issue is now filed with the Supreme Court. There are several claims that have not yet been filed in any court despite resolutions by the DOJ that there is probable cause.
b) Patents
Ramipril Canada Patent Litigation
Sanofi was involved in a number of legal proceedings involving companies which market generic ALTACE (ramipril) in Canada. In 2004, Sanofi unsuccessfully brought Notice of Compliance proceedings (NOC proceedings) at the end of which eight manufacturers obtained marketing authorizations from the Canadian Minister of Health for generic versions of ramipril in Canada. Sanofi filed unsuccessful patent infringement actions against all those companies and ultimately Sanofi was liable for damages under Section 8. Sanofi made payment in complete satisfaction of those awards.
In June 2011, Apotex commenced an action in the Ontario Superior Court of Justice asserting damages under the Ontario Statute of Monopolies, the UK Statute of Monopolies, and the Trade-marks Act (the “Ontario Action”).
At the request of the parties, in June 2021, the Court ordered that the action be stayed in view of the lower court’s decision in March in the Apotex vs. Lilly case. In the Lilly case, the Court dismissed Apotex’s Statute of Monopolies claim by way of summary judgment. In April 2023, the Canadian Supreme Court denied Apotex’s application for leave to appeal in the Lilly case and based on the Supreme Court decision, Apotex’s claim no longer has any basis. Sanofi is seeking the court’s assistance to conclude the case and recover appropriate costs.
PRALUENT (alirocumab)-related Amgen Patent Litigation in the US
In 2014, Amgen filed four separate complaints against Sanofi and Regeneron in the US District Court for the District of Delaware (“District Court”) asserting patent infringement relating to Sanofi and Regeneron’s PRALUENT product. Together these complaints alleged that PRALUENT infringed seven patents for antibodies targeting PCSK9 and sought injunctive relief and unspecified damages.
In February 2021, the Federal Circuit affirmed the District Court’s ruling invalidating the Amgen asserted patent claims. In November 2021, Amgen filed a petition with the US Supreme Court, asking it to overturn the Federal Circuit decision.
On November 4, 2022, the US Supreme Court granted Amgen’s petition for review. In May 2023, the Supreme Court issued a unanimous decision in favor of Sanofi and Regeneron regarding the patent infringement actions filed in 2014 by Amgen relating to Sanofi and Regeneron’s PRALUENT product. Sanofi is in the process of seeking certain legal costs from Amgen.
F-84 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
PRALUENT (alirocumab)-related Amgen Patent Litigation in Europe
In June 2023, Amgen filed an action for infringement of EP 3 666 797 against Sanofi and Regeneron concerning PRALUENT in the Munich Local Division of the Unified Patent Court. Amgen seeks a permanent injunction and unspecified damages and compensation from March 1, 2023. In June 2023, Sanofi filed a revocation action attacking the validity of EP 3 666 797 in the Munich Central Division of the Unified Patent Court. These cases are underway: a hearing in the revocation action is scheduled in June 2024 and a hearing in the infringement action is scheduled for October 2024.
JEVTANA (cabazitaxel)-related patent litigation in the US
JEVTANA is currently covered by four Orange Book listed patents US 7,241,907, US 8,927,592, US 10,583,110 and US 10,716,777. In May to July 2020, Sanofi filed patent infringement suits under Hatch-Waxman against 12 generic filers asserting the ‘110 patent and the ‘777 patent in the US District Court for the District of Delaware, adding the '592 patent after its amended claims issued in August 2021. Sanofi reached settlement agreements with most of the defendants and went to trial against the remaining defendant Sandoz on the ‘777 patent in January 2023. In June 2023, the US District Court for the District of Delaware issued a decision in favor of Sanofi in connection with the JEVTANA patent litigation against Sandoz and on August 2, 2023, Sandoz appealed to the Court of Appeals for the Federal Circuit. On October 5, 2023, Sanofi and Sandoz filed a joint stipulation voluntarily dismissing Sandoz’s Appeal, bringing this matter to final conclusion.
PLAVIX Litigation (Commonwealth) in Australia
In August 2007, GenRX (a subsidiary of Apotex) obtained registration of a generic clopidogrel bisulfate product on the Australian Register of Therapeutic Goods. At the same time, GenRX filed a patent invalidation action with the Federal Court of Australia, seeking revocation of Sanofi’s Australian enantiomer patent claiming clopidogrel salts (a “nullity action”). In September 2007, Sanofi obtained a preliminary injunction from the Federal Court preventing commercial launch of this generic clopidogrel bisulfate product until judgment on the substantive issues of patent validity and infringement.
In August 2008, the Australian Federal Court confirmed that the claim in Sanofi’s Australian enantiomer patent directed to clopidogrel bisulfate (the salt form in PLAVIX) was valid and the patent infringed. On appeal, the Full Federal Court of Australia held in September 2009 that all claims in the patent are invalid. Sanofi’s appeal to the Australia High Court was denied in March 2010. On conclusion of the proceedings in 2010, the Sanofi patent was invalidated.
In April 2013, the Australian Department of Health and Ageing (“Commonwealth”) filed an application before the Federal Court of Australia seeking payment of damages from Sanofi related to the Apotex preliminary injunction.
Sanofi and BMS settled the patent litigation with Apotex in November 2014. In April 2020, the Commonwealth’s claim was dismissed. In May 2020, the Commonwealth filed a Notice of Appeal to the Full Court of the Federal Court. On appeal, the Commonwealth reduced its claim to a range of AUD223.3 million (€137.8 million) to AUD280.2 million (€172.9 million) which, inclusive of interest to December 31, 2023, ranges from AUD360.5 million (€218.0 million) to AUD487.5 million (€294.3 million). In June 2023, the Full Court of the Federal Court of Australia unanimously dismissed the Commonwealth’s appeal following its application seeking payment of damages from Sanofi/BMS related to the preliminary injunction. On July 24, 2023, the Commonwealth filed an application for special leave to appeal to the High Court of Australia, which was granted on December 18, 2023.
c) Other litigation
PLAVIX (clopidogrel) – Attorney General Action in Hawaii
In March 2014, the Hawaii Attorney General (AG) filed a complaint that sets forth allegations related to the sale and marketing of and variability of response to PLAVIX. The Hawaii AG specifically alleged that PLAVIX had a diminished effect in patients of certain genetic backgrounds and that Sanofi and BMS had failed to make an earlier disclosure of this information.
In February 2021, the Court issued its decision, imposing penalties in the total amount of $834,012,000 against both Sanofi and Bristol Myers Squibb (BMS), with $417,006,000 being apportioned to each company. In June 2021, Sanofi and BMS appealed this judgment. The appeal was transferred directly to the Hawaii Supreme Court. In March 2023, the Hawaii Supreme Court vacated the judgment and ordered a new trial. A second trial was concluded in October 2023 and Sanofi is expecting a decision in Q1 2024. To the extent there is a new judgment entered by the court, this would be split evenly with BMS.
PLAVIX (clopidogrel)-related litigation in France
In France, in the claim concerning allegations that Sanofi’s communication and promotional practices inhibited the entry on the market of generics of clopidogrel (the active ingredient of PLAVIX), the French Antitrust Authority issued its decision on May 14, 2013, imposing on Sanofi a fine of €40.6 million. This decision was confirmed by the Supreme Court (Cour de cassation) in 2016. As a consequence of the May 2013 ruling, claims were filed by Sandoz and by Teva in 2014 before the Commercial Court of Paris for compensation of their alleged damages: loss of margin and other ancillary damages. In June and November 2016 respectively, settlement agreements were entered into with Sandoz and Teva. Consequently, they subsequently withdrew their civil claims, jointly and severally. In September 2017, Sanofi and its French affiliate received a summons before the Paris Commercial Court from the French Caisse Nationale d’Assurance Maladie – CNAM (French Social Security) claiming €115.8 million for their alleged damages. On October 1, 2019, the Paris Commercial Court dismissed the CNAM’s action as time barred. On February 9, 2022, the Paris Court of Appeals overturned the Paris Commercial Court's ruling, finding the CNAM’s action as not time-barred and designated an expert to determine the amount of damages. The expert report is expected to be issued in March 2024.
SANOFI FORM 20-F 2023 | F-85 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
340B Drug Pricing Program in the United States
Sanofi is currently involved in several matters relating to the 340B program in the US (a federal program that requires drug manufacturers to supply certain products to certain “covered entities” at reduced prices). In 2021, Sanofi filed a lawsuit against the Department of Health and Human Services (HHS), the Health Resources and Services Administration (HRSA), and certain of their administrators in the US District Court for the District of New Jersey challenging (i) HHS’s December 2020 Advisory Opinion (AO) stating that drug manufacturers are legally obligated to deliver discounts under the 340B program to an unlimited number of contract pharmacies; (ii) HHS’s December 2020 Administrative Dispute Resolution (ADR) Rule; and (iii) HRSA’s May 2021 letter to Sanofi concluding that Sanofi’s 340B integrity initiative (under which Sanofi collects limited, de-identified, claims data on 340B-priced drugs dispensed by contract pharmacies) violates section 340B and that Sanofi has therefore “overcharged” certain covered entities. The court issued its opinion in November 2021, upholding HRSA’s conclusion in the May 2021 letter, but did not impose any fines, penalties or refund obligations against Sanofi for any “overcharges”. The court also rejected Sanofi’s challenge to the ADR Rule and dismissed its challenge to the AO as moot. Sanofi appealed the court’s decision to the Third Circuit Court of Appeals (Third Circuit) and the government filed a cross-appeal.
In January 2023, the Third Circuit held that Sanofi’s restrictions on delivery to contract pharmacies do not violate Section 340B. It also enjoined HHS from enforcing against Sanofi its reading of Section 340B in the AO and the May 2021 violation letter. As to Sanofi’s challenge to the 340B ADR rule, the Third Circuit held in favor of the Department of Health and Human Services (HHS) and HHS is in the process of revising the ADR rule (HRSA has sent the final rule to the Office of Information and Regulatory Affairs for review). The Third Circuit remanded the case back to the US District Court for the District of New Jersey (District Court) and on May 24, 2023, the District Court issued an injunction and declaratory judgment consistent with the Third Circuit’s opinion. This ruling concluded the case as to Sanofi; however similar cases brought by other manufacturers remain pending.
ADR Proceedings
In January 2021, the National Association of Community Health Centers (NACHC) filed an ADR proceeding before HRSA on behalf of a number of covered entities, seeking to require Sanofi and AstraZeneca to supply contract pharmacies with 340B discounts without conditions. On August 10, 2022, the ADR panel granted the motions to dismiss filed both by Sanofi and AstraZeneca, holding that the Delaware district court’s decision granting AstraZeneca’s motion for summary judgment precluded NACHC’s ADR claims against both AstraZeneca and Sanofi.
In September 2023, the University of Washington Medical Center and Harborview Medical Center filed a petition for monetary and equitable relief against Sanofi before the ADR Panel. The petition alleges that Sanofi has violated Section 340B of the Public Health Service Act, 42 U.S.C. § 256b, by imposing data reporting requirements on “Covered Entities” that are authorized under that statute to receive discounts on certain prescription drugs and that in June 2023, Sanofi further restricted access to 340B discounted drugs. The parties are waiting for a panel to be assigned to the case.
In September 2021, HRSA referred Sanofi (as well as other manufacturers) to the HHS Office of the Inspector General (OIG) in accordance with the 340B Program Ceiling Price and Civil Monetary Penalties Final Rule. The Third Circuit’s decision and the District Court’s injunction and declaratory judgment (described above) would preclude action against Sanofi based on the particular program at issue in the Third Circuit case.
In February 2021, the Vermont Attorney General issued a Civil Investigative Subpoena seeking certain information about Sanofi’s participation in the 340B Drug Pricing Program. Sanofi cooperated with this investigation, including producing documents to the Vermont Attorney General’s office.
Mosaic Health
In July 2021, Mosaic Health Inc. and Central Virginia Health Services (covered entities) filed a nationwide antitrust class action complaint against Sanofi and three other manufacturers in the United States District Court for the Western District of New York. Plaintiffs allege that Sanofi and the other defendants conspired to eliminate favorable 340B pricing, particularly with respect to diabetes therapies. On September 2, 2022, the court granted Defendants’ motion to dismiss the complaint. On October 3, 2022, plaintiff filed a motion for leave to file a second amended complaint. On February 1, 2024, the Court denied plaintiffs’ motion for leave to file an amended complaint and dismissed the case. An appeal by plaintiffs is expected.
Adventist Health System/West
In June 2023, Adventist Health System/West sued several drug manufacturing companies, including Sanofi-Aventis US LLC, Sanofi US Services Inc. and Genzyme Corporation, alleging that the companies violated state and federal False Claims Acts through overcharging for 340B Program drugs in violation of federal “penny pricing” policy. The manufacturers jointly moved to dismiss.
Preliminary investigation by the Parquet National Financier (PNF) in France
In November 2023, Sanofi learnt through the press of an ongoing preliminary investigation by the French financial prosecutor (Parquet National Financier – PNF) started in March 2023 relating to allegations regarding Sanofi’s financial communication on the launch of DUPIXENT at the end of 2017. Sanofi considers these allegations as groundless and is cooperating with the PNF to respond to the potential questions relating to the ongoing investigation.
F-86 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
d) Contingencies arising from certain mergers & acquisitions transactions
As a result of divestitures, the Company is subject to a number of ongoing contractual and legal obligations regarding the state of the sold businesses, their assets, and their liabilities, some of which may be subject to dispute.
Aventis CropScience Retained Liabilities
The sale by Aventis Agriculture SA and Hoechst GmbH (both legacy companies of Sanofi) of their aggregate 76 % participation in Aventis CropScience Holding (ACS) to Bayer and Bayer CropScience AG (BCS), the wholly owned subsidiary of Bayer which holds the ACS shares, was effective on June 3, 2002. The Stock Purchase Agreement (SPA) dated October 2, 2001, contained customary representations and warranties with respect to the sold business, as well as a number of indemnifications subject to limitation periods and caps, in particular with respect to environmental liabilities for which some outstanding claims from Bayer remain unresolved.
Infraserv Hoechst Retained Liabilities
By the Asset Contribution Agreement dated December 19/20, 1996, as amended in 1997, Hoechst contributed all lands, buildings, and related assets of the Hoechst site at Frankfurt Hoechst to Infraserv GmbH & Co. Hoechst KG. Infraserv Hoechst undertook to indemnify Hoechst against environmental liabilities at the Hoechst site and with respect to certain landfills. As consideration for the indemnification undertaking, Hoechst transferred to Infraserv Hoechst approximately €57 million to fund reserves. In 1997, Hoechst also agreed it would reimburse current and future Infraserv Hoechst environmental expenses up to €143 million. As a former operator of the land and as a former user of the landfills, Hoechst may ultimately be liable for costs of remedial action in excess of this amount.
Boehringer Ingelheim (BI) Consumer Healthcare Liabilities
Sanofi and Boehringer Ingelheim (BI) have agreed upon respective indemnification obligations for liabilities as part of the swap of Sanofi’s Animal Health (AH) business for BI’s Consumer Health Care (CHC) business in January 2017 and under a Global Settlement Agreement concluded in September 2019 regarding notably the offset of respective AH and CHC claims notified under the SPAs.
In February 2020, BI initiated an arbitration against Sanofi seeking indemnification for losses it could incur as a result of the ZANTAC litigation in the US (see above). In an award rendered on June 19, 2023, the arbitral tribunal irrevocably dismissed BI’s indemnification claim against Sanofi and confirmed that Sanofi shall not be liable to indemnify BI for any potential losses in relation to the ongoing ZANTAC litigation in the U.S. The case is closed.
SANOFI FORM 20-F 2023 | F-87 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.23. Provisions for discounts, rebates and sales returns
Adjustments between gross sales and net sales, as described in Note B.13., are recognized either as provisions or as reductions in accounts receivable, depending on their nature.
The table below shows movements in these items:
| (€ million) | Government and State programs(a) | Managed care and GPO programs(b) | Chargeback incentives | Rebates and discounts | Sales returns | Other deductions | Total | |||||||||||||||||||
| Balance at January 1, 2021 | ||||||||||||||||||||||||||
| Changes in scope of consolidation | ( | |||||||||||||||||||||||||
| Provision related to current period sales | ||||||||||||||||||||||||||
| Net change in provision related to prior period sales | ( | ( | ( | ( | ( | ( | ||||||||||||||||||||
| Payments made | ( | ( | ( | ( | ( | ( | ( | |||||||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ( | ( | ( | |||||||||||||||||||
| Balance at December 31, 2021 | (c) | |||||||||||||||||||||||||
| Provision related to current period sales | ||||||||||||||||||||||||||
| Net change in provision related to prior period sales | ( | ( | ( | ( | ( | ( | ||||||||||||||||||||
| Payments made | ( | ( | ( | ( | ( | ( | ( | |||||||||||||||||||
| Currency translation differences | ||||||||||||||||||||||||||
| Balance at December 31, 2022 | (c) | |||||||||||||||||||||||||
| Changes in scope of consolidation | ( | ( | ( | ( | ||||||||||||||||||||||
| Provision related to current period sales | ||||||||||||||||||||||||||
| Net change in provision related to prior period sales | ( | ( | ( | ( | ( | ( | ||||||||||||||||||||
| Payments made | ( | ( | ( | ( | ( | ( | ( | |||||||||||||||||||
| Currency translation differences | ( | ( | ( | ( | ( | ( | ( | |||||||||||||||||||
| Balance at December 31, 2023 | (c) | |||||||||||||||||||||||||
(a) Primarily US government programs: Medicaid (€1,421 million in 2023, €1,307 million in 2022, €1,244 million in 2021) and Medicare (€1,099 million in 2023, €775 million in 2022 and €941 million in 2021).
(b) Mainly rebates and other price reductions granted to healthcare authorities in the United States (including Managed Care: €1,028 million in 2023, €934 million in 2022 and €896 million in 2021).
(c) Provisions related to US net sales amounted to €5,124 million as of December 31, 2023, €4,270 million as of December 31, 2022 and €4,057 million as of December 31, 2021.
D.24. Personnel costs
Total personnel costs (other than termination benefits, presented in Note D.27.) include the following items:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Salaries | |||||||||||
| Social security charges (including defined-contribution pension plans) | |||||||||||
Other employee benefits(a) | |||||||||||
| Total | |||||||||||
The total number of registered employees was 87,994 as of December 31, 2023, compared with 91,573 as of December 31, 2022 and 95,442 as of December 31, 2021.
D.25. Other operating income
Other operating income totaled €1,292 million in 2023, versus €1,969 million in 2022 and €859 million in 2021.
F-88 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.26. Other operating expenses
Other operating expenses totaled €3,516 million in 2023, compared with €2,531 million in 2022 and €1,805 million in 2021.
For 2023, this line item includes €3,206 million of expenses related to Regeneron (see Note C.1.), compared with €2,378 million for 2022 and €1,568 million for 2021 (as shown in the table below):
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Income & expense related to sharing of (profits)/losses under the Monoclonal Antibody Alliance | ( | ( | ( | ||||||||
Additional share of profit paid by Regeneron towards development costs(b) | |||||||||||
Reimbursement to Regeneron of selling expenses incurred | ( | ( | ( | ||||||||
| Total - Monoclonal Antibody Alliance | ( | ( | ( | ||||||||
| Immuno-Oncology Alliance | |||||||||||
Other (mainly ZALTRAP and LIBTAYO)(a) | ( | ||||||||||
Other operating income/(expenses), net related to Regeneron | ( | ( | ( | ||||||||
of which amount presented in Other operating income (Note D.25.) | |||||||||||
(a) Following the restructuring of the Immuno-Oncology agreement between Sanofi and Regeneron, applicable from July 1, 2022 (see Note C.1.).
Charges to provisions for litigation and environmental risks are also recorded within this line item.
D.27. Restructuring costs and similar items
Restructuring costs and similar items amounted to €1,490 million in 2023, €1,336 million in 2022 and €820 million in 2021, and were comprised of the following items:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Employee-related expenses | |||||||||||
Charges, gains or losses on assets(a) | |||||||||||
Costs related to transformation programs | |||||||||||
Other | |||||||||||
| Total | |||||||||||
(a) This line consists of impairment losses and accelerated depreciation charges related to site closures (including leased sites), and gains or losses on divestments of assets arising from reorganization decisions made by Sanofi.
Restructuring costs and similar items were €154 million higher in 2023 than 2022. For 2023 they include the impact of French pension reform on future annuities under the rules of each severance plan, while for 2022 they mainly comprised severance costs recognized further to the announcements made during that period. Also included in Restructuring costs and similar items are the impacts of ongoing transformational projects, primarily those associated with the creation of the standalone Consumer Healthcare entity and the implementation of Sanofi’s new digital strategy.
D.28. Other gains and losses, and litigation
Other gains and losses, and litigation for 2023 represent a charge of €38 million, including costs relating to the settlement of litigation with Bioverativ former shareholders.
For 2022, this line item represented a charge of €370 million, comprising the pre-tax loss arising on the deconsolidation of EUROAPI (see Note D.2.) and costs related to major litigation.
SANOFI FORM 20-F 2023 | F-89 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.29. Financial expenses and income
An analysis of Financial expenses and Financial income is set forth below:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
Cost of debt(a) | ( | ( | ( | ||||||||
Interest income(b) | |||||||||||
| Cost of net debt | ( | ( | ( | ||||||||
| Non-operating foreign exchange gains/(losses) | ( | ( | |||||||||
Unwinding of discounting of provisions(c) | ( | ( | ( | ||||||||
| Net interest cost related to employee benefits | ( | ( | ( | ||||||||
| Gains/(losses) on disposals of financial assets | ( | ||||||||||
| Net interest expense on lease liabilities | ( | ( | ( | ||||||||
Other(d) | ( | ||||||||||
| Net financial income/(expenses) | ( | ( | ( | ||||||||
| comprising: Financial expenses | ( | ( | ( | ||||||||
| Financial income | |||||||||||
(a) Includes net gains/(losses) on interest rate and currency derivatives used to manage debt: €(67 ) million in 2023, €(11 ) million in 2022, €14 million in 2021.
(b) Includes net gains on interest rate and currency derivatives used to manage cash and cash equivalents: €(13 ) million in 2023, €68 million in 2022, €51 million in 2021.
(c) Primarily on provisions for environmental risks, restructuring provisions, and provisions for product-related risks (see Note D.19.).
(d) Includes a financial expense of €541 million (zero
In 2023, 2022 and 2021, the impact of the ineffective portion of hedging relationships was not material.
D.30. Income tax expense
Sanofi has elected for tax consolidations in a number of countries, principally France, Germany, the United Kingdom and the United States.
The table below shows the allocation of income tax expense between current and deferred taxes:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Current taxes | ( | ( | ( | ||||||||
| Deferred taxes | |||||||||||
| Total | ( | ( | ( | ||||||||
| Income before tax and investments accounted for using the equity method | |||||||||||
The difference between the effective tax rate and the standard corporate income tax rate applicable in France is explained as follows:
(%) | 2023 | 2022 | 2021 | ||||||||
| Standard tax rate applicable in France | |||||||||||
Difference between the standard French tax rate and the rates applicable to Sanofi(a) | ( | ( | ( | ||||||||
Revisions to tax exposures and settlements of tax disputes | ( | ||||||||||
Probable reversal of temporary differences on investments in Consumer Healthcare subsidiaries(b) | |||||||||||
| Fair value remeasurement of contingent consideration | ( | ||||||||||
Other items(c) | |||||||||||
| Effective tax rate | |||||||||||
(a) The difference between the French tax rate and tax rates applicable to foreign subsidiaries reflects the fact that Sanofi has operations in many countries, most of which have lower tax rates than France.
(b) In accordance with IAS 12, a deferred tax liability was recognized in 2023 on the temporary differences arising on investments in subsidiaries which Sanofi expects will reverse in connection with the proposed separation of the Consumer Healthcare business, as announced in October 2023.
(c) In determining the amount of the deferred tax liability for 2023, 2022 and 2021, Sanofi took into account changes in the ownership structure of certain subsidiaries.
For the periods presented, the amount of deferred tax assets recognized in profit or loss that were initially subject to impairment losses at the time of a business combination is immaterial.
F-90 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.31. Share of profit/loss from investments accounted for using the equity method
The line item Share of profit/(loss) from investments accounted for using the equity method was a net loss of €115 million in 2023 (including an impairment loss of €231 million on the equity-accounted investment in EUROAPI – see Note D.6.), compared with net income of €68 million for 2022 and €39 million for 2021.
D.32. Net income attributable to non-controlling interests
The table below shows Net income attributable to non-controlling interests for the reporting periods presented:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
Share of net income attributable to non-controlling interests | |||||||||||
| Total | |||||||||||
D.33. Related party transactions
The principal related parties are companies over which Sanofi has control or significant influence, joint ventures, key management personnel, and principal shareholders.
Sanofi has not entered into any material transactions with any key management personnel. Financial relations with Sanofi’s principal shareholders fall within the ordinary course of business and were immaterial in the years ended December 31, 2023, 2022 and 2021.
Note F.1. lists the principal companies controlled by Sanofi; those companies are fully consolidated, as described in Note B.1. Transactions between those companies, and between the parent company and its subsidiaries, are eliminated when preparing the consolidated financial statements.
Transactions with companies over which Sanofi has significant influence, and with joint ventures, are presented in Note D.6.
Key management personnel include corporate officers and the members of the Executive Committee (an average of 10 members in 2023, and 11
The table below shows, by type, the compensation paid to key management personnel:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
Short-term benefits(a) | |||||||||||
| Post-employment benefits | |||||||||||
| Share-based payment | |||||||||||
| Total recognized in profit or loss | |||||||||||
(a) Compensation, employer’s social security contributions, directors’ compensation, and any termination benefits (net of reversals of termination benefit obligations).
The table below shows the aggregate obligation as of December 31 for each period presented for individuals who hold or have held executive positions within Sanofi during that period.
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Aggregate top-up pension obligation in favor of certain corporate officers and of Executive Committee members | |||||||||||
| Aggregate termination benefits and lump-sum retirement benefits in favor of key management personnel | |||||||||||
D.34. Disclosures about major customers and credit risk
Credit risk is the risk that customers (wholesalers, distributors, pharmacies, hospitals, clinics or government agencies) may fail to pay their debts; for Sanofi, that risk is mainly concentrated on amounts receivable from wholesalers in the United States. Sanofi manages credit risk by vetting customers in order to set credit limits and risk levels, and asking for guarantees or insurance where necessary; performing controls; and monitoring qualitative and quantitative indicators of accounts receivable balances, such as the period of credit taken and overdue payments.
Sales generated by Sanofi with its biggest customers, in particular certain wholesalers in the United States, represented 27 % of net sales in 2023. The three largest customers respectively accounted for approximately 11 %, 9 % and 7 % of Sanofi's net sales in 2023 (12 %, 8 % and 7 % in 2022; 10 %, 7 % and 6 % in 2021).
SANOFI FORM 20-F 2023 | F-91 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.35. Segment information
As indicated in Note B.26., Sanofi has two operating segments with effect from January 1, 2023: Biopharma and Consumer Healthcare.
In 2022, Sanofi reported three operating segments (Pharmaceuticals, Vaccines and Consumer Healthcare). The costs of the global support functions (Corporate Affairs, Finance, People & Culture, Legal, Ethics, Business Integrity & Global Security, Information Solutions & Technology, Sanofi Business Services, etc.), which are mainly managed centrally at group-wide level, were presented within the “Other” category.
In 2023, Sanofi reviewed the presentation of its segment information following adjustments to its internal reporting systems in order to reflect (i) progress on the “Play to Win” strategy leading to the creation of the standalone Consumer Healthcare Global Business Unit (GBU) which, in addition to integrated research, development and production functions now also has its own dedicated global support functions (including Finance, People & Culture, Legal, Ethics, Business Integrity & Global Security, Information Solutions & Technology, Global Business Services, etc.); and (ii) organizational changes to Sanofi’s Manufacturing & Supply global function (previously known as Industrial Affairs).
Consequently, with effect from January 1, 2023, Sanofi reports two operating segments: Biopharma and Consumer Healthcare.
The Biopharma operating segment comprises commercial operations and research, development and production activities relating to the Specialty Care, General Medicines and Vaccines franchises, for all geographical territories. The segment’s results include the costs of global support functions that are not within the managerial responsibility of the Consumer Healthcare GBU.
The Consumer Healthcare operating segment comprises commercial operations relating to Consumer Healthcare products, and research, development and production activities and global support functions (as listed above) dedicated to the segment, for all geographical territories. The Consumer Healthcare GBU segment’s results reflect all incurred costs of global support functions attributable to its business.
The “Other” category comprises reconciling items, primarily but not limited to (i) gains and losses on centralized foreign exchange risk hedging transactions that cannot be allocated to the operating segments and (ii) gains and losses on retained commitments in respect of previously divested operations.
F-92 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.35.1. Segment results
D.35.1.1. Analysis of net sales
The table below sets forth Sanofi’s net sales for the years ended December 31, 2023, 2022 and 2021:
| (€ million) | Europe | United States | Other countries | 2023 | Europe | United States | Other countries | 2022(a) | Europe | United States | Other countries | 2021(b) | |||||||||||||||||||||||||||||
Biopharma | |||||||||||||||||||||||||||||||||||||||||
| Specialty Care | |||||||||||||||||||||||||||||||||||||||||
of which | DUPIXENT | ||||||||||||||||||||||||||||||||||||||||
| AUBAGIO | |||||||||||||||||||||||||||||||||||||||||
| CEREZYME | |||||||||||||||||||||||||||||||||||||||||
| FABRAZYME | |||||||||||||||||||||||||||||||||||||||||
| MYOZYME/ LUMIZYME | |||||||||||||||||||||||||||||||||||||||||
| JEVTANA | |||||||||||||||||||||||||||||||||||||||||
| ALPROLIX | |||||||||||||||||||||||||||||||||||||||||
| ELOCTATE | |||||||||||||||||||||||||||||||||||||||||
General Medicines | |||||||||||||||||||||||||||||||||||||||||
| Core Assets | |||||||||||||||||||||||||||||||||||||||||
| of which | LOVENOX | ||||||||||||||||||||||||||||||||||||||||
TOUJEO | |||||||||||||||||||||||||||||||||||||||||
| PLAVIX | |||||||||||||||||||||||||||||||||||||||||
| Non-Core Assets | |||||||||||||||||||||||||||||||||||||||||
| of which | LANTUS | ||||||||||||||||||||||||||||||||||||||||
| Other non-core assets | |||||||||||||||||||||||||||||||||||||||||
| Industrial sales | |||||||||||||||||||||||||||||||||||||||||
| Vaccines | |||||||||||||||||||||||||||||||||||||||||
| of which | Polio/Pertussis/ Hib Vaccines | ||||||||||||||||||||||||||||||||||||||||
| Influenza Vaccines | |||||||||||||||||||||||||||||||||||||||||
Consumer Healthcare | |||||||||||||||||||||||||||||||||||||||||
| of which | Allergy | ||||||||||||||||||||||||||||||||||||||||
| Pain Care | |||||||||||||||||||||||||||||||||||||||||
| Digestive Wellness | |||||||||||||||||||||||||||||||||||||||||
| Total net sales | |||||||||||||||||||||||||||||||||||||||||
(a) 2022 figures have been adjusted to take account of the two new operating segments, Biopharma and Consumer Healthcare, effective January 1, 2023.
(b) Due to a lack of available data and the complex adjustments that would be required (particularly for our reporting tools), the 2021 figures have not been restated to reflect changes arising from our new organizational structure.
D.35.1.2. Business operating income
Sanofi reports segment results on the basis of “Business operating income”. This indicator is used internally by Sanofi’s chief operating decision maker to measure the performance of each operating segment and to allocate resources.
“Business operating income” is derived from Operating income, adjusted as follows:
•amortization and impairment losses charged against intangible assets (other than software and other rights of an industrial or operational nature), are eliminated;
•fair value remeasurements of contingent consideration relating to business combinations (IFRS 3) or business divestments, and presented within the line item Fair value remeasurement of contingent consideration, are eliminated;
•expenses arising from the remeasurement of inventories following business combinations (IFRS 3) or acquisitions of groups of assets that do not constitute a business within the meaning of paragraph 2b of IFRS 3, are eliminated;
•amounts reported within the line items Restructuring costs and similar items are eliminated;
•other gains and losses including gains and losses on major divestments, presented within the line item Other gains and losses, and litigation, are eliminated;
•other costs and provisions related to litigation, presented within the line item Other gains and losses, and litigation, are eliminated;
•upfront payments and regulatory milestone payments recognized within the line item Other operating income and related to transactions outside the ordinary activities of Sanofi are eliminated;
SANOFI FORM 20-F 2023 | F-93 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
•the share of profits/losses from investments accounted for using the equity method is added, to the extent that this relates to joint ventures and associates with which Sanofi has a strategic alliance; and
•net income attributable to non-controlling interests is deducted.
The table below shows Sanofi’s segment results for the years ended December 31, 2023, December 31, 2022 and December 31, 2021:
2023 | ||||||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Other(a) | Total Sanofi | ||||||||||
| Net sales | ||||||||||||||
| Other revenues | ||||||||||||||
| Cost of sales | ( | ( | ( | ( | ||||||||||
| Research and development expenses | ( | ( | ( | |||||||||||
| Selling and general expenses | ( | ( | ( | |||||||||||
| Other operating income and expenses | ( | ( | ( | |||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | ||||||||||||||
| Net income attributable to non-controlling interests | ( | ( | ( | |||||||||||
| Business operating income | ( | |||||||||||||
(a) This caption reconciles segment financial information to total consolidated financial information.
2022(a) | ||||||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Other(b) | Total Sanofi | ||||||||||
| Net sales | ||||||||||||||
| Other revenues | ||||||||||||||
| Cost of sales | ( | ( | ( | |||||||||||
| Research and development expenses | ( | ( | ( | |||||||||||
| Selling and general expenses | ( | ( | ( | |||||||||||
| Other operating income and expenses | ( | ( | ||||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | ||||||||||||||
| Net income attributable to non-controlling interests | ( | ( | ( | |||||||||||
| Business operating income | ||||||||||||||
(a) 2022 figures have been adjusted to take account of the two new operating segments, Biopharma and Consumer Healthcare, effective from January 1, 2023.
(b) This caption reconciles segment financial information to total consolidated financial information.
2021(a) | |||||||||||||||||
| (€ million) | Pharmaceuticals | Vaccines | Consumer Healthcare | Other(b) | Total Sanofi | ||||||||||||
| Net sales | |||||||||||||||||
| Other revenues | |||||||||||||||||
| Cost of sales | ( | ( | ( | ( | ( | ||||||||||||
| Research and development expenses | ( | ( | ( | ( | ( | ||||||||||||
| Selling and general expenses | ( | ( | ( | ( | ( | ||||||||||||
| Other operating income and expenses | ( | ( | ( | ||||||||||||||
| Share of profit/(loss) from investments accounted for using the equity method | |||||||||||||||||
| Net income attributable to non-controlling interests | ( | ( | ( | ( | ( | ||||||||||||
| Business operating income | ( | ||||||||||||||||
(a) Due to a lack of available data and the complex adjustments that would be required (particularly for our reporting tools), the 2021 figures have not been restated to reflect changes arising from our new organizational structure.
(b) This caption reconciles segment financial information to total consolidated financial information.
F-94 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
The table below, presented in compliance with IFRS 8, shows a reconciliation between aggregated “Business operating income” for the segments and Income before tax and investments accounted for using the equity method:
| (€ million) | 2023 | 2022 | 2021 | ||||||||
| Business operating income | |||||||||||
Share of profit/(loss) from investments accounted for using the equity method(a) | ( | ( | ( | ||||||||
Net income attributable to non-controlling interests(b) | |||||||||||
Amortization and impairment of intangible assets(c) | ( | ( | ( | ||||||||
| Fair value remeasurement of contingent consideration | ( | ( | |||||||||
Expenses arising from the impact of acquisitions on inventories(d) | ( | ( | ( | ||||||||
| Restructuring costs and similar items | ( | ( | ( | ||||||||
Other gains and losses, and litigation | ( | ( | ( | ||||||||
Income from out-licensing(e) | |||||||||||
| Operating income | |||||||||||
| Financial expenses | ( | ( | ( | ||||||||
| Financial income | |||||||||||
| Income before tax and investments accounted for using the equity method | |||||||||||
(a) Joint ventures and associates with which Sanofi has entered into a strategic alliance.
(b) Excludes (i) restructuring costs and (ii) other adjustments attributable to non-controlling interests.
(c) For 2023, this amount mainly comprises an impairment loss of €833 million, reflecting the impact of the strategic decision to de-prioritize certain R&D programs, in particular those related to the NK Cell and PRO-XTEN technology platforms. For 2022, this line includes a reversal of €2,154 million on ELOCTATE franchise products following FDA approval of ALTUVIIIO dated February 22, 2023, partially offset by an impairment loss of €1,586 million on intangible assets relating to SAR444245 (non-alpha interleukin-2).
(d) This line records the impact of the workdown of acquired inventories remeasured at fair value at the acquisition date.
(e) For 2022, this line includes an upfront payment of $900 million and a regulatory milestone payment of $100 million related to the out-licensing of LIBTAYO following the restructuring of the Immuno-Oncology Collaboration and License Agreement with Regeneron (see Note C.1.).
D.35.2. Other segment information
The tables below show the split by operating segment of (i) the carrying amount of investments accounted for using the equity method with which Sanofi has entered into a strategic alliance, (ii) acquisitions of property, plant and equipment, and (iii) acquisitions of intangible assets.
The principal investments accounted for using the equity method in the Biopharma segment are the interests in MSP Vaccine Company, and Infraserv GmbH & Co. Höchst KG (see Note D.6.).
Acquisitions of intangible assets and property, plant and equipment correspond to acquisitions paid for during the period.
| 2023 | |||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Total | ||||||||
| Investments accounted for using the equity method | |||||||||||
| Acquisitions of property, plant and equipment | |||||||||||
| Acquisitions of other intangible assets | |||||||||||
2022(a) | |||||||||||
| (€ million) | Biopharma | Consumer Healthcare | Total | ||||||||
| Investments accounted for using the equity method | |||||||||||
| Acquisitions of property, plant and equipment | |||||||||||
Acquisitions of other intangible assets | |||||||||||
(a) 2022 figures have been adjusted to take account of the two new operating segments, Biopharma and Consumer Healthcare, effective from January 1, 2023.
| (€ million) | 2021(a) | |||||||||||||
| Pharmaceuticals | Vaccines | Consumer Healthcare | Total | |||||||||||
Investments accounted for using the equity method | ||||||||||||||
| Acquisitions of property, plant and equipment | ||||||||||||||
Acquisitions of other intangible assets | ||||||||||||||
(a) Due to a lack of available data and the complex adjustments that would be required (particularly for our reporting tools), the 2021 figures have not been restated to reflect changes arising from our new organizational structure.
SANOFI FORM 20-F 2023 | F-95 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
D.35.3. Information by geographical region
The geographical information on net sales provided below is based on the geographical location of the customer. In accordance with IFRS 8, the non-current assets reported below exclude right-of-use assets relating to leases as determined under IFRS 16, investments accounted for using the equity method, other non-current assets, non-current income tax assets, and deferred tax assets.
| 2023 | ||||||||||||||||||||
| (€ million) | Total | Europe | of which France | North America | of which United States | Other countries | ||||||||||||||
| Net sales | ||||||||||||||||||||
| Non-current assets: | ||||||||||||||||||||
•property, plant and equipment owned | ||||||||||||||||||||
•goodwill | ||||||||||||||||||||
▪other intangible assets | ||||||||||||||||||||
2022 | ||||||||||||||||||||
| Total | Europe | of which France | North America | of which United States | Other countries | |||||||||||||||
| Net sales | ||||||||||||||||||||
| Non-current assets: | ||||||||||||||||||||
•property, plant and equipment owned | ||||||||||||||||||||
•goodwill | ||||||||||||||||||||
•other intangible assets | ||||||||||||||||||||
| (€ million) | 2021 | |||||||||||||||||||
| Total | Europe | of which France | North America | of which United States | Other countries | |||||||||||||||
Net sales | ||||||||||||||||||||
| Non-current assets: | ||||||||||||||||||||
•property, plant and equipment owned | ||||||||||||||||||||
•goodwill | ||||||||||||||||||||
•other intangible assets | ||||||||||||||||||||
As stated in Note D.5., goodwill is not allocated by geographical region.
F-96 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
E/ Principal accountants’ fees and services
PricewaterhouseCoopers Audit and Ernst & Young et Autres served as independent auditors of Sanofi for the year ended December 31, 2023 and for all other reporting periods presented. The table below shows fees charged by those firms and member firms of their networks to Sanofi and consolidated subsidiaries in the years ended December 31, 2023 and 2022.
| Ernst & Young | PricewaterhouseCoopers | |||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||
| (€ million) | Amount | % | Amount | % | Amount | % | Amount | % | ||||||||||||||||||
Audit: Statutory audit of separate and consolidated financial statements(a) | % | % | % | % | ||||||||||||||||||||||
Services other than statutory audit(b) | % | % | % | % | ||||||||||||||||||||||
Audit-related services(c)(d) | ||||||||||||||||||||||||||
| Tax | ||||||||||||||||||||||||||
| Other | ||||||||||||||||||||||||||
| Total | % | % | % | % | ||||||||||||||||||||||
(a) Includes services provided by the independent auditors of the parent company and French subsidiaries: Ernst & Young €7.9 million in 2023, €7.3 million in 2022; PricewaterhouseCoopers Audit €8.3 million in 2023, €7.7 million in 2022.
(b) Services other than statutory audit provided by Ernst & Young during 2023 comprised:
- contractual audits, including on the combined financial statements of the Consumer Healthcare business;
- additional procedures to enable reports previously signed by the firm to be incorporated by reference;
- assurance engagements, agreed-upon procedures, tax compliance work and technical consultancy; and
- issuance of the Independent Third Party’s report on the consolidated statement of extra-financial performance.
Services other than statutory audit provided by PricewaterhouseCoopers during 2023 comprised:
- contractual audits, including on the combined financial statements of the Consumer Healthcare business;
- additional procedures to enable reports previously signed by the firm to be incorporated by reference; and
- assurance engagements, agreed-upon procedures, tax compliance work and technical consultancy.
(c) Includes services provided by the independent auditors of the parent company and French subsidiaries: Ernst & Young: €5.2 million in 2023, €1.4 million in 2022; PricewaterhouseCoopers Audit €5.3 million in 2023, €0.3 million in 2022.
(d) Includes €0.5 million for services that can only be provided by the statutory auditors, such as comfort letters, attestation services required by regulation (which qualify as audit fees under SEC rules).
SANOFI FORM 20-F 2023 | F-97 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
F/ List of principal companies included in the scope of consolidation during 2023
F.1. Principal fully consolidated companies
The table below shows Sanofi’s principal subsidiaries and their country of incorporation:
| Europe | Financial interest (%) as of December 31, 2023 | |||||||
| Hoechst GmbH | Germany | |||||||
| Sanofi-Aventis Deutschland GmbH | Germany | |||||||
| A. Nattermann & Cie. GmbH | Germany | |||||||
| Sanofi-Aventis GmbH | Austria | |||||||
| Sanofi Belgium | Belgium | |||||||
Ablynx NV | Belgium | |||||||
Genzyme Flanders BV | Belgium | |||||||
| Sanofi A/S | Denmark | |||||||
Sanofi-Aventis SA | Spain | |||||||
Opella Healthcare Spain, SL | Spain | |||||||
| Sanofi Oy | Finland | |||||||
| Sanofi | France | |||||||
| Sanofi-Aventis France | France | |||||||
| Sanofi Winthrop Industrie | France | |||||||
| Sanofi-Aventis Recherche & Développement | France | |||||||
| Sanofi-Aventis Groupe | France | |||||||
| Sanofi Chimie | France | |||||||
| Sanofi-Aventis Participations | France | |||||||
| Sanofi Pasteur | France | |||||||
| Aventis Pharma S.A. | France | |||||||
| Sanofi Biotechnology | France | |||||||
| Sanofi Mature IP | France | |||||||
| Sanofi Pasteur NVL | France | |||||||
| Sanofi Pasteur Europe | France | |||||||
| SECIPE SAS | France | |||||||
Sanofi Pasteur Merieux SAS | France | |||||||
| Opella Healthcare International SAS | France | |||||||
| Opella Healthcare France SAS | France | |||||||
| Opella Healthcare Group SAS | France | |||||||
Genzyme Polyclonals SAS | France | |||||||
| Sanofi-Aventis A.E.B.E. | Greece | |||||||
| Sanofi-Aventis Private Co, Ltd | Hungary | |||||||
| Chinoin Private Co. Ltd | Hungary | |||||||
| Opella Healthcare Hungary Commercial K.F.T | Hungary | |||||||
Opella Healthcare Hungary K.F.T | Hungary | |||||||
| Carraig Insurance DAC | Ireland | |||||||
| Genzyme Ireland Limited | Ireland | |||||||
| Sanofi-Aventis Holdings (Ireland) Ltd | Ireland | |||||||
Sanofi SRL | Italy | |||||||
Opella Healthcare Italy SRL | Italy | |||||||
| Genzyme Global Sarl | Luxembourg | |||||||
| Genzyme Luxembourg Sarl | Luxembourg | |||||||
| Sanofi-Aventis Norge AS | Norway | |||||||
Sanofi BV | Netherlands | |||||||
Sanofi Foreign Participations BV | Netherlands | |||||||
| Sanofi-Aventis Sp. z.o.o. | Poland | |||||||
Opella Healthcare Poland sp.z.o.o. | Poland | |||||||
| Sanofi Pasteur Sp. z.o.o. | Poland | |||||||
| Sanofi Produtos Farmaceuticos Lda | Portugal | |||||||
| Sanofi-Aventis, s.r.o. | Czech Republic | |||||||
| Opella Healthcare Czech s.r.o | Czech Republic | |||||||
F-98 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
| Europe | Financial interest (%) as of December 31, 2023 | |||||||
| Sanofi Romania SRL | Romania | |||||||
Opella Healthcare Romania SRL | Romania | |||||||
| Sanofi-Aventis UK Holdings Limited | United Kingdom | |||||||
| Aventis Pharma Limited | United Kingdom | |||||||
| Sanofi-Synthelabo UK Ltd | United Kingdom | |||||||
| Aventis Pharma Holdings Ltd | United Kingdom | |||||||
| Opella Healthcare UK Limited | United Kingdom | |||||||
| AO Sanofi Russia | Russia | |||||||
| Opella Healthcare LLC | Russia | |||||||
| Sanofi AB | Sweden | |||||||
| Sanofi-Aventis (Suisse) SA | Switzerland | |||||||
| Genzyme Global Sarl Baar Intellectual Property Branch | Switzerland | |||||||
Sanofi Ilac Sanayi ve Ticaret AS | Turkey | |||||||
Sanofi Pasteur Asi Ticaret AS | Turkey | |||||||
Opella Healthcare Tüketici Sağlığı Anonim Şirketi | Turkey | |||||||
| Sanofi Saglik Urunleri Limited Sirketi | Turkey | |||||||
| United States | Financial interest (%) as of December 31, 2023 | |||||||
| Genzyme Therapeutic Products Limited Partnership | United States | |||||||
| Aventis Inc. | United States | |||||||
| Sanofi US Services Inc. | United States | |||||||
| Sanofi-Aventis U.S. LLC | United States | |||||||
| Chattem, Inc. | United States | |||||||
| Aventisub LLC | United States | |||||||
| Genzyme Corporation | United States | |||||||
| Sanofi Pasteur Inc. | United States | |||||||
| VaxServe, Inc. | United States | |||||||
| Bioverativ Inc. | United States | |||||||
| Bioverativ U.S. LLC | United States | |||||||
| Bioverativ Therapeutics Inc. | United States | |||||||
| Principia Biopharma Inc. | United States | |||||||
| Sanofi Research Invest LLC | United States | |||||||
| Sanofi Bioverativ Holdings LLC | United States | |||||||
| RPR US Ltd. | United States | |||||||
| Kadmon Corporation, LLC | United States | |||||||
| Amunix | United States | |||||||
| Synthorx, Inc | United States | |||||||
| Provention Bio | United States | |||||||
QRIB Intermediate Holding | United States | |||||||
QRI | United States | |||||||
TargeGen Inc. | United States | |||||||
Chattem (GB) Holding | United States | |||||||
Translate Bio, Inc. | United States | |||||||
SANOFI FORM 20-F 2023 | F-99 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
| Other Countries | Financial interest (%) as of December 31, 2023 | |||||||
| Sanofi-Aventis South Africa (Pty) Ltd | South Africa | |||||||
| Sanofi-Aventis Algérie | Algeria | |||||||
| Sanofi Arabia Trading Company Limited | Saudi Arabia | |||||||
Sanofi-Aventis Argentina SA | Argentina | |||||||
| Sanofi-Aventis Healthcare Pty Ltd | Australia | |||||||
| Sanofi-Aventis Australia Pty Ltd | Australia | |||||||
| Sanofi Medley Farmaceutica Ltda | Brazil | |||||||
Opella Healthcare Brazil Ltda. | Brazil | |||||||
| Sanofi-Aventis Canada Inc. | Canada | |||||||
| Sanofi Pasteur Limited | Canada | |||||||
| Merieux Canada Holdings ULC (Canada) | Canada | |||||||
| Sanofi (Hangzhou) Pharmaceuticals Co., Ltd | China | |||||||
| Sanofi (China) Investment Co., Ltd | China | |||||||
| Sanofi (Beijing) Pharmaceuticals Co.Ltd | China | |||||||
| Sanofi Pasteur Biologies Co., Ltd | China | |||||||
| Shenzhen Sanofi pasteur Biological Products Co, Ltd | China | |||||||
| Shanghai Rongheng Pharmaceutical Co, Ltd | China | |||||||
Sanofi-Aventis de Colombia SA | Colombia | |||||||
| Sanofi-Aventis Korea Co. Ltd | South Korea | |||||||
| Sanofi Pasteur Ltd | South Korea | |||||||
| Opella healthcare Korea Inc. | South Korea | |||||||
| Sanofi-Aventis Gulf FZE | United Arab Emirates | |||||||
| Sanofi Egypt | Egypt | |||||||
| Sanofi Hong-Kong Limited | Hong Kong | |||||||
| Sanofi India Limited | India | |||||||
| Sanofi Healthcare India Private Limited | India | |||||||
| Sanofi-Aventis Israël Ltd | Israel | |||||||
| Sanofi K.K. | Japan | |||||||
| SSP Co.,Ltd | Japan | |||||||
| Sanofi-Aventis (Malaysia) SDN. BHD. | Malaysia | |||||||
| Sanofi-Aventis Maroc | Morocco | |||||||
Sanofi-Aventis de Mexico SA de CV | Mexico | |||||||
Sanofi Pasteur SA de CV | Mexico | |||||||
Azteca Vacunas, SA de CV | Mexico | |||||||
Sanofi-Aventis de Panama SA | Panama | |||||||
Opella Healthcare Panama SA | Panama | |||||||
| Sanofi-Aventis Puerto Rico Inc | Puerto Rico | |||||||
| Sanofi-Aventis Philippines Inc. | Philippines | |||||||
| Opella Healthcare Philippines Inc. | Philippines | |||||||
| Sanofi-Aventis Singapore Pte. Ltd | Singapore | |||||||
| Aventis Pharma (Manufacturing) Pte. Ltd | Singapore | |||||||
| Sanofi Manufacturing Pte Ltd | Singapore | |||||||
| Sanofi Taiwan Co., Ltd | Taiwan | |||||||
| Sanofi-Aventis (Thailand) Ltd | Thailand | |||||||
| Sanofi Pasteur Ltd | Thailand | |||||||
Sanofi-Aventis de Venezuela SA | Venezuela | |||||||
| Sanofi-Aventis Vietnam Company Limited | Vietnam | |||||||
| Sanofi Vietnam Shareholding Company Limited | Vietnam | |||||||
F-100 | SANOFI FORM 20-F 2023 | ||||
| NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS | ||
F.2. Principal investments accounted for using the equity method
| Financial interest (%) as of December 31, 2023 | ||||||||
Haleon US, LP | United States | |||||||
| Infraserv GmbH & Co. Höchst KG | Germany | |||||||
| Maphar | Morocco | |||||||
MCM Vaccine BV | Netherlands | |||||||
| MSP Vaccine Company (formerly MCM company) | United States | |||||||
| EUROAPI | France | |||||||
G/ Event subsequent to December 31, 2023
On January 23, 2024 Sanofi announced the entry into a merger agreement with Inhibrx, Inc. (Inhibrx), a publicly traded, clinical-stage biopharmaceutical company focused on developing a broad pipeline of novel biologic therapeutic candidates in oncology and orphan diseases (the Merger Agreement), pursuant to which Sanofi has agreed to acquire Inhibrx following the spin-off of Inhibrx's non-INBRX-101 assets and liabilities into a new publicly traded company ("New Inhibrx"). Under the terms of the Merger Agreement, Sanofi has agreed to: (i) provide Inhibrx's stockholders with consideration of $30 per share of Inhibrx common stock at the closing of the merger (approximately $1.7 billion), and to additionally issue one non-transferable contingent value right per share of Inhibrx common stock, which will entitle its holder to receive a deferred cash payment of $5 , conditioned upon the achievement of a regulatory milestone (approximately $0.3 billion, if the regulatory milestone is achieved); (ii) pay off Inhibrx’s outstanding third-party debt (approximately $0.2 billion); and (iii) provide a capital contribution to New Inhibrx (up to $0.2 billion). At the closing of the merger, Sanofi will acquire 100 % of the equity interests in Inhibrx, which will become a 100 % wholly owned subsidiary of Sanofi. In addition, Inhibrx will retain a minority stake (approximately 8 % equity interest) in New Inhibrx. INBRX-101 is a human recombinant protein that holds the promise of allowing Alpha-1 Antitrypsin Deficiency (AATD) patients to achieve normalization of serum AAT levels with less frequent (monthly vs. weekly) dosing. AATD is an inherited rare disease characterized by low levels of AAT protein, predominantly affecting the lung with progressive deterioration of the tissue. INBRX-101 may help to reduce inflammation and prevent further deterioration of lung function in affected individuals. INBRX-101 acquisition is expected to support Sanofi’s portfolio growth strategy and complements the Company’s 30-year heritage in rare diseases and track record in immunology and inflammation. The transaction is subject to various closing conditions, including the receipt of regulatory approvals and completion of the spin-off of New Inhibrx. Assuming satisfaction of those closing conditions, Sanofi currently anticipates that the transaction will close in the second quarter of 2024.
SANOFI FORM 20-F 2023 | F-101 | ||||
| Notes | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
F-102 | SANOFI FORM 20-F 2023 | ||||
| Notes | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
SANOFI FORM 20-F 2023 | F-103 | ||||
| Notes | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
| ................................................................................................................................................................................................................................................................. | ||
F-104 | SANOFI FORM 20-F 2023 | ||||
 | ||||||||
English translation and language consultancy: Stephen Reynolds & Jane Lambert. Photo credits: Front cover: Nils Libert, Associate Scientist, Ghent, Belgium © Simon Buxton – Key Figures : © Simon Buxton - p. 89 : © Yann Audic - p. 90 : © Jean Chiscano - p. 91 : © Alain BUU - p. 92 : © GE China - p. 93 : © Christel Sasso/Capa Pictures - p. 94 : © Lisbeth Holten, Denmark - p. 95 : Christel Sasso/Capa Pictures - p. 96 : © Julien LUTT/Capa Pictures - p. 97 : © Pierre-Olivier / Capa Pictures - p. 98 : © Marie Etchegoyen/Capa Pictures - p. 99 : © Legrand - p. 100 : © Franck Parisot - p. 101 : © Augustin Detienne/Capa Pictures - p. 103 : © Julien LUTT/Capa Pictures - p.104 : ©Oscar Timmers/Capa Pictures – p. 105 : ©Jennifer Altman/Capa Pictures | ||||||||
 | ||||||||