UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|||
|
☒ |
|
Smaller reporting company |
|
||
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on June 30, 2023 was $
The number of shares of Registrant’s Common Stock outstanding as of March 22, 2024 was
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement relating to the 2024 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. Such Proxy Statement, or an amendment to this Annual Report on Form 10-K, will be filed with the Securities and Exchange Commission within 120 days after the end of the registrant’s fiscal year ended December 31, 2023.
Table of Contents
|
|
Page |
PART I |
|
|
Item 1. |
5 |
|
Item 1A. |
18 |
|
Item 1B. |
55 |
|
Item 1C. |
55 |
|
Item 2. |
56 |
|
Item 3. |
56 |
|
Item 4. |
57 |
|
|
|
|
PART II |
|
|
Item 5. |
58 |
|
Item 6. |
58 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
59 |
Item 7A. |
70 |
|
Item 8. |
70 |
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
70 |
Item 9A. |
70 |
|
Item 9B. |
71 |
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
71 |
|
|
|
PART III |
|
|
Item 10. |
72 |
|
Item 11. |
72 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
72 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
72 |
Item 14. |
72 |
|
|
|
|
PART IV |
|
|
Item 15. |
73 |
|
Item 16. |
77 |
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this Annual Report on Form 10-K, including statements relating to our financial condition, results of operations, plans, objectives, future performance and business, are forward-looking statements. In some cases, you can identify forward-looking statements because they contain words such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “would,” “potential,” “likely,” or “continue” or the negative of these terms or other similar expressions. Forward-looking statements contained in this Annual Report on Form 10-K include, but are not limited to, statements about:
1
We have based the forward-looking statements contained in this Annual Report on Form 10-K primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations, prospects, business strategy, and financial needs. The outcome of the events described in these forward-looking statements is subject to risks, uncertainties, assumptions, and other factors described in the section titled “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Other sections of this Annual Report on Form 10-K describe additional factors that could adversely impact our business and financial performance. Furthermore, new risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this Annual Report on Form 10-K. This Annual Report on Form 10-K therefore does not contain an exhaustive list of all potential risks. We cannot assure you that the results, events, and circumstances reflected in the forward-looking statements will be achieved or occur, and actual results, events, or circumstances could differ materially from those described in the forward-looking statements.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject, based upon information available to us as of the date of this Annual Report on Form 10-K. While we believe that information forms a reasonable basis for such statements, it may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and investors are cautioned not to rely upon them unduly.
The forward-looking statements in this Annual Report on Form 10-K are made as of the date hereof. We undertake no obligation to update any forward-looking statements after the date of this Annual Report on Form 10-K or to conform such statements to actual results or revised expectations, except as required by law.
You should read this Annual Report on Form 10-K and the documents to which we refer herein and have filed as exhibits completely and with the understanding that our actual future results, levels of activity, performance, and achievements may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
2
RISK FACTOR SUMMARY
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below beginning at “Risks Related to Our Business and Strategy” within Item 1A., "Risk Factors" and should be carefully considered, together with other information in this Annual Report on Form 10-K and our other filings with the Securities and Exchange Commission before making investment decisions regarding our common stock.
3
4
PART I
Item 1. Business.
Overview
Alpha Teknova, Inc. (referred to herein as the Company, Teknova, we, us or our) is a leading producer of critical reagents for the discovery, development, and commercialization of novel therapies, vaccines, and molecular diagnostics. Our more than 2,500 customers span the continuum of the life sciences market and include leading pharmaceutical and biotechnology companies, contract development and manufacturing organizations, in vitro diagnostics franchises, and academic and government research institutions. Our Company is built around our knowledge, methods, and know-how in our manufacturing processes and infrastructure, which are highly adaptable and configurable. These proprietary processes enable us to manufacture and deliver high-quality, custom, made-to-order products with short turnaround times and at scale, across all stages of our customers’ product development, including commercialization.
We have substantial expertise in manufacturing customer-specified formulations and have demonstrated the ability to manufacture and deliver our products to customers quickly. Due to our expertise in raw materials sourcing, chemical formulation, and quality control, developed over more than two decades, we are typically able to move a new custom product into production in a matter of weeks from order receipt. This can allow our customers to receive their products in weeks as compared to months from alternative suppliers operating in traditional production environments. Our processes are designed to handle a diverse array of customer-requested inputs, which may vary by volume, chemical formulation, quality specifications, container types, and transportation requirements, enabling broad use of our products across the life sciences market. Our proprietary capabilities and products underpin the value we provide to customers across their product development and commercialization activities and allow us to scale with our clients as they grow, supporting their need for materials in greater volumes and that meet increasingly stringent regulatory requirements.
We offer three primary product types: pre-poured media plates for cell growth and cloning; liquid cell culture media and supplements for cellular expansion; and molecular biology reagents for sample manipulation, resuspension, and purification. We typically begin working with customers in the discovery phase of development, in which they use our off-the-shelf (catalog) formulations for initial experimentation. As customers’ product development progresses and they begin to need products with improved performance, in greater volumes, and that meet GMP requirements (see below), they routinely go on to order higher value, custom, and GMP-grade products. We believe the bespoke nature of our portfolio makes us a critical, trusted supplier to our customers.

Due to extensive validation and customer loyalty due, in part, to fast turnaround times for our custom products, our customers frequently integrate them as components into the lifecycle of their own products and, we believe, are therefore unlikely to substitute Teknova’s components with alternatives. As a result, our customer
5
relationships typically span many years and help drive recurring business. Moreover, we are committed to delivering high levels of customer satisfaction through continued investment in our customer service, operating infrastructure, quality systems, and manufacturing processes. During 2023, we achieved an annual customer retention rate of approximately 96% for customers purchasing more than $10,000 annually, which represented approximately 15% of our customer base and approximately 90% of our annual revenue during that period. We believe the Teknova brand is well established in the life sciences industry as a result of our track record of delivering high quality, custom products and providing superior customer service.
We participate in multiple market segments because customers use our products across the life sciences, including in high growth areas like cell and gene therapy research, development, and production. In the five years from late 2017 through 2022, the U.S. Food and Drug Administration (FDA) approved five gene therapies for rare genetic diseases. Meanwhile, 2023 was a breakthrough year for cell and gene therapies, with seven FDA approvals in the U.S. and one in the European Union, according to the Alliance for Regenerative Medicine. Looking to 2024, the Alliance for Regenerative Medicine estimates that the sector could see up to a combined 17 approvals in the U.S. and European Union.
We believe our prospects for growth will also benefit from developments in other fields, including mRNA vaccines, synthetic biology, and molecular diagnostics and genomics. We believe the key industry factors that will drive our future growth include:
The nature of many of our products and their uses require that they be manufactured by highly skilled personnel in contamination-controlled environments, following exacting procedures to ensure quality. We manufacture our products at our facilities in Hollister, California, which were purpose-built to address our customers’ needs for custom-made, RUO, or GMP-grade input components.
Our Portfolio
Our products are used across all stages of biopharmaceutical and diagnostic development workflows from discovery to commercialization. They include essential formulations for common research applications and highly customized formulations for customer-specific applications in genomics and bioproduction. Our customers also use our GMP-grade products as components in diagnostic kits and in the development and production of therapeutics.
Product Categories
We have two primary product categories: Lab Essentials and Clinical Solutions. Our products cross all stages of clinical development, from early research through commercialization.
Lab Essentials
We are a leader in providing highly complex chemical formulations for use in biological research and drug discovery. Our core Lab Essentials products consist of commonly used, catalog solutions and customer-specified formulations. During discovery, our products are used regularly in small, bench-scale experiments. As customers optimize their processes and begin to scale up in volume, they tend to order more custom products. Our Lab
6
Essentials products include essential formulations for common research applications and highly customized formulations for customer-specific applications in fields such as genomics, synthetic biology, and bioproduction. We sometimes refer to our Lab Essentials products as "research use only" or "RUO." For the year ended December 31, 2023, our Lab Essentials business contributed approximately 79% of our total revenue.
Clinical Solutions
We are ISO 13485:2016 certified, enabling us to meet the Quality System Regulation (QSR) of products for use in diagnostic and therapeutic applications. Our Clinical Solutions products are custom products used in the development and production of protein therapies, gene therapies, mRNA vaccines, and diagnostic kits. We sometimes refer to our Clinical Solutions products as "GMP" or "GMP-grade". Since offering GMP-grade products, we have achieved substantial growth in the number of customers seeking these products annually. For the year ended December 31, 2023, our Clinical Solutions business contributed approximately 18% of our total revenue.
Product Types
We offer three primary product types: pre-poured media plates for cell growth and cloning; liquid cell culture media and supplements for cellular expansion; and molecular biology reagents for sample manipulation, resuspension, and purification, among other applications. Within each of the three product types we offer products from each of our two primary product categories, except pre-poured media plates, which we only offer in our Lab Essentials product category.
7
Pre-poured Media Plates
We have an extensive selection of standard and specialty pre-poured media plates for a wide variety of applications including bacteria, fungi, and nematode growth. Pre-poured media plates, also referred to as agar plates, are the industry standard for growing microorganisms. The agar contains nutrients for the microorganism to grow and often contains compounds, such as antibiotics, to identify and select for microorganisms of interest. Microbes are spread on agar media to produce colonies, which are identical sets or clones of the original microorganism. The use of media plates is essential in the drug development process as it enables scientists to perform discovery experiments, express proteins, select cells for further expansion, and monitor the sterility of a bioproduction environment. Our ability to manufacture specialty pre-poured media plates across a wide range of formulations and plate formats makes them suitable for the most complex biological experiments and high throughput robotic applications.
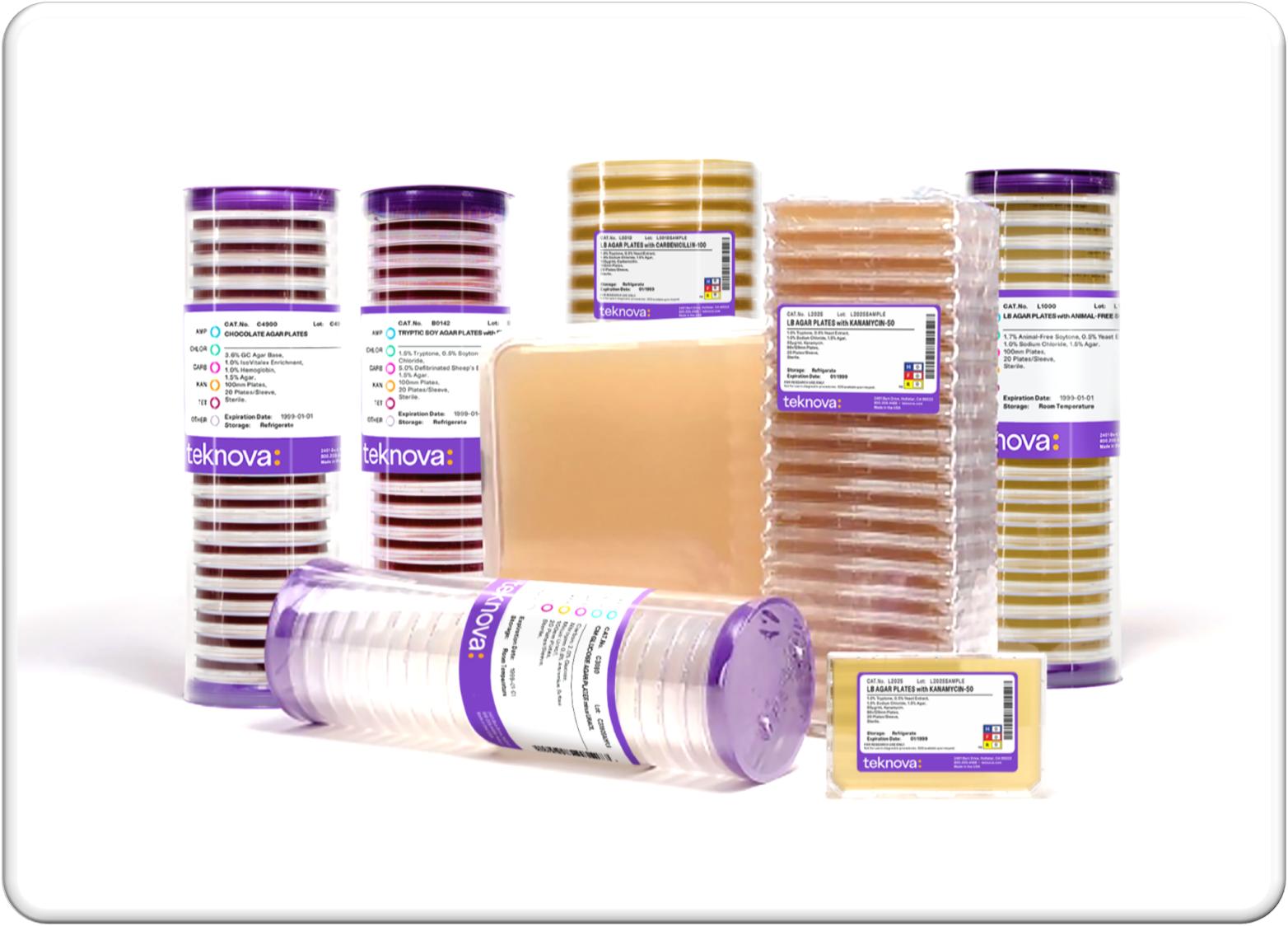
8
Cell Culture Media and Supplements
Cell culture media and supplements are used to expand, or grow, a particular cell of interest under controlled conditions. Cell culture media is composed of essential nutrients, such as amino acids and carbohydrates, growth factors, and hormones. To maintain the cells in culture, supplements (such as growth factors and sugars) are added to the culture over time. Expansion of cell lines is fundamental to the production of enzymes, antibodies, vaccines, and protein therapeutics. Different cells, based on species of origin or cell type, require different nutrients for efficient growth. The ability to customize cell culture media and supplements for a specific cell line is necessary to optimize bioproduction purity and yield. Given our customers’ desire to optimize cell culture processes early in development, combined with our ability to offer low production volumes for custom formulations, and then to scale production volumes over time, we believe we are a critical supplier for cell culture development and optimization. In addition, we are a leader in the production of bacterial cell culture media and supplements, which are critical inputs into mRNA vaccine and cell and gene therapy production processes.

9
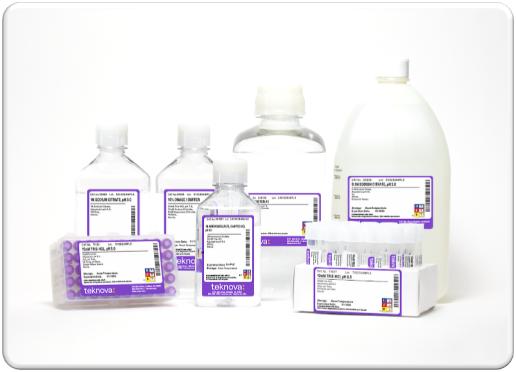
Molecular Biology Reagents
Molecular biology reagents are a cornerstone of biological research, molecular diagnostics, drug development, and bioproduction. Molecular biology reagents are used routinely for a wide variety of applications, including, but not limited to: washing samples; resuspending samples; purifying nucleic acids or proteins; analyzing samples, cell lysis, and sample management. Our diverse offering simplifies widely used biological protocols for our customers. As customers begin to scale production volumes and require increased manufacturing precision, customers frequently seek to specify formulations and product packaging requirements—which we specialize in providing—to achieve their goals of increasing product performance and realizing manufacturing efficiencies.
Competitive Strengths
Expertise in Complex, Custom Chemical Formulation Manufacturing
We work closely with our customers to provide highly customized formulations across a variety of workflows. Our customers routinely specify the raw material source, chemical composition, packaging, labeling, and quality control specifications required for their desired product. Through two decades of capital investment and process optimization, we have created a production system designed to develop and manufacture customer-specified formulations, which we believe enables us to produce and quality control custom products faster than our competitors. We leverage our proprietary chemical formulation and production expertise, supported by a product database consisting of the formulations of thousands of previously made products. This database, along with our experienced staff, allows us to quickly determine the optimal production process and meet the associated complexity requirements for custom orders. We believe our ability to rapidly customize has contributed significantly to the adoption of our products.
10
Quality and Regulatory Expertise Drives Deep Customer Relationships
The life sciences industry is subject to rigorous regulatory scrutiny in areas such as quality, reliability, and performance. Our customers rely on us to meet these high standards while also facilitating the development of novel, innovative products. During the early stages of product development, we manufacture formulations specified by our customers to aid in the optimization of therapeutic or diagnostic production processes. Our customers frequently validate these custom-made research and GMP-grade components into their production processes, allowing them to remain with us as a supplier as they scale up from research to commercial production. As a result of the extensive validation and regulatory requirements applicable to these therapeutic and diagnostic products, we believe these components are often used for the life of a product, as evidenced by our customer retention rates. We are focused on developing and fostering long-term relationships with our customers, which has resulted in increased purchasing volumes from our customers over time.
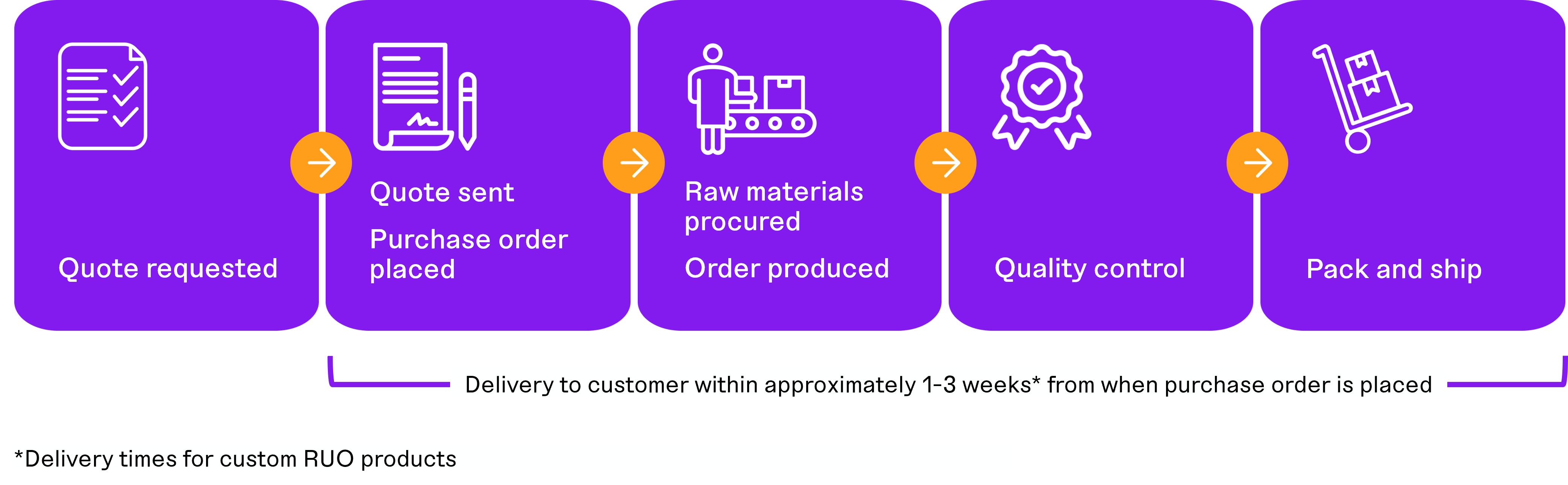
Industry-Leading Delivery Time for Custom Products
Our operations, built upon our proprietary manufacturing processes developed over the past 20 years, enable adaptable, versatile, and rapid production of complex chemical formulations. Our production process is designed to handle diverse inputs in volume and product type, allowing us to deliver custom, made-to-order products for our clients across the life sciences industry. We seek to collaborate with our customers to gain visibility into their product development and purchasing requirements and are positioned to react quickly to meet their needs. Due to our expertise in raw materials sourcing, product creation, chemical formulation, and quality control, we are typically able to move a new custom product into production in a matter of weeks from order receipt. In addition, we can provide custom solutions at low minimum volumes and increase in scale by up to 100-fold within the same production environment. This means our customers can receive their products in weeks rather than months compared to other suppliers operating in traditional production environments. We ship approximately 70% of our custom RUO products less than three weeks from order placement.
For the year ended December 31, 2023, two of our suppliers each made up 10% or more of our total inventory purchases, which suppliers comprised 50% of our total inventory purchases in the aggregate. One supplier, a distributor, accounted for 40% of total inventory purchases and one of our other suppliers accounted for 10% of total inventory purchases. For the year ended December 31, 2022, three of our suppliers each made up 10% or more of our total inventory purchases, which suppliers comprised 63% of our total inventory purchases in the aggregate. One supplier, a distributor, accounted for 37% of our total inventory purchases and two of our other suppliers accounted for 14% and 12% of total inventory purchases, respectively.
11
Well-Positioned in Evolving Cell and Gene Therapy Market
We work closely with our cell and gene therapy customers to provide customized, made-to-order formulations across a variety of workflows. Our products are critical components frequently used in the research and development of cell-and-gene-therapy-derived pharmaceuticals and vaccines. In particular, we are a leading provider of research and GMP-grade bacterial cell culture media and specialized chromatography solutions—reagents required for cell and gene therapies—which we believe positions us especially well to capture share in these growing markets.
A report commissioned by us predicts that, compared to spending during phase 1 clinical trials, average spend by customers developing cell and gene therapies increases by 1.4 times during phase 2 trials, 3.2 times during phase 3 trials and 29.8 times during commercial production, following FDA approval. Our data shows that in calendar year 2023, of our approximately 110 customers purchasing more than $5,000 annually and active in cell and gene therapy development, 60% of them purchased $5,000 or more annually of catalog products from us, 24% purchased $5,000 or more annually of custom products, and 16% purchased $5,000 or more annually of GMP-grade products. We therefore believe our customers will spend more with us over time as cell and gene therapies move through the FDA approval process and they purchase more GMP-grade products. Combined with our existing strengths and planned investments in areas valued by developers of cell and gene therapies, which we discuss elsewhere in this Annual Report on Form 10-K, we therefore aim to significantly increase our overall revenue from sales to customers active in cell and gene therapy in the years ahead.
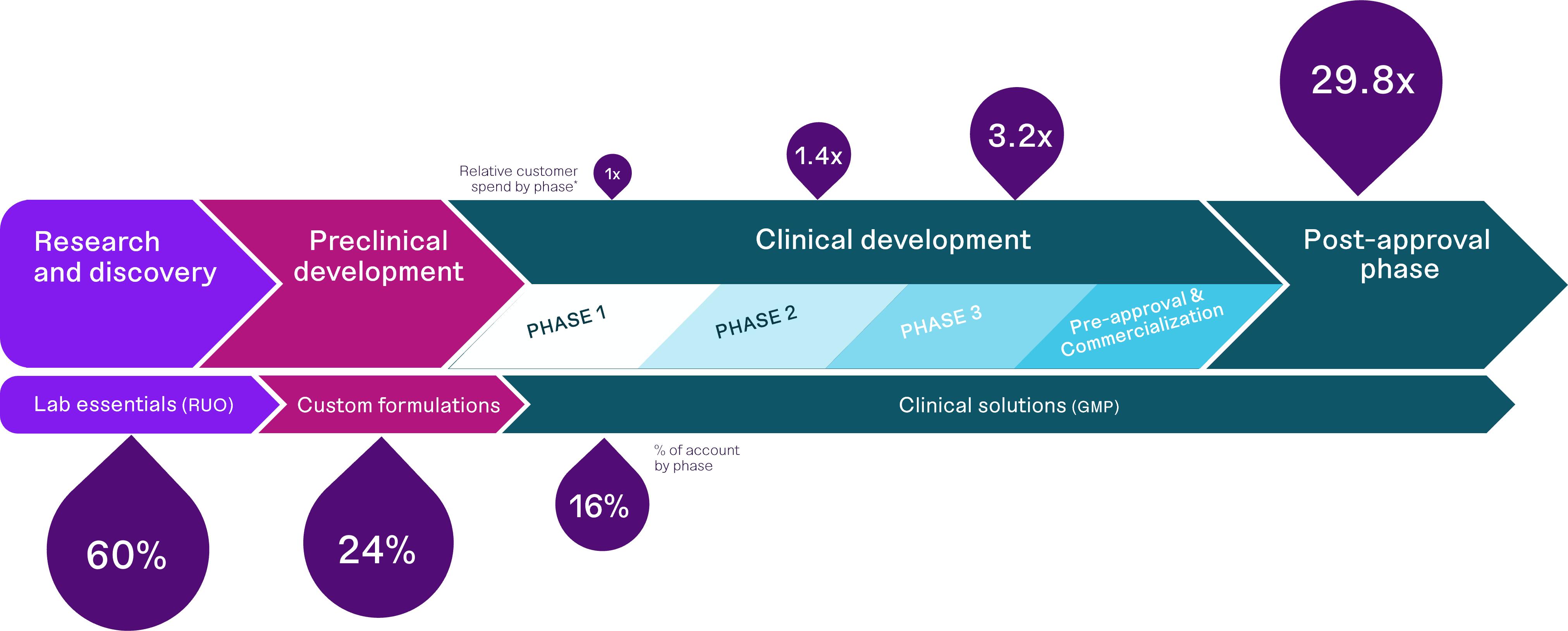
Source: Fletcher Spaght Growth Report, a report commissioned by us
Experienced Leadership and Talented Workforce
Our senior management team has deep experience across the life sciences, diagnostics, and biopharmaceutical market segments. Our senior management team has served in numerous leadership roles at both large, multinational organizations and small growth companies. Our employees provide tailored support, guidance, and service for our customers. We believe the quality of our personnel is critical to our ability to maintain collaborative, long-standing relationships with our customers.
12
Our Markets
We participate in multiple market segments, because customers use our products across the life sciences industry, including in areas like cell and gene therapy research, development, and production. We believe our prospects for growth will also benefit from developments in other fields, including the validation of mRNA vaccines and their possible use in therapies, continued significant investment in synthetic biology, and growing interest in molecular diagnostics and genomics. Within these market segments, we have benefited from and expect to continue to benefit from favorable industry preferences for customized products, high quality, and short turnaround times. Among the key factors underpinning the long-term attractiveness of our market opportunity are the expansion of cell and gene therapy, the development and deployment of mRNA vaccines and therapies, and the growing acceptance of molecular diagnostics and genomics.
The following are some of the other factors benefiting our core markets:
In addition to our core markets, we believe there are additional factors that will drive long-term growth including:
Cell and Gene Therapy
As a supplier to approximately 110 leading cell and gene therapy organizations, we are well positioned to benefit from long-term growth in this market through our high quality, custom, and made-to-order products. Factors driving this long-term growth will include, we believe, an increasing incidence of previously untreatable cancers and other chronic diseases, a corresponding rise in the number of clinical trials, and FDA approvals of cell and gene therapy products. In the five years from late 2017 through 2022, the FDA approved five gene therapies for rare genetic diseases. Meanwhile, 2023 was a breakthrough year for cell and gene therapies, with seven FDA approvals in the U.S. and one in the European Union, according to the Alliance for Regenerative Medicine. Looking to 2024, the Alliance for Regenerative Medicine estimates that the sector could see up to a combined 17 approvals in the U.S. and European Union.
We support the development of these therapies by providing customer-specified chemical formulations for bioprocessing, scale-up, and commercialization. Our products are used early in the product development cycle. We believe our product portfolio and our expertise in custom formulations allow us to work closely with our customers at the early stages of product development to optimize manufacturing processes for their particular therapies, and then to scale as their production needs evolve. Therefore, we are able to play an integral role in therapeutic development and, ultimately, commercialization. We believe that because our products are often customized for a specific therapy and then validated, it is unlikely these customers would switch suppliers once their therapies enter clinical trials. In addition, we have recently launched a portfolio of novel products to address certain critical pain points in gene therapy bioproduction. We believe the introduction of these novel products provides additional long-term revenue opportunities and helps position us as key partners to customers in the growing gene therapy market.
13
Increasing Use of mRNA Vaccines and Therapies
As a leader in bacterial cell culture media and supplements, lysis buffers, and nucleic acid and protein purification reagents, we are a supplier to the mRNA vaccine and therapeutics market and are well positioned to benefit from the increasing use of mRNA vaccines and therapies over time. We believe the demand for mRNA will continue to increase and therefore drive the need for more customized, research and GMP-grade bacterial cell culture media and associated formulations. The short development timeline and proven effectiveness of the COVID-19 mRNA vaccines have demonstrated the promise of mRNA therapies. The production process for mRNA requires the use of bacteria for plasmid production and a substantial number of chemical formulations for producing, purifying, and re-suspending nucleic acid sequences.
Growth in Molecular Diagnostics and Genomics Markets
According to third-party research, the global molecular diagnostics market was estimated to have been at $13.6 billion in 2022 and is projected to reach $27.0 billion by 2030, while the global genomics market is expected to grow from an estimated $37.9 billion in 2024 to $94.9 billion by 2030. We expect this growth to continue to drive demand for our research- and clinical- grade reagents in the long-term because diagnostics and genomic market leaders use our formulations as critical components in their manufacturing processes and saleable kits. For example, synthetic biology, enzyme, and antibody manufacturers often use our bacterial cell culture media and related cell lysis and purification buffers to produce their cell lines or proteins of interest. A number of our customers in the life science tools and molecular diagnostic market segments, such as spatial transcriptomics, single cell sequencing, and liquid biopsy, use our molecular biology reagents as critical subcomponents in the kits they sell to their end users.
Our Strategy
Our goal is to provide our customers the products necessary to accelerate their therapeutic and diagnostic development efforts, from basic research to commercialization of therapies that improve human health. The key elements of our business strategy to achieve this goal include:
Increase Integration of Our Products into Our Customers’ Workflows
Building lasting relationships and embedding our products within our customers’ key workflows are at the core of our strategy. During the early stages of product development, we manufacture formulations specified by our customers to aid in the optimization of therapeutic or diagnostic production processes. Our customers validate these custom-made research and GMP-grade components into their production processes, and because of the extensive validation required for customers' therapeutic and diagnostic products, we believe these components are often used for the life of a product, as evidenced by our customer retention rates. As customers move from catalog to custom and, ultimately, to clinical production, their total expenditure increases. Based on our purchase data from 2023, customers who purchased our custom products spent approximately 19 times more on average per account with us than those who solely purchased catalog products. Over the same period, our customers who purchased our GMP-grade products, purchased 62 times more per account with us than those who solely purchased catalog products and approximately 3 times more than those who purchased catalog and custom research-grade products. In 2023, customers who purchased $5,000 or more of catalog products, custom products, and GMP-grade products constituted approximately 79%, 16% and 5%, respectively, of these larger customers during the period. We aim to increase the proportion of our customers purchasing custom products and GMP-grade products by building lasting relationships and embedding our products within our customers’ key workflows as our customers’ product development matures.
Provide Superior Customer Service Through Operational Excellence
We are committed to providing superior customer service and fulfilling the expectations of our customers by making the investments required to perpetuate our operational excellence. We have extended our rapid custom production capability by further investing in automation, facilities, and operating infrastructure to substantially increase the manufacturing capacity at our facilities, improve operating efficiency, and reduce delivery time for our custom research and GMP-grade products. We believe these investments position us for future growth by allowing
14
us to continue to exceed our customers’ expectations in quality and delivery time and enabling us to maintain lasting relationships with our customers as they advance their products through key phases of product development.
Competition
We operate in a highly competitive environment with a diverse base of competitors, many of whom focus on specific regions, products and/or customer segments. Many of the companies selling or developing competitive products, which in some cases are also large customers, have greater financial, personnel, R&D, manufacturing, and marketing resources than we do. We also compete with other smaller, niche competitors and specialized companies that focus on certain areas of the life sciences market. A portion of our target customers have established in-house production capabilities to manufacture products that are substantially similar to our products. In-house production may prove to be a less costly or more desirable alternative to purchasing our products due to prior investments in production infrastructure and workforce.
Our Lab Essentials and Clinical Solutions products compete on the basis of delivery time, performance, and quality with products offered by numerous large, established life science companies such as Thermo Fisher, Millipore (Merck KGaA), Cytiva (Danaher), Hardy Diagnostics, and Lonza. We are differentiated by our ability to offer customer-specified RUO and GMP formulations with short turnaround times in volumes and product characteristics matching customer needs, our Teknova brand reputation established over more than 25 years, and our technical expertise.
Government Regulation
We market the products we manufacture as ancillary reagents and materials that our customers can use for research purposes or in the further manufacture of their products, which may include therapies, vaccines, and molecular diagnostics. As ancillary reagents and materials, our products are not subject to regulation under the U.S. Federal Food, Drug and Cosmetic Act, and therefore none of our current products are registered with the FDA. We do not make any claims related to the safety, effectiveness, or diagnostic utility of any of our products because they are not intended for clinical, therapeutic, or diagnostic use.
At the same time, the quality of our ancillary reagents and materials is critical to our biopharmaceutical and other life sciences customers who are subject to extensive regulation by the FDA, and by corresponding regulatory authorities in other countries, regarding the conduct of clinical trials and the marketing approval for and commercialization of products for diagnostic and therapeutic uses. The regulatory oversight of our customers necessitates that they impose rigorous quality requirements on us, as their supplier, through supplier qualification processes, quality agreements, and routine customer audits. We therefore choose to maintain a quality system compliant with our customers’ requirements and expectations, including records of our manufacturing, testing, and quality control activities, and we must be able to provide our customers with corresponding records on a periodic basis, upon their request. These customers may seek to requalify us on a regular basis to ensure our quality system, processes, and facilities continue to meet their needs and requirements outlined in relevant customer agreements.
Because quality is so important to our customers, and because many of them may further process and validate the products they purchase from us, we voluntarily built our quality system to comply with specific sections of the ISO 13485:2016 standards established by the International Organization for Standardization (ISO). We are certified to manufacture our products in accordance with those standards. We sell products that we manufacture and process with additional, even more exacting quality and validation controls as “Clinical Solutions” or “GMP-grade,” specifically to meet the needs of customers who use our materials in the further manufacture of their diagnostic, vaccine, or therapeutic products.
Compliance with “Research Use Only” Labeling Guidance
In November 2013, the FDA issued Final Guidance for Industry and Food and Drug Administration Staff on “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” (RUO/IUO Guidance). The RUO/IUO Guidance, while generally not legally binding, explains that the FDA will review the totality of the circumstances when evaluating whether equipment and testing components are properly
15
labeled as RUO. Merely including a labeling statement that a product is intended for research use only will not necessarily exempt the product from FDA regulation or oversight, if the circumstances surrounding the distribution of the product indicate that the manufacturer intends its product to be used for clinical, therapeutic, or diagnostic use. These circumstances may include written or verbal marketing claims or links to articles regarding a product’s performance in clinical applications, a manufacturer’s provision of technical support for clinical validation or clinical applications, or solicitation of business from clinical laboratories, all of which could be considered evidence of intended uses that conflict with RUO labeling. We do not market any of our products for use in clinical, therapeutic, or diagnostic settings. We believe that all of the products we label and sell as intended for “Research Use Only” are properly labeled and marketed as such in accordance with the RUO/IUO Guidance. If the FDA were to determine, based on the totality of circumstances, that any of our products are intended for diagnostic or therapeutic purposes, then those products would be considered medical products and would require approval from the FDA prior to their commercialization.
Environmental Laws and Regulations
We are subject to federal, state, and local laws and regulations relating to the protection of human health and the environment. In the conduct of our business, we handle, store, and dispose of certain chemicals and biohazardous waste. The laws and regulations applicable to our operations include provisions that regulate the discharge of materials into the environment. Some of these environmental laws and regulations impose “strict liability,” rendering a party liable without regard to negligence or fault on the part of such party. Such environmental laws and regulations may expose us to liability for environmental contamination, including remediation costs, natural resource damages and other damages as a result of the conduct of, or conditions caused by, us or others or for acts that complied with all applicable laws at the time such acts were performed. In addition, where contamination may be present, it is not uncommon for neighboring landowners and other third parties to file claims for personal injury, property damage, and recovery of response costs. Although it is our policy to use generally accepted operating and disposal practices in accordance with applicable environmental laws and regulations, hazardous substances or wastes may have been disposed or released on, under, or from properties owned, leased, or operated by us or on, under, or from other locations where such substances or wastes have been taken for disposal. These properties may be subject to investigation, remediation, and monitoring requirements under federal, state, and local environmental laws and regulations.
We believe that our operations comply in all material respects with applicable environmental laws and regulations. However, failure to comply with these environmental laws and regulations may result in the imposition of administrative, civil, and criminal penalties or other liabilities. Because the requirements imposed by such laws and regulations may frequently change and new environmental laws and regulations may be adopted, we are unable to predict the cost of compliance with such requirements in the future, or the effect of such laws on our capital expenditures, results of operations or competitive position.
Intellectual Property
Our success depends, in part, on our ability to obtain and maintain intellectual property protection for our products and trade secrets, to operate our business without infringing, misappropriating or otherwise violating the intellectual property rights of others, and to defend and enforce our intellectual property rights.
We rely on trade secrets, including know-how, confidential information, unpatented technologies, and other proprietary information, to strengthen or enhance our competitive position, and prevent competitors from reverse engineering or copying our technologies. We maintain, as trade secrets, information relating to our current products and our products currently in development, as well as information related to our business strategy, client lists, and business methods. However, trade secrets and confidential know-how are difficult to protect. To avoid inadvertent and improper disclosure of trade secrets, and to avoid the risks of former employees using these trade secrets to gain future employment, it is our policy to require employees, consultants, and independent contractors to assign to us all rights to intellectual property they develop in connection with their employment with or services for us. We also protect our existing and developing intellectual property expressly through confidentiality provisions in agreements with third parties. There can be no assurance, however, that these agreements will provide meaningful protection for our trade secrets or other intellectual property or proprietary information, or afford adequate remedies in the event of the unauthorized use or disclosure of such trade secrets or other intellectual property or proprietary information. We
16
also seek to preserve the integrity and confidentiality of our trade secrets and other confidential information by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in the measures we take to protect and preserve our trade secrets, such measures can be breached, and we may not have adequate remedies for any such breaches. In addition, our trade secrets may otherwise become known or be independently discovered by competitors.
We intend to pursue additional intellectual property protection to the extent we believe it would advance our business objectives. Despite our efforts to protect our intellectual property rights, these rights may not be respected in the future or may be circumvented or challenged (and potentially invalidated) in a legal proceeding in any jurisdiction where we have intellectual property rights. In addition, the laws of various foreign countries may not afford the same protections or assurances to the same extent as the laws in the U.S. See the section titled “Risk Factors—Risks Related to Our Intellectual Property” for additional information regarding these and other risks related to intellectual property.
Human Capital
As of December 31, 2023, we had 211 employees, of which 210 were full-time and one was part-time. This includes 109 employees in our operations organization, 54 in administrative functions, 25 in sales and marketing and 23 in engineering and research and development. None of our employees are represented by a labor union or are subject to a collective bargaining agreement.
On January 11, 2024, we announced a reduction in our workforce that affected approximately 15% of our employees at that time. The reduction in workforce is expected to be substantially complete in the first quarter of 2024. Refer to the “Notes to Financial Statements—Note 17. Subsequent Events” in our financial statements for details regarding the workforce reduction.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing, and motivating our existing and future employees. The principal purposes of our equity incentive plans are to attract, retain, and motivate selected employees, consultants, and directors through the granting of stock-based compensation awards.
Facilities
Our headquarters are located in Hollister, California, where we lease approximately 220,000 square feet of commercial, office, manufacturing, and warehouse space at six separate locations in the same vicinity, which we refer to collectively as our Hollister campus. Our Hollister campus includes dedicated space for us to carry out product formulation, dispensing, manufacturing, and packaging of our products. The Hollister campus includes space used for quality control, packaging, and storage of “retains” for quality control purposes and approximately 12,500 square feet of clean room space. Space used to store our finished goods inventory, ship our products, and house our engineering and quality departments is also located at our Hollister campus, along with a receiving warehouse and raw materials storage. Our management offices, labs, engineering, and customer service groups are also located at the Hollister campus.
We believe that the facilities we currently lease are adequate to meet our needs for the immediate future, and that, should it be needed, additional space can be acquired or leased to accommodate future growth.
Corporate Information
The Company was founded in 1996 and initially incorporated in California on May 30, 2000, under the name “eTeknova, Inc.” On January 11, 2019, the Company filed a certificate of merger and merged with and into Alpha Teknova, Inc., a Delaware corporation, which continued as the surviving entity bearing the corporate name of “Alpha Teknova, Inc.”
In June 2021, we completed the initial public offering of our common stock (IPO). Our common stock trades on the Nasdaq Global Market under the symbol "TKNO". Following the IPO, Telegraph Hill Partners Management Company LLC (Telegraph Hill Partners), through its affiliates Telegraph Hill Partners IV, L.P. (THP LP) and THP
17
IV Affiliates Fund, LLC (THP LLC, and collectively with THP LP, THP), continues to be our controlling stockholder.
Our principal executive offices are located at 2451 Bert Dr., Hollister, California 95023. Our telephone number is (831) 637-1100. Our website address is www.teknova.com. Information contained on, or that can be accessed through, our website is not part of and is not incorporated by reference in, or a part of, this or any other report we file with, or furnish to, the United States Securities and Exchange Commission (SEC).
The name “Teknova”, "teknova:", and the “Teknova Science Matters” logos, and other registered or common law trademarks or service marks of Alpha Teknova, Inc. appearing in this Annual Report on Form 10-K are the property of Alpha Teknova, Inc. Other trademarks and tradenames referred to in this Annual Report on Form 10-K are the property of their respective owners. Solely for convenience, trade names, trademarks, and service marks referred to in this Annual Report on Form 10-K may appear without the ® or TM symbols. Such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to such trade names, trademarks, and service marks.
Item 1A. Risk Factors.
Risks Related to Our Business and Strategy
We have incurred operating losses in the past and may incur losses in the future.
We have incurred operating losses in the past, may incur operating losses in the future and may never achieve or maintain profitability. For the years ending December 31, 2023 and 2022, we incurred net losses of $36.8 million and $47.5 million, respectively. We have incurred and will continue to incur costs in connection with legal, accounting, and other administrative expenses related to operating as a public company and we expect that our operating expenses will increase modestly with the growth of our business. Since our inception, we have financed our operations primarily through revenue from our products, the sale of our equity securities (including through our June 2021 IPO and September 2023 registered direct offering, and private placements), and debt. While our revenue has generally grown over the last several years, it decreased in 2023 compared to 2022. If our revenue continues to decline or fails to grow at a rate sufficient to offset our operating expenses, we will not be able to achieve and maintain profitability in future periods. We may never be able to generate sufficient revenue to achieve or maintain profitability, and our more recent growth and historical profitability should not be considered predictive of our future performance.
Our operating results may fluctuate significantly in the future, making them difficult to predict, and they could fall below expectations or any guidance we may provide.
Our quarterly and annual operating results may fluctuate significantly, making them difficult to predict. These fluctuations may not fully reflect the underlying performance of our business. They may occur due to a variety of factors, including, but not limited to:
18
The impact of any one of the factors set out above, or the cumulative effects of a combination of such factors, could result in significant fluctuations and unpredictability in our quarterly and annual operating results. As a result, comparisons of our operating results on a period-to-period basis may not be meaningful. Furthermore, our historical results are not necessarily indicative of results expected for any future period, and quarterly results are not necessarily indicative of the results to be expected for the full year or any other period, and accordingly should not be relied upon as indicative of future performance.
As a result of variability and unpredictability, we may also fail to meet the expectations of industry or financial analysts or investors for any period. If our revenue or operating results fall short of the expectations of analysts or investors or any guidance we may provide, or if the guidance we provide falls short of the expectations of analysts or investors, the price of our common stock could decline substantially. Such a stock price decline could occur even when we have met or exceeded any publicly stated guidance we may have provided and could in turn negatively impact our business, financial condition, results of operations, cash flows, and prospects.
We have invested a significant amount of capital in our new and legacy manufacturing facilities. Our efforts to scale our manufacturing capabilities in these facilities could be disruptive and adversely affect our results of operations and financial condition. We may not realize some or all of the anticipated benefits of this investment in the time frame anticipated, or at all.
We have invested a significant amount of capital in our new and legacy manufacturing facilities in both equipment and infrastructure to substantially increase the effective manufacturing capacity at our facilities, improve operating efficiency through the use of automation, and reduce delivery time for our custom Lab Essentials and Clinical Solutions products. Our efforts to scale our manufacturing capabilities could be disruptive to our operations, divert the attention of management, and require additional investments. Our ability to increase our manufacturing capacity is dependent upon a number of uncertainties inherent in all new manufacturing operations, including but not limited to ongoing compliance with regulatory requirements and the pace of bringing production equipment and processes online with the capability to manufacture high-quality products at scale. If we experience any problems or delays in meeting our projected timelines for expansion of operating capacity or efficiency, if the actual production capacity yielded by our recent expansion efforts does not meet our projections, or if additional investment is needed, our business, financial condition, results of operations, cash flows, and prospects may suffer.
If our products do not possess the required or expected quality characteristics or perform as expected, or if the reliability of the technology on which our products are based is questioned, we could experience lost revenue, delayed or reduced market acceptance of our products, increased costs, and damage to our reputation.
Our success depends in large measure on the market’s confidence that we can provide reliable, high-quality reagents that our customers can use for the development and commercialization of therapies, novel vaccines, and molecular diagnostics. We believe that customers in our target markets are particularly sensitive to product nonconformances, defects, and errors given the potential for impact on their own products and processes, which in
19
many cases are regulated. Our reputation and the public image of our products and capabilities may be impaired if our products fail to perform as expected.
Although we operate a rigorous quality control system, nonconformances, defects, or errors could nonetheless occur or be present in products that we release for shipment to customers. Our operating results depend on our ability to execute and, when necessary, improve our quality management strategy and systems, our ability to effectively train and maintain our employee base with respect to quality management, and our ability to consistently meet international quality standards, including those set out in ISO 13485:2016 and meet the product specifications and quality requirements specified in agreements with customers. A failure of our quality control systems could result in problems with facility operations, the manufacture or delivery of our products, or our ability to maintain our ISO certification. These and related problems could arise for a variety of reasons, including equipment malfunctions, the failure to follow specific manufacturing and quality control and assurance protocols and procedures or other human error, defects in our engineering, design, manufacturing, and delivery processes, problems with third-party components or raw materials, environmental factors, and damage to, or loss of, our quality systems. The consequences could affect production of a particular batch or series of batches of products, requiring the disposal of those products or a stop to production altogether. Furthermore, some of the products we manufacture are subsequently incorporated into products that are sold by other life sciences companies; we have no control over any aspect of those products.
Although we have established internal procedures to reduce the risks that may arise from product quality issues, there can be no assurance that we will be able to eliminate or mitigate occurrences of these issues and associated liabilities. In addition, identifying the root cause of quality issues, particularly those affecting reagents and third-party components, may be difficult, which increases the time needed to address quality issues as they arise and increases the risk that similar problems could recur. Finding solutions to quality issues can be expensive and we may incur significant costs or lost revenue in connection with, for example, shipment holds, product recalls or replacements, or the disposal of unsaleable products.
In addition, if we or our suppliers fail to meet applicable quality standards and if our products experience, or are perceived to experience, a material nonconformance, defect, or error, our products could be recalled or we may be unable to timely deliver products to our customers, which in turn could damage our relationships with new and existing customers and our reputation for quality and service. Although we continually take steps to improve our quality procedures, we cannot guarantee that we will not experience quality assurance issues with our products in the future. Any such failure could, among other things, lead to increased costs, delayed or lost revenue, delayed or reduced market acceptance, damage to our relationships with new and existing customers and our reputation, diversion of development resources, legal claims, reimbursement to customers, other customer claims, damage to and possible termination of existing customer relationships, increased insurance costs, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other batches or products, any of which could harm our business, financial condition, results of operations, cash flows, and prospects. Such nonconformances, defects, or errors could also narrow the scope of the use of our products, which could hinder our success in the market.
Even after any underlying quality or related concerns or problems are resolved, any lingering concerns in our target markets regarding our products or services, or any manufacturing defects or performance errors in our products, could continue to result in lost revenue, delayed or reduced market acceptance, damage to our reputation, and claims against us.
In addition, we may be unable to maintain the quality, reliability, robustness, and expected turnaround times of our products and services to continue to satisfy customer demand as we grow. Fast delivery time is of crucial importance, especially to the cell and gene therapy market segment and our customers rely on us to provide timely delivery of their custom-made formulations. We must continuously improve our operational, manufacturing, quality control and assurance and monitoring systems and processes, and other aspects of our business, and effectively train and manage our personnel. Failure to meet those objectives could adversely affect our operations and negatively impact our business and financial results. Over time, we may need to purchase additional equipment (some of which can take several months or more to procure, set up, and validate), establish new production processes, and hire additional personnel to meet increased demand. There can be no assurance that we will meet any of these anticipated challenges successfully. Failure to manage future growth could result in delays in turnaround times, higher product
20
costs, declining product quality, deteriorating customer service, and slower responses to competitive challenges. A failure in any one of these areas could make it difficult for us to meet market expectations for our products and could damage our reputation and our business, financial condition, results of operations, cash flows, and prospects could be adversely affected.
We are dependent on our customers’ spending on and demand for our products. A reduction in spending or demand, including as a result of changes in economic conditions, could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
Our chemical formulations are sold primarily to biopharmaceutical companies, life science research companies, contract research organizations (CROs), contract development and manufacturing organizations (CDMOs), in vitro diagnostics franchises, and academic and government research institutions developing novel vaccines, diagnostics and therapies and performing basic research. Our customers' spending on research, development, production, and marketing, as well as the outcomes of such research, development, and marketing activities, has a substantial impact on our revenues and profitability, particularly the amount our customers choose to spend on our products. Available resources, the need to develop new products, and consolidation in the industries in which our customers operate may have an impact on that spending. Many of our customers finance their research and development spending with capital raised from private investors and the public capital markets.
The success of our business depends primarily on the number and size of purchases from these customers. Research and development spending by our customers and the availability of government and academic research funding of, or capital markets investment in, life sciences research and development can fluctuate due to changes in available resources, mergers of pharmaceutical and biotechnology companies, spending priorities, general economic conditions and institutional and governmental budgetary policies. Changes in governmental and academic funding of, or capital markets investment in, life sciences research and development, or overall reductions in healthcare spending, could negatively impact us or our customers and, consequently, our sales to them. A substantial majority of our sales are made on a purchase order basis, which permits our customers to cancel, change, or delay their product purchase commitments with little or no notice to us and often without penalty to them. Changes in the number of orders received and filled can cause fluctuations in our quarterly revenue and earnings.
For example, over a period of several years, we benefited from growing demand for our products attributable to the ongoing expansion of the global biologics and diagnostics market segments, robust research and development budgets, and a trend toward greater outsourcing by our customers. These market conditions changed substantially in the middle of 2022, when private and public funding available to small and emerging biotechnology companies, in particular, contracted sharply as tailwinds from the COVID-19 pandemic subsided and led to a reduction of or deferral in spending by some of our customers. These negative market dynamics remained through 2023. It is unclear whether these market conditions will continue in the future. If these economic pressures on the life sciences industry persist, they could have an ongoing and substantial adverse effect on the demand for our products.
In addition to these industry trends, our customers’ willingness and ability to utilize our products are also subject to, among other things, their own financial performance, changes in their available resources, their decisions to acquire in-house manufacturing capacity, their spending priorities, their budgetary policies and practices including inventory levels and their need to develop new biological products, which, in turn, are dependent upon a number of factors, including their competitors’ discoveries, developments and commercial manufacturing initiatives and the anticipated market, clinical and reimbursement scenarios for specific products and therapeutic areas. In addition, consolidation in the industries in which our customers operate may have an impact on our customers’ spending as they integrate acquired operations, including research and development departments and associated budgets. If our customers reduce their spending on our products as a result of any of these or other factors, our business, financial condition, results of operations, cash flows, and prospects would be materially and adversely affected.
Our customers' research and development, and the clinical and market success of their products, may significantly influence our business, financial condition, and results of operations.
Our customers are engaged in research, development, production, and marketing of pharmaceutical and biotechnology products. We depend on, and have no control over, end market demand for the products our customers manufacture. End market demand for our customers’ products could be adversely affected by, among
21
other things, delays in regulatory approvals, the inability of our customers to demonstrate the efficacy and safety of their products, the loss of patent and other intellectual property rights protection, the emergence of competing or alternative products, including generic drugs, the degree to which private and government payment subsidies for a particular product offset the cost to consumers, and changes in the marketing strategies for such products. Additionally, if the products our customers manufacture do not gain market acceptance, our revenues and profitability may be adversely affected.
Ongoing changes to the healthcare industry, including ongoing healthcare reform, adverse changes in government or private funding of healthcare products and services, legislation or regulations governing the privacy of patient information or patient access to care, or the delivery, pricing, or reimbursement of pharmaceuticals and healthcare services or mandated benefits, may cause healthcare industry participants to purchase fewer products and services from us or influence the price that others are willing to pay for our products and services. Changes in the healthcare industry’s pricing, selling, inventory, distribution, or supply policies or practices could also significantly reduce our revenue and profitability.
If our customers are not successful in attaining or retaining product sales due to market conditions, reimbursement issues, or other factors like those set out above, or if our customers' orders otherwise decline, our financial condition and results of operations may be adversely affected.
If we cannot maintain our current relationships with customers, if we fail to sustain recurring sources of revenue with our existing customers, or if we fail to enter into new customer relationships, our future operating results will be adversely affected.
The revenue attributable to our top customers on a quarterly basis has fluctuated in the past and may fluctuate in the future, especially in our Clinical Solutions product category, within which orders are on average of higher value than orders within our Lab Essentials category. This could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects. A substantial majority of our customers buy from us on a purchase order basis, and therefore these relationships are subject to termination. The termination of these relationships could result in a temporary or permanent loss of revenue.
Our future success depends on our ability to maintain these relationships, to increase our penetration among these existing customers and to establish new relationships. We engage in conversations with other companies and institutions regarding potential commercial opportunities on an ongoing basis, which can be time consuming. There is no assurance that any of these conversations will result in a commercial agreement, or if an agreement is reached, that the resulting relationship will be successful. Speculation in the industry about our existing or potential commercial relationships can be a catalyst for adverse speculation about us, our products, and our capabilities, which can adversely affect our reputation and our business. In addition, if our customers order our products but fail to pay on time or at all, our liquidity, financial condition, results of operations, cash flows, and prospects could be materially and adversely affected.
We compete with life science, pharmaceutical, and biotechnology companies, some of whom are our customers, who are substantially larger than we are and potentially capable of developing new approaches that could make our products and technology obsolete or develop their own internal capabilities that compete with our products, making it difficult for us to implement our strategies for revenue growth.
The market for biologics components products and services in the biopharmaceutical development, life science research, and diagnostics space is intensely competitive, rapidly evolving, significantly affected by new product introductions and other market activities by industry participants and subject to rapid technological change. We also expect increased competition as additional companies enter our market and as more advanced technologies become available. We compete with other providers of outsourced biologics components products and services. We also compete with the in-house discovery, development, and commercial manufacturing functions of pharmaceutical and biotechnology companies. Many of our competitors, which in some cases are also our customers, are large, well-capitalized companies with significantly greater resources and market share than we have. They may undertake their own development of products that are substantially similar to or compete with our products and they may succeed in developing products that are more effective or less costly than any that we may develop. Customers may believe that larger companies are better able to compete as sole source suppliers, and therefore prefer to purchase
22
from such businesses. Additionally, our competitors may be able to spend more aggressively on product and service development, marketing, sales and other initiatives than we can. Many of these competitors also have:
These factors, among others, may enable our competitors to market their products and services at lower prices or on terms more advantageous to customers than we can offer. Competition may result in price reductions, reduced gross margins, and loss of market share, any of which could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects. Moreover, consolidation trends in the pharmaceutical, biotechnology, and diagnostics industries have served to create fewer customer accounts and to concentrate purchasing decisions for some customers, resulting in increased pricing pressure on us. Additionally, our current and future competitors, including certain of our customers, may at any time develop additional products and services that compete with our products, and new approaches by these competitors may make our products, capabilities, and methodologies obsolete or noncompetitive. We may not be able to compete effectively against these organizations. Failure to anticipate and respond to competitors' actions may impact our future revenue and profitability.
Certain of our products are used by customers in the development and production of novel vaccines, therapies, and molecular diagnostics, some of which represent relatively new and still-developing modes of treatment and testing. Unforeseen adverse events, negative clinical outcomes, or increased regulatory scrutiny of these treatments and their financial cost may damage public perception of the safety, utility, or efficacy of these vaccines and therapies or other modes of treatment and may harm our customers’ ability to conduct their business. Such events may negatively impact our revenue and have an adverse effect on our performance.
Cell and gene therapy and mRNA vaccines remain relatively new and are under active development, with only a few cell and gene therapies and mRNA vaccines authorized or approved to date by regulatory authorities. Public perception may be influenced by claims that cell and gene therapy or mRNA vaccines are unsafe or ineffective, and cell and gene therapy may not gain the acceptance of the public or the medical community. In addition, ethical, social, legal, and financial concerns about cell and gene therapy and mRNA vaccines could result in additional regulations or limitations or even prohibitions on certain gene therapies or vaccine-related products, or reduced access to funding for our customers in these market segments. More restrictive regulations or negative public perception could reduce certain of our customers’ use of our products, which could negatively affect our revenue and performance. In addition, certain vaccine development and diagnostic testing programs utilize our bacterial cell culture media and our molecular biology reagents, which we manufacture subject to GMP requirements. There can be no assurance that any cell and gene therapy, vaccine programs, or diagnostic tests will proceed to clinical trials or result in a commercial product, or that any resulting gene therapies, vaccines, or diagnostic tests will incorporate or utilize our products.
Our products are highly complex and are subject to quality control and assurance requirements.
We believe all of our products are exempt from compliance with the U.S. Food, Drug, and Cosmetic Act (FDCA) and the current GMP regulations of the FDA, because all of our products are intended for research use only or for further processing by our customers. We do not make any claims related to the safety, effectiveness, or diagnostic utility of any of our products because they are not intended for clinical, therapeutic, or diagnostic use.
23
Nevertheless, the quality of our products is critical to our customers. We apply quality control procedures, including inspection of our products and/or the materials used in their manufacture, the verification of stability and/or performance, and, for certain products, additional validation procedures, whether a product we offer is designed and manufactured by us or purchased from outside suppliers. All of our quality control processes are administered under a system designed to adhere to aspects of ISO 13485:2016. Some of our customers also validate the products they purchase from us for their applications, and they may qualify us against their quality system requirements, which can include supplier questionnaires, quality agreements, and on-site audits. In the event we or our suppliers manufacture products that fail to comply with applicable quality standards or expectations, we may incur delays in fulfilling orders, recalls, and/or harm to our reputation.
If our customers do not qualify our quality systems, or if we are unable to maintain our ISO certification, our operating results could suffer.
We believe our quality system is adequate and that our activities comply with the qualification and technical standards established in our quality system. However, our customers often require that our quality system meets their qualification standards and that we be certified as in compliance with international quality standards, including with those set out in ISO 13485:2016. We are ISO 13485:2016 certified, and we must periodically pass audits in order to maintain certification. We may also encounter quality issues in the future as a result of the expansion or reconfiguration of existing manufacturing facilities, automation or other changes in our manufacturing processes, or the introduction of new products. We may be unable to obtain, or could experience delays in obtaining, customer qualification of our quality system. Any failure by us to obtain and maintain qualification of our quality systems by our customers, or to remain ISO certified, could have a material adverse effect on our business, financial condition, results of operations, cash flows, reputation, and prospects.
If we cannot provide quality technical and applications support, we could lose customers and our business and prospects would suffer.
The introduction of our products into our customers’ existing laboratory workflows and ongoing customer support for our products can be challenging. Accordingly, we need highly trained technical support personnel. Hiring technical support personnel is very competitive in our industry due to the limited number of people available with the necessary scientific and technical backgrounds and ability to understand our products and their uses at a technical level. To effectively support potential new customers and the expanding needs of current customers, we will need to substantially expand our technical support staff over time. If we are unable to attract, train, or retain the number of highly qualified technical services personnel that our business needs, our business and prospects will suffer.
We depend on a stable and adequate supply of quality raw materials from our suppliers, and price increases or interruptions of such supply could have an adverse impact on our business, financial condition, results of operations, cash flows, and prospects.
Our operations depend upon our ability to obtain raw materials at reasonable prices. If we are unable to obtain the materials we need at reasonable prices, whether due to inflation in the broader economy, supply chain disruptions, or other factors, we may not be able to produce certain of our products at marketable prices or at all, which could have a material adverse effect on our results of operations. Certain of our raw materials are sourced from a limited number of suppliers. For each of the years ended December 31, 2023 and 2022, purchases from suppliers making up 10% or more of our total inventory purchases represented 50% and 63% of total inventory purchases, respectively. However, we note that one of these suppliers is a distributor that sells products on behalf of a diversified supply chain where purchases from this supplier made up 40% and 37% of our total inventory purchases for the years ended December 31, 2023 and 2022. Delays or difficulties in securing these raw materials or other laboratory materials could result in an interruption in our production operations if we cannot obtain an acceptable substitute. Any such interruption could significantly affect our business, financial condition, results of operations, cash flows, and prospects.
Although we believe that we have stable relationships with our existing suppliers, we cannot ensure that we will be able to secure a stable supply of raw materials going forward. Our suppliers may not be able to keep up with our pace of growth or may reduce or cease their supply of raw materials to us at any time. While we may identify
24
other suppliers, raw materials we purchase from those replacement suppliers may require us to alter our production operations or perform extensive validations, which may be time consuming and expensive. In addition, we cannot assure you that our suppliers have obtained and will be able to obtain or maintain all licenses, permits, and approvals necessary for their operations or comply with all applicable laws and regulations, and the failure to do so by them may lead to interruption in their business operations, which in turn may result in shortages of raw materials supplied to us. Some of our suppliers are based overseas and therefore may need to maintain export or import licenses. If the supply of raw materials is interrupted, our business, financial condition, results of operations, cash flows, and prospects may be adversely affected.
If we are unable to manufacture or ship our products to meet demand, our operating results will suffer.
Our revenue and other operating results depend in large part on our ability to manufacture and ship our products in sufficient quantities and on specific timelines. Any interruptions we experience in the manufacturing or shipping of our products could delay our ability to recognize revenue in a particular quarter. Manufacturing problems can and do arise, and as demand for our products increases, any such problems could have an increasingly significant impact on our operating results. While we have not experienced significant problems with, or delays in our production capabilities that resulted in delays in our ability to ship finished products, there can be no assurance that we will not encounter such problems in the future. The COVID-19 pandemic caused disruptions to the global supply chain, affecting service providers, logistics, and the flow and availability of supplies and products. While not significant, we experienced some disruptions to parts of our supply chain as a result of the pandemic, and we could experience other such disruptions in the future. We also adjust our supply chain requirements based on changing customer needs and demands, and such adjustments could cause delays. We may not be able to ship products quickly and recognize anticipated revenue for a given period if we experience significant delays in the manufacturing process. In addition, we must maintain sufficient production capacity in order to meet anticipated customer demand, and we may be unable to offset the associated fixed costs if orders slow, which would adversely affect our operating margins. Furthermore, our customers rely on us for fast delivery of their custom-made formulations, and if our production timeline slows down, we may not be able to meet their expectations and our relationships could suffer. If we are unable to manufacture and ship our products consistently, in sufficient quantities and on a timely basis, our business, financial condition, results of operations, cash flows, and prospects will be materially and adversely affected.
Future strategic investments or transactions may require us to seek additional financing, which we may not be able to secure on favorable terms, if at all.
Over time, we may pursue various strategic investments and transactions, including licensing or acquiring products, technologies, or businesses complementary to our existing portfolio of products and services. We may need to seek additional financing to fund these strategic investments or transactions. Should we need to do so, we may not be able to secure such financing, or obtain such financing on favorable terms. In addition, future acquisitions may require the issuance or sale of additional equity, or equity-linked securities, which may result in additional dilution to our stockholders. The Amended Credit Agreement imposes significant restrictions on our ability to make acquisitions or certain other investments and our ability to make such acquisitions or other investments may be further limited by the terms of any future debt or preferred securities we may issue or any future credit facilities we may enter into.
Natural disasters (including earthquakes, fire, and drought), geopolitical unrest, war (including the war in Ukraine and the Israeli-Hamas war), terrorism, public health issues (like the COVID-19 pandemic) or other catastrophic events, some possibly related to the increasing effects of climate change, could disrupt the supply, production, delivery, or demand of our products, which could negatively affect our operations and performance.
We are subject to the risk of damage, destruction, and business disruption caused by the increasing effects of climate change; earthquakes, hurricanes, floods, droughts, and other natural disasters; fire; power shortages; geopolitical unrest, war, terrorist attacks and other hostile acts; public health issues, epidemics or pandemics, such as the COVID-19 pandemic; and other events beyond our control and the control of the third parties on which we depend. Any of these catastrophic events, whether in the U.S. or abroad, may have a significant negative impact on the global economy, our employees, facilities, critical equipment, partners, suppliers, distributors, or customers and could decrease demand for our products, create delays and inefficiencies in our supply chain and make it difficult or
25
impossible for us to manufacture products or perform services for our customers. We may not carry sufficient business insurance to compensate for damage or other losses relating to our facilities and equipment. In addition, any legislative or regulatory responses to these events, including to address the effects of or to mitigate climate change, could increase compliance costs and impose additional operating restrictions, each of which could have a negative impact on the Company's operations.
We rely upon our internal manufacturing, packaging, and distribution operations to produce many of the products we sell and on our warehouse facilities to store products pending sale. Our manufacturing and storage operations are located in California, near major earthquake faults, which makes us susceptible to earthquake and fire risks. A catastrophic event that results in damage to specific equipment that would be difficult to replace, in the destruction or disruption of our research and production facilities or in the disruption of our critical business or information technology systems would severely affect our ability to conduct normal business operations. Any disruptions in our operations could adversely affect our business, financial condition, and results of operations and harm our reputation. We may not carry sufficient business insurance to compensate for losses that may occur. Any such losses or damages could have a material adverse effect on our business, financial condition, and results of operations. In addition, the facilities of our suppliers and customers may be harmed or rendered inoperable by such catastrophic events, which may cause disruptions, difficulties or otherwise materially and adversely affect our business.
We rely upon the use of water to produce many of the products we sell, including the sale of water products themselves. Lack of sufficient water to manufacture our products could severely impact our operations and performance. Extended periods of drought in California may put pressure on the use and availability of water for manufacturing purposes, and in some cases, governmental authorities could divert, or already have diverted, water to other uses. As California has grown in population, there are increasing and multiple pressures on the use and distribution of water, which many view as a finite resource. We believe we have access to adequate supplies of water for our manufacturing operations and currently do not anticipate that future drought conditions will have a material impact on our operating results. However, if future drought conditions are worse than prior drought conditions or if regulatory responses to such conditions limit our access to water, our business could be negatively affected.
Because we rely heavily on third-party package-delivery services, a significant disruption in these services, damages or losses sustained during shipping or significant increases in prices could adversely affect our business, financial condition, results of operations, cash flows, and prospects.
We ship a significant portion of our products to our customers through independent package delivery companies, such as FedEx, UPS, and FedEx Freight. If one or more of these third-party package-delivery providers were to experience a significant service disruption, preventing our products from being delivered in a timely fashion or causing us to incur additional shipping costs we could not pass on to our customers, our costs could increase and our relationships with certain of our customers could be adversely affected. In addition, if one or more of these third-party package-delivery providers were to increase prices, and we were not able to find comparable alternatives or make adjustments to our delivery network, our business could be adversely affected. Furthermore, if one or more of these third-party package-delivery providers were to experience performance problems or other difficulties, it could negatively impact our operating results and our customers’ experience. In the past, some of our products have sustained serious damage in transit such that they were no longer usable. Although we have taken steps to improve our packaging and shipping containers, there is no guarantee our products will not become damaged or lost in transit in the future. If our products are damaged or lost in transit, it may result in a substantial delay in the fulfillment of our customers' orders and, depending on the type and extent of the damage, it may result in a substantial financial loss. If our products are not delivered in a timely, cost-effective fashion, or are damaged or lost during the delivery process, our customers could become dissatisfied and cease using our products, which would adversely affect our business, financial condition, results of operations, cash flows, and prospects.
If we are unable to continue to hire and retain skilled personnel, we will have trouble developing and marketing our products.
We are highly dependent, and our success depends largely, upon the continued service of our management, technical, and other staff and our ability to attract, retain, and motivate highly skilled personnel who deliver
26
high-quality and timely products and services to our customers. We also face significant competition in the hiring and retention of such personnel from other companies, other providers of outsourced biologics services, research and academic institutions, government and other organizations who have superior funding and resources. Each of our executive officers may terminate their employment with us at any time. The loss of key personnel or our inability to hire and retain skilled personnel could materially adversely affect the development of our products and our business, financial condition, results of operations, cash flows, and prospects. We do not maintain “key person” insurance for any of our executives or employees.
In addition, at times, we have relied on and may again utilize consultants to assist us in developing and implementing engineering and operational advancements. Our consultants and advisors may be contracted by companies other than ours and therefore may have commitments that may limit their availability to us.
Our corporate culture has contributed to our success, and if we cannot maintain this culture as we grow, we could lose the innovation, creativity, and teamwork fostered by our culture and our business may be harmed.
We believe that our culture has been and will continue to be a critical contributor to our success. If we do not continue to develop our corporate culture or maintain and preserve our guiding principles as we grow and evolve, we may be unable to foster the innovation, curiosity, creativity, focus on execution, teamwork, and the facilitation of critical knowledge transfer and knowledge sharing we believe we need to support our growth. Recent reductions in our workforce could result in a change to our corporate culture, which could harm our business.
We face risks arising from our recent workforce reductions, including adverse effects on employee morale, risks to our ability to meet customer demand with adequate turnaround times, and uncertainty around our ability to achieve anticipated cost savings from the workforce reductions.
During the roughly twelve-month period from February 2023 and January 2024, we undertook a strategic reduction in our workforce designed both to align the costs of our business with our near-term revenue expectations and to create operational and management-level efficiencies. These workforce reductions may result in unintended consequences, such as unwelcome attrition and reduced employee morale, which may cause our employees who were not affected by the reduction in workforce to seek alternative employment. Employees whose positions were eliminated or those who determine to seek alternative employment may seek employment with our competitors.
We cannot provide assurance that we will not undertake additional workforce reductions or that we will be able to realize the cost savings and other anticipated benefits from our previous or any future workforce reduction. In addition, our previous and any future workforce reductions, may adversely affect our ability to respond rapidly to any new product, growth, or revenue opportunities, to meet customer demand with adequate turnaround times, and otherwise to execute on our business plans. Additionally, reductions in workforce may make it more difficult to recruit and retain new employees. If we need to increase the size of our workforce in the future, we may encounter a competitive hiring market due to labor shortages, increased employee turnover, changes in the availability of workers, and increased wage costs.
We may enter into additional distribution arrangements and marketing alliances for certain products and services or certain geographic areas, and any failure to successfully identify and implement these arrangements on favorable terms, if at all, may impair our ability to effectively distribute and market our products.
We may pursue additional arrangements regarding the sales, marketing, and distribution of one or more of our products and our future revenue may depend, in part, on our ability to enter into and maintain successful arrangements with other companies having sales, marketing, and distribution capabilities. Any failure to enter into such arrangements and marketing alliances on favorable terms, if at all, could delay or impair our ability to distribute or market our products and could increase our costs of distribution and marketing. Any use of distribution arrangements and marketing alliances to commercialize our products will subject us to a number of risks, including the following:
27
Our success depends on the market acceptance of our bacterial cell culture media, specialized chromatography solutions, and molecular biology reagents, which we manufacture subject to GMP quality standards. Our bacterial cell culture media, specialized chromatography solutions, and molecular biology reagents may not achieve or maintain significant commercial market acceptance.
Our commercial success is dependent upon our ability to continue to successfully market and sell our bacterial cell culture media, specialized chromatography solutions, and molecular biology reagents, which are manufactured subject to GMP quality standards. Our ability to achieve and maintain market acceptance of our products will depend on a number of factors, including:
We cannot assure you that we will be successful in addressing these or other risk factors that might affect the market acceptance of our products. If we are unsuccessful in achieving and maintaining market acceptance of our products, our business, financial condition, results of operations, cash flows, and prospects could be adversely affected.
Our estimates of market sizes and opportunity may prove to be inaccurate, and even if the market in which we compete achieves the forecasted growth, our business could fail to grow at similar rates, if at all.
Addressable market estimates and growth forecasts, including the cell and gene therapy market as well as the molecular diagnostics and genomics markets, are subject to significant uncertainty and are based on assumptions and estimates that may be inaccurate. These estimates and forecasts are based on a number of complex assumptions and third-party estimates and other business data, including assumptions and estimates relating to our ability to
28
generate revenue from existing products and the development of new products and services. Our estimates and forecasts relating to the size and expected growth of our markets may prove to be inaccurate. Even if the markets in which we compete meet the size estimates and growth forecasted by us, our business could fail to grow at the rate we anticipate, if at all.
Product liability lawsuits against us could cause us to incur substantial liabilities, limit sales of our existing products, and limit commercialization of any products that we may develop.
Our business exposes us to the risk of product liability claims that are inherent in the development, production, distribution, and sale of biotechnology products. We face an inherent risk of product liability exposure related to the use of our products and product liability lawsuits may allege that our products failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of or inappropriate reliance upon, the information we provide in the ordinary course of our business activities. If any of our products harm people due to our negligence, willful misconduct, unlawful activities, or material breach, or if we cannot successfully defend ourselves against claims that our products caused injuries, we could incur substantial liabilities. While we seek to limit our product liability exposure, including in our contracts and terms and conditions of sale with our customers, we may not be successful in reducing or eliminating potential liability. Regardless of merit or eventual outcome, liability claims may result in the following, any of which could impact our business, financial condition, results of operations, cash flows, and prospects:
We maintain product liability insurance, but this insurance is subject to deductibles, limits, and exclusions and may not fully protect us from the financial impact of defending against product liability claims or the potential loss of revenue that may result. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future.
We are subject to stringent and evolving data privacy and information security laws, regulations, and standards, as well as policies and contractual obligations related to data privacy and security, and changes in these could adversely affect our business.
We are subject to data privacy and information security laws and regulations that apply to the collection, transmission, storage, and use of proprietary information and personal information. Failure to comply with any of these laws and regulations could result in enforcement actions against us, including fines, claims for damages by affected individuals, damage to our reputation, and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects. Additionally, if we are unable to properly protect the privacy and security of information, we could be found to have breached our contracts.
In the U.S., numerous federal and state laws, including state data breach notification laws and federal and state health information privacy and consumer protection laws, govern the collection, use, disclosure, and security of personal information. These laws continue to change and evolve and are increasing in breadth and impact. Many states in which we operate have laws that protect the privacy and security of personal information. For example, the California Consumer Privacy Act of 2018 (CCPA), which protects the privacy rights of California residents and imposes obligations on companies that process their personal information, came into effect on January 1, 2020. Among other things, the CCPA requires covered companies to provide disclosures to California residents and afford them data protection and privacy rights, including the ability to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for certain data breaches that
29
result in the loss of personal information. In addition, the California Privacy Rights Act (CPRA) took effect January 1, 2023. The CPRA amends the CCPA, giving California residents additional control over their personal information and imposing further obligations on businesses processing the personal information of California residents. The CPRA includes the creation of a privacy-specific enforcement agency, the first of its kind in any U.S. state, which will be responsible for enforcing the new law. These laws subject us to increased regulatory and overall risk. State laws are changing rapidly and there is discussion in Congress of a new federal data protection and privacy law to which we would become subject, if it is enacted. Without an overarching federal law driving privacy compliance in the U.S., however, a patchwork of privacy legislation formed by individual state laws could also create risks, similar to the patchwork created by differing state data breach notification obligations. Requirements to comply with varying state laws not only increase costs for compliance, but also create the potential for enforcement by individual state attorneys general.
Various foreign countries also have, or are developing, laws that govern the collection, use, disclosure, security, and cross-border transmission of personal information. The legislative and regulatory landscape for data privacy and information security continues to evolve, and there has been an increasing focus on data privacy and information security issues that have the potential to affect our business. To the extent applicable, we are, or could be subject to these laws, rules, and regulations, and we cannot guarantee that we are, or will be, in compliance with all applicable laws, rules, and regulations as they are enforced now or as they evolve.
We use third-party credit card processors to process payments from our customers. Through our agreements with our third-party credit card processors, we are subject to payment card association operating rules, including the Payment Card Industry Data Security Standard (PCI-DSS), which governs a variety of areas, including how consumers and customers may use their cards, the security features of cards, security standards for processing, data security and allocation of liability for certain acts or omissions, including liability in the event of a data breach. Any change in these rules or standards and related requirements could make it difficult or impossible for us to comply. Additionally, any data breach or failure to hold certain information in accordance with PCI-DSS may have an adverse effect on our business and results of operations.
It is possible that the laws governing data privacy and information security may be interpreted and applied in a manner that is inconsistent with our practices and our efforts to comply with the evolving data protection rules may be unsuccessful. We must devote significant resources to understanding and complying with this changing landscape. Failure to comply with U.S. federal and state and non-U.S. laws regarding data privacy and information security could expose us to penalties under such laws, orders requiring that we change our practices, claims for damages or other liabilities, regulatory investigations and enforcement action, litigation, and significant costs for remediation, any of which could adversely affect our business. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
Our internal computer systems, or those of our suppliers, customers, or contractors, have been and may in the future be subject to cyberattacks or security breaches, which could result in a material disruption of our business or otherwise adversely affect our business, financial condition, results of operations, cash flows, and prospects.
Despite the implementation of security measures, our internal computer systems and those of our suppliers, customers, and contractors, are vulnerable to damage from computer viruses and unauthorized access. We and our suppliers, including security and infrastructure suppliers, manage and maintain our data using a combination of on-site systems and cloud-based data centers. We face a number of risks related to protecting information, including inappropriate use or disclosure, unauthorized access or acquisition, or inappropriate modification of information. Cyberattacks are increasing in their frequency, sophistication, and intensity and have become increasingly difficult to detect. Cyberattacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering, and other means to affect service reliability and threaten the confidentiality, integrity, and availability of information. Cyberattacks also could include phishing attempts or e-mail fraud to cause unauthorized payments or information to be transmitted to an unintended recipient, or to permit unauthorized access to systems. A material cyberattack or security incident could cause interruptions in our operations and could result in a material
30
disruption of our business operations, damage to our reputation, financial condition, results of operations, cash flows, and prospects.
In the ordinary course of our business, we collect and store data that we are required to protect, including, among other things, personal information about our employees, credit card data, intellectual property, and proprietary business information. Any cyberattack or security incident that leads to the unauthorized access, acquisition, use or disclosure of personal or proprietary information could harm our reputation, cause us not to comply with U.S. federal and/or state, or non-U.S., data breach notification laws, or our contractual obligations, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information. In addition, we could be subject to risks caused by misappropriation, misuse, leakage, falsification, or intentional or accidental release or loss of information maintained in our information systems and networks and those of our suppliers, including personal information of our employees and Company, customer, and supplier confidential data. In addition, outside parties have previously attempted and may in the future attempt to penetrate our systems or those of our suppliers or fraudulently induce our personnel or the personnel of our suppliers to disclose information in order to gain access to our data and/or systems or make unauthorized payments to third parties. The number and complexity of these threats continue to increase over time. If a material breach of our information technology systems or those of our suppliers occurs, the market perception of the effectiveness of our security measures could be harmed and our reputation and credibility could be damaged.
Our insurance coverage may not be adequate to cover losses associated with security incidents, and in any case, such insurance may not cover all of the types of costs, expenses, and losses we could incur to address a security incident. As a result, we may be required to expend significant additional resources to protect against the threat of these issues or to alleviate problems caused by the same. In addition, we could be subject to regulatory actions and/or claims made by individuals and groups in private litigation related to data collection and use practices and other data privacy laws and regulations, including claims for misuse or inappropriate disclosure of data, as well as unfair or deceptive practices. Although we develop and maintain systems and controls designed to prevent these events from occurring, and we have a process to identify and mitigate threats, the development and maintenance of these systems, controls, and processes is costly and requires ongoing monitoring and updating as technologies change and efforts to overcome security measures become increasingly sophisticated. Moreover, despite our efforts, the possibility of these events occurring cannot be eliminated entirely and there can be no assurance that any measures we take will prevent cyberattacks or security incidents that could adversely affect our business, financial condition, results of operations, cash flows, and prospects.
We are dependent upon information technology systems, which are subject to disruption, damage, and failure.
To conduct our business, we rely on information technology systems, networks, and services, many of which are managed, hosted, and provided by third parties. System failure, malfunction, or loss of data that is housed in the Company’s or its third-party service providers’ critical information systems could disrupt our ability to perform critical functions, which in turn could materially and adversely affect our business and operating results, financial position, and cash flows. Our information systems could be damaged or cease to function properly due to a number of other reasons, including power outages or other catastrophic events. As a result, we may experience interruptions in our ability to manage our daily operations, which could adversely affect our business, financial condition, and results of operations.
Changes in political, economic, or governmental regulations may reduce demand for our products or increase our expenses.
We compete in many markets in which we and our customers must comply with federal, state, local and international regulations, such as environmental, health and safety, and food and drug regulations. We develop, configure, and market our products to meet customer needs created by those regulations. The U.S. and international healthcare industry is subject to changing political, economic, and regulatory influences that could significantly affect the drug development process, research and development costs, and the pricing and reimbursement for pharmaceutical and other therapeutic products. Any significant change in regulations could have an adverse effect on both our customers’ business and our business, which could result in reduced demand for our products and
31
services or increases in our expenses. For example, we provide products used for basic research and input components used by biopharmaceutical customers for further processing.
Changes in the FDA’s regulation of the drug discovery and development process may have a negative impact on the ability of our customers to conduct and fund clinical trials, which could have a material adverse effect on the demand for the products we supply to these customers. Additionally, the U.S. government and governments worldwide have increased efforts to expand healthcare coverage while at the same time curtailing and better controlling the increasing costs of healthcare. If cost-containment efforts limit our customers’ profitability, they may decrease research and development spending, which could decrease the demand for our products and materially adversely affect our growth prospects. Any of these factors could harm our customers’ businesses, which, in turn, could materially adversely affect our business, financial condition, results of operations, cash flows, and prospects.
Future acquisitions, if any, may expose us to risks that could adversely affect our business, and we may not achieve the anticipated benefits of acquisitions of businesses or technologies.
We may, in the future, make selected opportunistic acquisitions of complementary businesses, products, services, or technologies. Any acquisition involves numerous risks, uncertainties, and operational, financial, and managerial challenges, including the following, any of which could adversely affect our business, financial condition, results of operations, cash flows, and prospects:
32
In addition, the successful integration of acquired businesses requires significant effort and expense across all operational areas, including sales and marketing, research and development, manufacturing, finance, legal, and information technologies. We may not be able to identify or complete promising acquisitions for many reasons, including competition among buyers, the high valuations of businesses in our industry, the need for regulatory and other approvals, and the availability of capital. Even if we are able to complete acquisitions in the future, there can be no assurance that such acquisitions will be successful or profitable. Our failure to successfully address the foregoing risks may prevent us from achieving the anticipated benefits from any future acquisitions in a reasonable time frame, or at all.
We and our customers’ respective business operations are and will continue to be subject to extensive laws and regulations, and assessing the applicability and relevant requirements of, and maintaining compliance with, these laws and regulations can be expensive and time consuming.
We are subject to various local, state, federal, foreign, and transnational laws and regulations, and, in the future, any changes to such laws and regulations could adversely affect us. We offer certain products that may be deemed medical devices and become subject to related regulation. Additionally, we provide products used by third parties for the development and commercialization of drug therapies, novel vaccines, and molecular diagnostics by biopharmaceutical companies, life science research companies, CROs, CDMOs, in vitro diagnostics franchises, laboratories, and academic and government research institutions that are also subject to extensive regulation.
The quality of our products is critical to our customers, including researchers looking to develop novel vaccines and drug therapies and for biopharmaceutical customers who use our products as components in their preclinical studies and clinical trials. Biopharmaceutical customers are subject to extensive regulations by the FDA and similar regulatory authorities in other countries for conducting clinical trials and commercializing products for therapeutic or diagnostic use. This regulatory scrutiny results in our customers imposing rigorous quality requirements on us as their supplier through supplier qualification processes and customer contracts, including quality agreements. Regulatory authorities and our customers may conduct scheduled or unscheduled periodic inspections of our facilities to monitor our regulatory compliance or compliance with our quality agreements with our customers. There are significant risks at each stage of the regulatory scheme for our customers.
Regulatory agencies may in the future take action against us or our customers for failure to comply with applicable regulations governing clinical trials and the development and testing of diagnostic and therapeutic products, as well as requirements to fall within certain regulatory categories to qualify for exemption from marketing authorization, or where applicable to obtain clearance, authorization, or approval prior to marketing of regulated products. Failure by us or by our customers to comply with the requirements of these regulatory authorities, including without limitation, remediating any inspectional observations to the satisfaction of these regulatory authorities, could result in warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution, restrictions on our operations, civil or criminal sanctions, or withdrawal of existing or denial of pending approvals, including those relating to products or facilities. In addition, such a failure could expose us to contractual or product liability claims, contractual claims from our customers, including claims
33
for reimbursement, as well as ongoing remediation and increased compliance costs, any or all of which could be significant.
We are also subject to a variety of federal, state, local, and international non-U.S. laws and regulations that govern, among other things, the importation and exportation of products, the handling, transportation, and manufacture of substances that could be classified as hazardous, and our business practices in the U.S. and abroad such as anti-corruption and anti-competition laws. Any noncompliance by us with applicable laws and regulations or the failure to maintain, renew, or obtain necessary permits and licenses could result in criminal, civil, and administrative penalties and could have an adverse effect on our results of operations.
Establishing policies, procedures, and monitoring and oversight with consideration of both legal requirements and industry best practices in these areas are costly and time consuming. Defending against any actions for non-compliance of such laws can also be costly, time consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
Our products could become subject to regulation by the FDA or other regulatory agencies in the future, which could increase our costs and delay or prevent commercialization of our products, thereby materially and adversely affecting our business, financial condition, results of operations, cash flows, and prospects.
We voluntarily follow the quality standards set out in specific sections of ISO 13485:2016 for the manufacture of our products. Nevertheless, we believe all of our products, including those we market as “GMP-grade” or as being within our “Clinical Solutions” category of products, are exempt from FDA regulations applicable to medical devices and drugs because all of our products are ancillary materials and reagents that are intended for research use or for further processing by our customers. We believe our products are properly labeled and marketed as such. The FDA could nonetheless disagree and conclude that our products are in fact subject to the FDCA and decide to take enforcement action against us, including requiring us to stop the sale of our products until we comply, which would adversely affect our business, financial condition, results of operations, cash flows, and prospects. There can be no assurance that the FDA would find our operations to be in compliance in a timely manner, or at all, and our results of operations could suffer.
In addition, we make certain of our products available to customers as RUO products. Those products must bear a label with the statement: “For Research Use Only,” and companies must comply with the FDA’s November 2013 Final Guidance for Industry and Food and Drug Administration Staff on “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only” (RUO/IUO Guidance) when labeling and marketing RUO products. The FDA could disagree with our assessment that our RUO products are properly labeled and marketed as RUO or could conclude that our products labeled and marketed as RUO are actually intended for diagnostic or clinical use. The FDA could take enforcement action against us under the FDCA, including requiring us to stop the sale of our RUO products until we are in compliance with applicable regulations, which would adversely affect our business, financial condition, results of operations, cash flows, and prospects. There can be no assurance that we could come into compliance with those regulations in a timely manner, or at all.
We rely on assumptions, estimates, and data to calculate certain of our key metrics, and real or perceived inaccuracies in such metrics may harm our reputation and negatively affect our business.
In addition to our financial results, our management regularly reviews a number of operating and financial metrics to evaluate our business, measure our performance, identify trends affecting our business, formulate financial projections, and make strategic decisions. As both the industry in which we operate and our business continue to evolve, so too might the metrics by which we evaluate our business and the Company. In addition, while the calculation of these metrics is based on what we believe to be reasonable estimates, our internal tools are not independently verified by a third party and have a number of limitations. Our methodologies for tracking these metrics may also change over time. If these metrics are not accurate representations of our business or perceived to be accurate, or if we discover material inaccuracies with respect to these figures, our reputation may be significantly harmed, and our operating and financial results could be negatively impacted. Accordingly, investors should not place undue reliance on these metrics.
34
We have recorded, and may be required to record in the future, a significant charge to earnings if our intangible or long-lived assets, or other investments become impaired.
We are required under U.S. generally accepted accounting principles (GAAP) to test indefinite lived intangibles for impairment at least annually and to review our intangible assets, and other assets for impairment when events or changes in circumstance indicate the carrying value may not be recoverable. Factors that could lead to impairment of intangible or long-lived assets, or other investments include significant adverse changes in the business climate and actual or projected operating results (affecting our company as a whole or affecting any particular segment) and declines in the financial condition of our business.
During the year ended December 31, 2022, the market price of our common stock and market capitalization declined significantly. Given the significance of this decline, we performed interim goodwill impairment testing. As a result of that testing, we determined goodwill was fully impaired and recorded an impairment charge of $16.6 million during the year ended December 31, 2022, adversely impacting our financial results.
As of December 31, 2023, intangible assets represented approximately 11% of our total assets. In addition, in the future we may acquire other businesses, products, or technologies as well as pursue strategic alliances, join ventures, technology licenses, or investments in complementary businesses, resulting in goodwill and other intangible assets. Such goodwill and intangible assets must be tested and reviewed as described above. If in the future we again determine that there has been impairment, we may be required to record charges to earnings, and our financial results for the relevant period would be reduced by the amount of the impairment, net of tax effects, if any.
Similarly, long-lived assets must be evaluated for impairment when events or changes in circumstances indicate a possible inability to recover carrying amounts. During the years ended December 31, 2023 and 2022, we recorded impairment charges related to long-lived assets of $2.2 million and $4.2 million, respectively. If in the future we again determine that there has been impairment to long-lived assets, we may be required to record charges to earnings, and our financial results for the relevant period would be reduced by the amount of the impairment, net of tax effects, if any.
Changes in accounting principles and guidance could result in unfavorable accounting charges or effects.
We prepare our financial statements in accordance with GAAP. These principles are subject to interpretation by the SEC and various bodies formed to create and interpret appropriate accounting principles and guidance. The adoption of new or revised accounting principles may require us to make changes to our systems, processes, and internal controls, which could have a significant effect on our reported financial results and internal controls, cause unexpected financial reporting fluctuations, retroactively affect previously reported results, or require us to make costly changes to our operational processes and accounting systems upon our following the adoption of these standards.
For example, in 2016, the Financial Accounting Standards Board issued Accounting Standard Update No. 2016-02, Leases, and its related interpretations, which as updated requires lessees to generally recognize operating and financing lease liabilities and corresponding right-of-use assets on the balance sheet. We adopted this new standard effective January 1, 2022, using the modified retrospective approach, applied at the beginning of the period of adoption, and elected the package of transitional practical expedients. The adoption of this standard resulted in recording operating right-of-use lease assets of $20.3 million, which included reclassifying approximately $0.2 million of deferred rent as a component of the operating lease asset as of January 1, 2022. The adoption also resulted in recording operating lease liabilities of $20.5 million as of January 1, 2022. The standard did not have an impact on the condensed statements of operations, and cash flows.
Our revenue recognition and other factors may impact our financial results in any given period and make them difficult to predict.
Under Accounting Standards Codification (ASC) 606, Revenue from Contracts with Customers, (ASC 606), we recognize revenue when our performance obligations have been satisfied in an amount that reflects the consideration that we expect to receive in exchange for those performance obligations. Our revenue primarily includes revenue from the sale of manufactured products, including products from our catalog or available for purchase on our website, and custom manufactured products (such as custom bacterial cell culture media and specialized chromatography solutions). Contracts for such sales contain a single performance obligation, namely the
35
delivery of consumable products. Our application of ASC 606 with respect to the nature of future contractual arrangements could impact the forecasting of our revenue for future periods, as both the mix of products we will sell in a given period, as well as the size of contracts, is difficult to predict.
Furthermore, the presentation of our financial results requires us to make estimates and assumptions that may affect revenue recognition. In some instances, we could reasonably use different estimates and assumptions, and changes in estimates may occur from period to period. See the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates—Revenue Recognition.”
Given the foregoing factors, comparing our revenue and operating results on a period-to-period basis may not be meaningful, and our past results may not be indicative of our future performance.
Our ability to use net operating loss carryforwards to reduce future tax payments may be limited.
As of December 31, 2023, we had $50.2 million of U.S. federal and $53.6 million of state net operating loss carryforwards (NOLs) available to reduce taxable income in future years. Our ability to utilize those NOLs may be limited based on our operating performance and tax laws in effect at the time of the proposed use. Under the Tax Cuts and Jobs Act (the Tax Act), as modified by the Coronavirus Aid, Relief, and Economic Security Act (the CARES Act), federal NOLs incurred in taxable years beginning after December 31, 2017, may be carried forward indefinitely, but the deductibility of such federal NOLs in taxable years beginning after December 31, 2020, is limited to 80% of taxable income. It is uncertain if and to what extent various states will conform to the Tax Act or the CARES Act.
Separately, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, and corresponding provisions of state law, if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50% change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOLs and other pre-change tax attributes, such as research tax credits, to offset its post-change income or taxes may be limited. We may experience ownership changes as a result of subsequent shifts in our stock ownership, some of which may be outside of our control. As a result, our ability to use our pre-change NOLs to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
In evaluating our ability to recover our deferred tax assets, in full or in part, we consider all available positive and negative evidence, including our past operating results, and our forecast of future earnings, future taxable income and prudent and feasible tax planning strategies. The assumptions utilized in determining future taxable income require significant judgment and are consistent with the plans and estimates we are using to manage the underlying business. Actual operating results in future years could differ from our current assumptions, judgments and estimates. For the year ended December 31, 2023, we recorded a net increase in valuation allowances of $9.7 million comprised primarily of additional valuation allowance on certain operating losses being carried forward which are not expected to be realizable.
Our business is subject to risks relating to environmental, health, and safety laws and regulations.
We are subject to environmental, health, and safety laws and regulations, incur costs to comply with such laws and regulations, and could be exposed to liabilities or other obligations imposed under such laws or regulations. The costs of compliance with environmental, health, and safety laws and regulations are significant. Any violations, even if inadvertent or accidental, of current or future environmental, health, and safety laws or regulations, and the cost of compliance with any resulting order or fine, could adversely affect our business, financial condition, and results of operations.
36
Our management has limited experience in operating a public company.
Our executive officers have limited experience in the management of a publicly traded company. Our management team may not successfully or effectively manage our ongoing transition to a mature public company that will be subject to significant regulatory oversight and reporting obligations under federal securities laws. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage because they may devote more time to these activities rather than to the management and growth of our business.
We may not have adequate personnel with the appropriate knowledge, experience, and training in the accounting policies, practices, or internal controls over financial reporting required of public companies in the U.S. The development and implementation of the standards and controls necessary for us to meet the accounting standards required of a public company in the U.S. may require costs greater than expected.
Risks Related to Our Intellectual Property
Our inability to protect our intellectual property could have a material adverse effect on our business. In addition, third parties may claim that we infringe or have disclosed their intellectual property or proprietary information, and we could suffer significant litigation or licensing expense as a result.
Our success depends on our ability to obtain and maintain intellectual property protection in the U.S. and other countries with respect to our proprietary products. We rely primarily upon a combination of trade secret protection and confidentiality agreements to protect our technology, manufacturing processes, and products. Our commercial success depends in part on obtaining and maintaining trade secret protection of our current and future products, if any, and the methods used to manufacture them, as well as successfully defending such trade secrets against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell, or importing our products is dependent upon the extent to which we have valid and enforceable intellectual property rights that cover these activities.
Although we do not currently own any issued patents covering our proprietary products or manufacturing processes, we may in the future file patent applications or acquire or license intellectual property rights, including patents and patent applications. The patent prosecution process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or in all jurisdictions where protection may be commercially advantageous. It is also possible that we may fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. In addition, we or our collaborators may only pursue, obtain, or maintain patent protection in a limited number of countries. Even if patents do successfully issue, such patents may not adequately protect our intellectual property, provide exclusivity for our current or future product and service offerings, prevent others from designing around our claims, or otherwise provide us with a competitive advantage.
Additionally, the laws of some countries do not protect intellectual property rights to the same extent as the laws of the U.S., and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of intellectual property rights, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement, misappropriation, or other violation of our intellectual property rights, including the unauthorized use or reproduction of our manufacturing or other trade secrets. Any of these outcomes could impair our ability to prevent competition from third parties, which may have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
We rely primarily on trade secret laws, as well as confidentiality and non-disclosure agreements, and other contractual protections, to protect our intellectual property. If we are unable to protect the confidentiality of our intellectual property, the value of our technology and products could be materially adversely affected.
In addition to trade secret protection, we also rely on confidentiality agreements with our employees, consultants, contractors, collaborators, CDMOs, CROs, and others to protect our technology and other proprietary information. These agreements require that all confidential information developed by the individual or entity or made known to the individual or entity by us during the course of the individual’s or entity’s relationship with us be
37
kept confidential and not disclosed to third parties. Our agreements with employees as well as our personnel policies also generally provide that any inventions conceived by the individual in the course of rendering services to us shall be our exclusive property or that we may obtain full rights to such inventions at our election. However, trade secrets are difficult to protect.
Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, collaborators, CDMOs, CROs, and others may unintentionally or willfully disclose our proprietary information to competitors, notwithstanding the existence of a valid confidentiality or similar non-disclosure agreement. We also face the risk that present or former employees could continue to hold rights to intellectual property used by us, demand the registration of intellectual property rights in their name, and seek payment of damages for our use of such intellectual property.
Enforcing a claim that a third party illegally obtained or is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. We may not have adequate remedies in the event of unauthorized use or disclosure of our trade secrets or other proprietary information in the case of a breach of any such agreements, and our trade secrets and other proprietary information could be disclosed to third parties, including our competitors. Many of our partners also collaborate with our competitors and other third parties. The disclosure of our trade secrets to our competitors, or more broadly, would impair our competitive position and may materially harm our business, financial condition, results of operations, cash flows, and prospects. Costly and time-consuming litigation could be necessary to determine the scope of and enforce our rights, and failure to maintain trade-secret protection could adversely affect our competitive business position. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction. Courts outside the U.S. are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop substantially equivalent or superior knowledge, methods, and know-how, and the existence of our own trade secrets affords no protection against such independent discovery.
If we are sued for infringing, misappropriating, or otherwise violating intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing our current or future products.
Our products may infringe on, or be accused of infringing on, one or more claims of an issued patent or may fall within the scope of one or more claims in a published patent application that may subsequently be issued and to which we do not hold a license or other rights.
Because patent applications in the U.S. and many foreign jurisdictions typically are not published until 18 months after filing, or in some cases not at all, and publications in the scientific literature often lag behind actual discoveries, we cannot be certain whether others have filed patent applications for technology that we were the first to invent. Others, including our competitors, may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over any future patent applications or patents that we may file or obtain, which could further require us to obtain rights to issued patents by others covering such technologies. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the United States Patent and Trademark Office (the USPTO) to determine priority of invention in the U.S. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful if, unbeknownst to us, the other party had independently arrived at the same or similar invention prior to our own invention, resulting in a loss of our U.S. patent position with respect to such inventions.
Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our current or future products or the use of our current or future products. After issuance, the scope of patent claims remains subject to construction based on interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. These third parties could bring claims against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages.
38
The life sciences industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Third parties have, and may in the future have, U.S. and non-U.S. issued patents and pending patent applications that may cover our current or future products. Such a third party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third party’s patent rights and may go to court or a tribunal to stop us from engaging in our normal operations and activities, including making or selling our current or future products. In the event that any of these patent rights were asserted against us, we believe that we may have defenses against any such action, including that such patents would not be infringed by our current or future products and/or that such patents are not valid. However, if any such patent rights were to be asserted against us and our defenses to such assertion were unsuccessful, unless we obtain a license to the patents concerned, we could be liable for damages, which could be significant and include treble damages and attorneys’ fees if we are found to have willfully infringed. We could also be precluded from commercializing any future products that were ultimately held to infringe such patents, all of which could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
If we are found to infringe the patent rights of a third party, or in order to avoid potential claims, we or our collaborators may choose or be required to seek a license from a third party and be required to pay license fees or royalties or both. These licenses may not be available on reasonable terms, or at all. In particular, any of our competitors that control intellectual property that we are found to infringe may be unwilling to provide us a license under any terms. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent. Further, if a patent infringement suit is brought against us or our third-party service providers and if we are unable to successfully obtain rights to required third-party intellectual property, we may be required to expend significant time and resources to redesign our current or future products, or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis, and may delay or require us to abandon our development, manufacturing or sales activities relating to our current or future products. A finding of infringement could prevent us from commercializing our future products or force us to cease some of our business operations, which could harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
Intellectual property litigation and other proceedings could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, intellectual property litigation or other legal proceedings relating to our, our licensors’ or other third parties’ intellectual property claims may cause us to incur significant expenses and could distract our personnel from their normal responsibilities. Patent litigation and other proceedings may also absorb significant management time. If not resolved in our favor, litigation may require us to pay any portion of our opponents’ legal fees. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Our competitors or other third parties may be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially greater resources. Uncertainties resulting from our participation in patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Furthermore, because of the substantial amount of discovery required in certain jurisdictions in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, the
39
perceived value of our current or future products or intellectual property could be diminished. Accordingly, the market price of our common stock may decline. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our business, financial condition, results of operations, and prospects.
We may be subject to claims by third parties asserting that we or our employees, consultants, or independent contractors have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property and proprietary technology.
We try to ensure that our employees do not use the proprietary information or know-how of others in their work for us. We may, however, be subject to claims that we or these employees have inadvertently or otherwise used or disclosed intellectual property, trade secrets, or other proprietary information of any such employee’s former employer or that patents and applications we have filed to protect inventions of these individuals, even those related to one or more of our current or future products, are rightfully owned by their former or concurrent employer. Litigation may be necessary to defend against these claims.
In addition, while we typically require our employees, consultants, and independent contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, or such agreements may be breached or alleged to be ineffective, and the assignment may not be self-executing, which may result in claims by or against us related to the ownership of such intellectual property or may result in such intellectual property becoming assigned to third parties. If we fail in enforcing or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business, financial condition, results of operations, cash flows, and prospects may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names or marks, which we need for name recognition by potential partners or customers in our markets of interest. During trademark registration proceedings, we may receive rejections. Although we would be given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business, financial condition, results of operations, cash flows, and prospects may be adversely affected.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our proprietary and intellectual property rights is uncertain because such rights offer only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
40
Should any of these events occur, they could significantly harm our business, financial conditions, results of operations, cash flows, and prospects.
We may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our current or future products, and we cannot provide any assurances that we would be able to do so.
We may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our current or future products, and we cannot provide any assurances that third-party patents do not exist that might be enforced against our current or future products in the absence of such a license. We may fail to obtain any of these licenses on commercially reasonable terms, if at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. If we could not obtain a license, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products, which could materially harm our business. The third parties owning such intellectual property rights could also seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation.
Licensing of intellectual property involves complex legal, business, and scientific issues. Disputes may arise between us and our licensors regarding intellectual property subject to a license agreement, including:
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain the licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product successfully, or the dispute may have an adverse effect on our results of operations.
We may, in the future, grant licenses under our intellectual property. Like in-licenses, out-licenses are complex, and disputes may arise between us and our licensees, such as the types of disputes described above. Moreover, our licensees may breach their obligations, or we may be exposed to liability due to our failure or alleged failure to satisfy our obligations. Any such occurrence could have an adverse effect on our business.
41
Risks Related to Our Indebtedness
Our existing indebtedness could adversely affect our business and growth prospects.
Our indebtedness, including the indebtedness we have incurred, and may in the future incur, under the Amended Credit Agreement or otherwise, could require us to divert funds identified for other purposes to debt service and impair our liquidity position. If we cannot generate sufficient cash flow from operations to service our debt, we may need to refinance our debt, dispose of assets, or issue equity to obtain necessary funds. We do not know whether we will be able to take any of these actions on a timely basis, on terms satisfactory to us, or at all.
Our indebtedness, the cash flow needed to service our debt, and the covenants contained in the Amended Credit Agreement may have important consequences, including:
The U.S. Federal Reserve has raised, and may again raise, interest rates in response to concerns about inflation. Fluctuations in interest rates, as a result of actions by the Federal Reserve or otherwise, can increase borrowing costs. Increases in interest rates, such as those increases observed during 2023, may directly impact the amount of interest we are required to pay and reduce earnings accordingly. In addition, tax laws, including the disallowance or deferral of tax deductions for interest paid on outstanding indebtedness, could have an adverse effect on our liquidity and our business, financial condition, results of operations, cash flows, and prospects. In addition, our Amended Credit Agreement contains customary affirmative and negative covenants and certain restrictions on operations that could impose operating and financial limitations and restrictions on us, including restrictions on our ability to enter into particular transactions and to engage in other actions that we may believe are advisable or necessary for our business.
We expect to use cash on hand to meet current and future financial obligations, including funding our operations, debt service requirements, and capital expenditures. The ability to meet these obligations depends on our financial and operating performance, which is subject to prevailing economic, industry, and competitive conditions and to certain financial, business, economic, and other factors beyond our control and as set forth herein.
Despite current indebtedness levels, we may incur substantially more indebtedness, which could further exacerbate the risks associated with our substantial indebtedness.
We may incur significant additional indebtedness in the future, including in connection with investments in joint ventures or acquisitions. If new debt is added to our current indebtedness levels, the related risks that we face could intensify.
The terms of the Amended Credit Agreement may restrict our current and future operations, particularly our ability to respond to changes or to take certain actions. If we fail to comply with the covenants and other obligations under the Amended Credit Agreement, the lender may be able to accelerate amounts owed under the facilities and may foreclose upon the assets securing our obligations.
The Amended Credit Agreement contains a number of restrictive covenants that impose significant operating and financial restrictions on us and may, unless waived by the lender, limit our ability to engage in acts that may be in our long-term best interests, including restrictions on our ability to:
42
These restrictions could limit, potentially significantly, our operational flexibility and affect our ability to finance our future operations or capital needs or to execute our business strategy. Our indebtedness under the Amended Credit Agreement is secured by substantially all of our assets. If we fail to comply with the covenants and our other obligations under the Amended Credit Agreement, the lender would be able to accelerate the required repayment of amounts due and, if they are not repaid, could foreclose upon the assets securing our obligations with respect to such indebtedness.
Risks Related to Our Common Stock
Telegraph Hill Partners Management Company LLC, through its affiliates THP LP and THP LLC, controls us, and its interests may conflict with ours or yours in the future.
THP controls approximately 62.8% of the voting power of our outstanding common stock, which means that THP can control the vote of all matters submitted to a vote of our stockholders. This control enables THP to control the election of the members of our board of directors and all other corporate decisions. In particular, for so long as THP continues to own a majority of our common stock, THP will be able to cause or prevent a change of control of us or a change in the composition of our board of directors and could preclude any unsolicited acquisition of us. Pursuant to our investors’ rights agreement and our amended and restated certificate of incorporation, THP has certain rights, and the ability to take certain actions, that are not otherwise available to all stockholders. For example, our investors’ rights agreement provides THP the right, subject to certain conditions, to demand that we file a registration statement or request that their shares of our common stock be covered by a registration statement that we are otherwise filing. In addition, until such time as THP first ceases to own greater than 50% of the outstanding voting power of our common stock, our amended and restated certificate of incorporation effectively provides THP with the ability to fill vacancies on the board, remove directors (with or without cause), call a special meeting of our stockholders, amend our certificate of incorporation (subject to approval of our board of directors) and amend our bylaws. Even when THP ceases to control a majority of the total voting power, for so long as THP continues to own a significant percentage of our common stock, THP will still be able to significantly influence the composition of our board of directors and the approval of actions requiring stockholder approval. Accordingly, for such period of time, THP will have significant influence with respect to our management, business plans, and policies. The concentration of ownership and availability of the foregoing rights could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of us and ultimately might affect the market price of our common stock.
THP and its affiliates engage in a broad spectrum of activities, including investments in our industry generally. In the ordinary course of their business activities, THP and its affiliates may engage in activities where their interests conflict with our interests or those of our other stockholders, such as investing in or advising businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours. Our amended and restated certificate of incorporation provides that none of THP and its affiliates and any person or entity who, while a stockholder, director, officer, or agent of the Company or any of its affiliates, is a director, officer, principal, partner, member, manager, employee, agent, and/or other representative of THP and its affiliates (each, an Identified Person) has any duty to refrain from (i) engaging in a corporate opportunity in the same or similar business activities or lines of business in which we or our affiliates are engaged or that are deemed to be competing with us or any of our affiliates or (ii) otherwise investing in or providing services to any person that competes with us or our affiliates or engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. In addition, to the fullest extent permitted by law, no Identified Person has any obligation to offer to us or our subsidiaries or affiliates the right to participate in any corporate opportunity in the same or similar business activities or lines of business in which we or our affiliates are engaged or that are deemed to be competing with us or any of our affiliates. This means that THP may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities
43
may not be available to us. In addition, THP may have an interest in pursuing acquisitions, divestitures, and other transactions that, in its judgment, could enhance its investment, even though such transactions might involve risks to you or may not prove beneficial.
Our shares of common stock are listed on the Nasdaq Global Market, and we are a “controlled company” within the meaning of the rules and listing standards of The Nasdaq Stock Market LLC (Nasdaq). As a result, we qualify for, and intend to rely on, exemptions from certain corporate governance requirements. Our stockholders therefore do not have the same protections as those afforded to stockholders of companies that are subject to such governance requirements.
THP controls a majority of the voting power of our outstanding common stock. As a result, we are a “controlled company” within the meaning of the corporate governance standards of Nasdaq. Under these corporate governance standards, a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements. For example, controlled companies, within one year of the date of the listing of their common stock:
We utilize, and intend to continue to utilize, certain of these exemptions. For example, a majority of our directors have not been, and in the future for so long as we rely on such exemptions, will not be, affirmatively determined to be independent, nor will our compensation committee or nominating and corporate governance committee of the board be comprised entirely of directors who have been determined to be independent. Accordingly, our stockholders do not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
If we fail to comply with Nasdaq listing rules or California laws governing the diversity of our board of directors, we could be exposed to financial penalties and suffer reputational harm.
In August 2021, the SEC announced that it had approved Nasdaq’s proposed rule change to advance board diversity and enhance transparency of board diversity statistics through new listing requirements. Under these new listing rules, Nasdaq listed companies will be required, subject to certain exceptions, to disclose annually diversity statistics regarding their directors’ voluntary self-identified characteristics and include on their boards of directors at least two “Diverse” directors or publicly disclose why their boards do not include such “Diverse” directors. Under the phase-in period for these new listing rules, for companies like ours that are listed on the Nasdaq Global Market, this disclosure requirement regarding the existence of at least one “Diverse” director applies starting on the later of August 7, 2023, or the date that the company files its proxy statement for its annual shareholder meeting during 2023, and regarding the existence of at least two “Diverse” directors applies starting on the later of August 6, 2025, or the date that the company files its proxy statement for its annual shareholder meeting during 2025. Under the proposed rule, a “Diverse” director is someone who self-identifies either as (i) female or (ii) Black or African American, Hispanic or Latinx, Asian, Native American or Alaska Native, Native Hawaiian or Pacific Islander, or two or more races or ethnicities, or (iii) lesbian, gay, bisexual, transgender or a member of the queer community. Smaller reporting companies, such as Teknova, can satisfy the Nasdaq rules by having two females on its board.
In addition, in September 2018, California’s Senate Bill 826 (SB 826) was signed into law. SB 826 generally requires public companies with principal executive offices in California to have a minimum number of females on its board of directors. As of December 31, 2021, each public company was required to have at least two females on its board of directors if the company had at least five directors, and at least three females on its board of directors if the company had at least six directors as of December 31, 2021. On May 13, 2022, the Superior Court of California
44
for the County of Los Angeles entered an order striking down SB 826, holding that the statute violates the Equal Protection Clause of the California Constitution. The California Secretary of State has appealed the order and such appeal is currently pending. On September 16, 2022, the appellate court ruled to temporarily stay enforcement of the trial court’s order, which prevented the California Secretary of State from collecting diversity data on corporate disclosure forms pursuant to SB 826, pending a further order of the appellate court. On December 1, 2022, the appellate court vacated the temporary stay order and on February 3, 2023, a record on appeal was filed and such appeal is currently pending. To the extent that this ruling of the appellate court permits the Secretary of State of California to collect and report diversity data, we may be required to comply with additional disclosure requirements. However, ultimate enforceability of SB 826 remains uncertain.
Additionally, on September 30, 2020, Assembly Bill 979 (AB 979) was signed into law. AB 979 generally requires public companies with principal executive offices in California to include specified numbers of directors from “underrepresented communities.” A director from an “underrepresented community” means a director who self-identifies as Black, African American, Hispanic, Latino, Asian, Pacific Islander, Native American, Native Hawaiian, Alaska Native, gay, lesbian, bisexual, or transgender. As of December 31, 2021, each public company with principal executive offices in California was required to have at least one director from an underrepresented community. By December 31, 2022, a public company with more than four but fewer than nine directors was required to have a minimum of two directors from underrepresented communities, and a public company with nine or more directors will need to have a minimum of three directors from underrepresented communities. These laws do not provide a transition period for newly listed companies. On April 1, 2022, the Superior Court of California for the County of Los Angeles entered an order striking down AB 979, holding that the statute violates the Equal Protection Clause of the California Constitution. On June 6, 2022, a notice of appeal was filed. On September 16, 2022, the appellate court ruled to temporarily stay enforcement of the trial court’s order, which prevented the California Secretary of State from collecting diversity data on corporate disclosure forms pursuant to AB 979, pending a further order of the appellate court. On December 1, 2022, the appellate court vacated the temporary stay order and on February 3, 2023, a record on appeal was filed and such appeal is currently pending. To the extent that this ruling of the appellate court permits the Secretary of State of California to collect and report diversity data, we may be required to comply with additional disclosure requirements. In June 2023, the federal district court for the Eastern District of California granted the plaintiffs a summary judgment and determined that AB 979 was unconstitutional on its face. The Eastern District of California's decision is currently on appeal. Litigation regarding AB 979 will continue.
Our board of directors currently includes three female directors, and no directors who self-identify as coming from “underrepresented communities.” We are currently in compliance with the Nasdaq’s board diversity rules. However, if our current or future female or other “diverse” directors no longer serve on our board, we could be out of compliance with the Nasdaq’s diversity rules. In addition, we would be in compliance with SB 826 were it to become applicable to us. However, if the current composition of our board of directors does not change and AB 979 becomes applicable to us, we would be out of compliance with these regulations. We cannot ensure that we can recruit, attract, and/or retain qualified members of the board and meet gender and diversity requirements under Nasdaq’s listing rules or any California law that may become applicable to us, which may expose us to financial penalties and adversely affect our reputation.
Because we are a public company, we are obligated to develop and maintain proper and effective internal control over financial reporting in order to comply with Section 404 of the Sarbanes-Oxley Act of 2002 (the Sarbanes-Oxley Act). These internal controls may not be determined to be effective, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
We are required, pursuant to Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment includes disclosure of any material weaknesses identified by our management in our internal control over financial reporting. We are also required to disclose changes made to our internal controls and procedures on a quarterly basis. However, we expect that our independent registered public accounting firm will not be required to report on the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until the date we are no longer an “emerging growth company” as defined in the JOBS Act, if we take advantage of the
45
exemptions contained in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse if it is not satisfied with the level at which our controls are documented, designed, or operating.
Additionally, the existence of any material weakness or significant deficiency would require management to devote significant time and incur significant expense to remediate any such material weaknesses or significant deficiencies and management may not be able to remediate any such material weaknesses or significant deficiencies in a timely manner. The existence of any material weakness in our internal control over financial reporting could also result in errors in our financial statements that could require us to restate our financial statements, cause us to fail to meet our reporting obligations, and cause stockholders to lose confidence in our reported financial information, all of which could materially and adversely affect our business and the price of our common stock.
Material weaknesses in our internal control over financial reporting may cause us to fail to timely and accurately report our financial results or result in a material misstatement of our financial statements.
In connection with the audits of our financial statements for the fiscal year ended December 31, 2022 as well as for fiscal years ended December 31, 2020 and 2019, we identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. The material weakness identified during the year ended December 31, 2022 resulted from not having the appropriate complement of tax resources commensurate with the nature and complexity associated with the Company's income tax accounting process which led to adjustments to correct errors in our accounting for income taxes during the same period. Our audited financial statements presented income taxes in accordance with GAAP, however, such adjustments amounted to a material weakness. The material weakness was remediated as of December 31, 2023. See Part II, Item 9A “Controls and Procedures” in this Annual Report on Form 10-K.
While we have remediated all prior material weaknesses, we can give no assurance that additional material weaknesses in our internal control over financial reporting will not be identified in the future. If we identify future material weaknesses in our internal control over financial reporting, the accuracy and timing of our financial reporting may be adversely affected. If additional material weaknesses exist or are discovered in the future, and we are unable to remediate any such material weaknesses, our reputation, financial condition, and operating results could suffer. Moreover, we could become subject to investigations by regulatory authorities, which could require additional financial and management resources.
We are an “emerging growth company” and a “smaller reporting company,” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act and, for as long as we continue to be an emerging growth company, we may choose to take advantage of certain exemptions and relief from various public reporting requirements, including: the requirement that our internal control over financial reporting be audited by our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act; the exemption from any rules that could be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotations or a supplement to the auditor’s report on financial statements; reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and not being required to hold nonbinding advisory votes on executive compensation or stockholder approval of any golden parachute payments not previously approved.
Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (the Securities Act), for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We have elected to take advantage of the extended transition period to comply with new or revised accounting standards and to adopt certain of the reduced disclosure requirements available to emerging growth companies. As a
46
result of the accounting standards election, we are not subject to the same implementation timing for new or revised accounting standards as other public companies that are not emerging growth companies, which may make comparison of our financials to those of other public companies more difficult. Additionally, because we have taken advantage of certain reduced reporting requirements, the information contained herein may be different from the information you receive from other public companies in which you hold stock.
We will remain an “emerging growth company” until the earliest to occur of (i) the last day of the fiscal year in which we have more than $1.235 billion in annual revenue; (ii) the date we qualify as a “large accelerated filer,” with at least $700.0 million of equity securities held by non-affiliates; (iii) the date on which we have issued, in any three-year period, more than $1.0 billion in non-convertible debt securities; and (iv) the last day of the fiscal year ending after the fifth anniversary of the completion of our IPO.
We are also a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company for so long as either (i) the market value of our common shares held by non-affiliates is less than $250.0 million as of the end of our second fiscal quarter or (ii) we have annual revenues of less than $100.0 million and the market value of our common shares held by non-affiliates is less than $700.0 million as of the end of our second fiscal quarter. To the extent we take advantage of such reduced disclosure obligations, it may also make comparison of our financial statements with other public companies difficult or impossible.
Investors may find our common stock less attractive to the extent we rely on the exemptions and relief granted by the JOBS Act. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and the price of our stock decline or become more volatile.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business, particularly after we are no longer an “emerging growth company” or if we cease to be a “smaller reporting company”.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the Exchange Act) and the Sarbanes-Oxley Act, the listing requirements of Nasdaq and other applicable securities rules and regulations. The Exchange Act requires that we file annual, quarterly, and current reports with respect to our business, financial condition, results of operations, cash flows, and prospects. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective internal controls and procedures for financial reporting. Furthermore, the need to continue to establish and maintain the corporate infrastructure demanded of a public company may divert our management’s attention from implementing our growth strategy, which could prevent us from improving our business, financial condition, results of operations, cash flows, and prospects. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a public company. However, the measures we take may not be sufficient to address all risks or wholly satisfy our obligations as a public company. In addition, these rules and regulations have increased our legal and financial compliance costs and have made, and are expected to continue to make, some activities more time-consuming and costly and to increase demand on our systems and resources, particularly after we are no longer an “emerging growth company” or if we cease to be a “smaller reporting company”. For example, these rules and regulations have made, and are expected to continue to make, it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to incur substantial costs to maintain the same or similar coverage. These additional obligations could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
In addition, changing laws, regulations, and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations, and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations, and standards, and this investment may result in increased general and administrative expenses and a diversion of our management’s time and attention from
47
revenue-generating activities. If our efforts to comply with new laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us, which could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
Provisions of our corporate governance documents could make acquiring us more difficult and may prevent attempts by our stockholders to replace or remove our current directors or management, even if beneficial to our stockholders.
Our amended and restated certificate of incorporation and amended and restated bylaws and the General Corporation Law of the State of Delaware, as amended (the DGCL), contain provisions that could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our stockholders. Among other things, these provisions:
Section 203 of the DGCL generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder. We have expressly elected not to be governed by Section 203 of the DGCL, until such time as THP beneficially owns, in the aggregate, less than a majority of the total voting power of all outstanding shares of our common stock entitled to vote generally in the election of directors. At that time, such election shall be automatically withdrawn and we will thereafter be governed by Section 203 of the DGCL, except that THP will not be deemed to be an interested stockholder under Section 203 of the DGCL regardless of its percentage ownership of our common stock. These provisions could discourage, delay, or prevent a transaction involving a change in control of our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing and cause us to take other corporate actions you desire, including actions that you may deem advantageous, or could negatively affect the trading price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team.
These and other provisions in our amended and restated certificate of incorporation, amended and restated bylaws, and Delaware law could make it more difficult for stockholders or potential acquirers to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including actions to
48
delay or impede a merger, tender offer, or proxy contest involving our company. The existence of these provisions could negatively affect the price of our common stock and limit opportunities for you to realize value in a corporate transaction.
Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the exclusive forum for certain litigation that may be initiated by our stockholders and the federal district courts of the United States as the exclusive forum for litigation arising under the Securities Act, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us.
Pursuant to our amended and restated certificate of incorporation, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) will be the sole and exclusive forum for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our current or former directors, officers, employees or stockholders to us or our stockholders, (3) any action asserting a claim against us, or any of our current or former directors, officers, employees, or stockholders arising pursuant to any provision of the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws, (4) any claim or cause of action seeking to interpret, apply, enforce, or determine the validity of our amended and restated certificate of incorporation or our amended and restated bylaws, (5) any action or proceeding asserting a claim against us or any of our current or former directors, officers, employees, or stockholders as to which the DGCL confers jurisdiction to the Court of Chancery of the State of Delaware, and (6) any action asserting a claim governed by the internal affairs doctrine of the law of the State of Delaware; provided that, for the avoidance of doubt, the foregoing forum selection provision will not apply to claims arising under the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our amended and restated certificate of incorporation also provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Our amended and restated certificate of incorporation further provides that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have notice of and consented to the provisions of our amended and restated certificate of incorporation described above.
The forum selection provisions in our amended and restated certificate of incorporation may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings and there is uncertainty as to whether a court would enforce such provisions. In addition, investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. If the enforceability of our forum selection provisions were to be challenged, we may incur additional costs associated with resolving such a challenge. While we currently have no basis to expect any such challenge would be successful, if a court were to find our forum selection provisions to be inapplicable or unenforceable with respect to one or more of these specified types of actions or proceedings, we may incur additional costs associated with having to litigate in other jurisdictions, which could have an adverse effect on our business, financial condition, results of operations, cash flows, and prospects and result in a diversion of the time and resources of our employees, management, and board of directors.
An active, liquid trading market for our common stock may not be sustained, which may limit your ability to sell your shares.
Our shares of common stock began trading on the Nasdaq Global Market on June 25, 2021. Prior to our IPO, there was no public market for our common stock. Although our common stock is listed on the Nasdaq Global Market, an active trading market for our common stock may not be sustained. A public trading market having the desirable characteristics of depth, liquidity, and orderliness depends upon the existence of willing buyers and sellers at any given time, which is dependent upon the individual decisions of buyers and sellers over which neither we nor any market maker has control. The failure of an active and liquid trading market to persist would likely have a material adverse effect on the value of our common stock. An inactive market may also impair our ability to raise capital to continue to fund operations by issuing additional shares of our common stock or other equity or equity-linked securities and may impair our ability to acquire other companies or technologies by using any such securities as consideration.
49
A significant portion of our total outstanding shares of common stock are available for immediate resale and may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. All shares sold in our IPO were freely tradable upon such sale without restriction or further registration under the Securities Act, except for any shares held by our affiliates, as that term is defined under Rule 144 of the Securities Act (Rule 144), including our directors, executive officers, and other affiliates (including THP), which may be sold only in compliance with certain limitations. The shares of our common stock issued in the course of our September 2023 registered direct offering and private placements are now also freely tradable, subject to the same limitations applicable to our directors, executive officers, and other affiliates (including THP).
As of December 31, 2023, we have 40,793,848 shares of common stock outstanding, substantially all of which are held by directors, executive officers, and other affiliates and will be subject to volume, manner of sale, and other limitations under Rule 144. Registration of any of these outstanding shares of common stock would result in such shares becoming freely tradable without compliance with Rule 144 upon effectiveness of the registration statement.
The market price of our stock could decline if the holders of currently restricted shares of common stock sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our shares of common stock or other securities. In addition, shares of our common stock that are issued pursuant to our equity incentive plans and our Employee Stock Purchase Plan (ESPP) will become eligible for sale in the public market, subject to provisions relating to various vesting agreements, lock-up agreements, and Rule 144, as applicable.
As of December 31, 2023, there were 312,174, 1,611,509 and 2,273,904 shares of common stock reserved for issuance pursuant to outstanding stock option awards under the 2016 Stock Plan, as amended (2016 Plan), the 2020 Equity Incentive Plan, as amended (2020 Plan) and the 2021 Equity Incentive Plan (2021 Plan), respectively. In addition, the 2021 Plan and the ESPP provide for annual automatic increases in the number of shares reserved thereunder. As of January 1, 2024, a total of 4,825,264 and 976,045 shares of common stock were available and have been reserved for future issuance under the 2021 Plan and our ESPP, respectively. In the future, we may also issue our securities in connection with investments or acquisitions. The amount of shares of our common stock issued in connection with an investment or acquisition could constitute a material portion of our then-outstanding shares of our common stock. Any issuance of additional securities in connection with investments or acquisitions may result in additional dilution to you.
Because we have no current plans to pay regular cash dividends on our common stock and are prohibited from paying cash dividends under the Amended Credit Agreement, you may not receive any return on investment unless you sell your common stock for a price higher than you paid for it.
We do not anticipate paying any regular cash dividends on our common stock. Any decision to declare and pay dividends in the future will be made at the discretion of our board of directors and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions, and other factors that our board of directors may deem relevant. In addition, the terms of the Amended Credit Agreement prohibit us from paying dividends, other than dividends payable in our stock, without the prior written consent of the lender. Our future ability to pay cash dividends on our common stock may also be limited by the terms of any future debt or preferred securities we may issue or any future credit facilities we may enter into. Therefore, any return on investment in our common stock is solely dependent upon the appreciation of the price of our common stock on the open market, which may not occur.
50
We may issue shares of preferred stock in the future, which could make it difficult for another company to acquire us or could otherwise adversely affect holders of our common stock, which could depress the price of our common stock.
Our amended and restated certificate of incorporation authorizes us to issue one or more series of preferred stock. Our board of directors has the authority to determine the preferences, limitations, and relative rights of the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series, without any further vote or action by our stockholders. Our preferred stock could be issued with voting, liquidation, dividend, and other rights superior to the rights of our common stock. The potential issuance of preferred stock may delay or prevent a change in control of us, discouraging bids for our common stock at a premium to the market price, and materially adversely affect the market price and the voting and other rights of the holders of our common stock.
Our future capital needs are uncertain and we may need to seek additional financing in the future, which we may not be able to secure on favorable terms, if at all.
Our available capital resources may not be sufficient for us to continue to meet our obligations as they become due over the next twelve months if we cannot improve our operating results or increase our operating cash inflows. Our future funding requirements will depend on many factors, including:
We cannot assure you that we will be able to obtain additional funds on acceptable terms, or at all. If we raise additional funds by issuing equity or equity-linked securities, our stockholders may experience dilution. Future debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt or equity financing may contain terms that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. Moreover, we cannot assure you that we will be able to comply with the financial covenants in our Amended Credit Agreement. If we are unable to comply with the financial covenants in our Amended Credit Agreement, we may be unable to maintain the Amended Credit Agreement as an external source of funds. We also may have to reduce marketing, customer support, or other resources devoted to our products, or cease operations. Any of these factors could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects.
Claims for indemnification by our directors and officers may reduce our funds available to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our amended and restated certificate of incorporation and amended and restated bylaws provide for indemnification of our directors and officers, in each case to the fullest extent permitted by Delaware law. In addition, as permitted by Section 145 of the DGCL, our amended and restated bylaws, and the indemnification agreements that we have entered into with our directors and officers, provide that:
51
While we maintain a directors’ and officers’ insurance policy, such insurance may not be adequate to cover all liabilities that we may incur, which may reduce our available funds to satisfy third-party claims and may adversely impact our cash position.
General Risk Factors
We have identified conditions and events that raise substantial doubt about our ability to continue as a going concern.
The accompanying financial statements included in Part IV, Item 15(a)(1) and 15(a)(2) of this Annual Report on Form 10-K, have been prepared assuming we will continue as a going concern, which contemplates continuity of operations, realization of assets, and the satisfaction of liabilities in the normal course of business for one year following the issuance of these audited financial statements. However, we have identified certain negative conditions and events, described further below, that raise substantial doubt about our ability to continue as a going concern.
Our available capital resources may not be sufficient for us to continue to meet our obligations as they become due over the next twelve months if we cannot improve our operating results or increase our operating cash inflows. If these capital resources are not sufficient, we may need to raise additional capital through the sale of equity or debt securities, enter into strategic business collaboration agreements with other companies, seek other funding sources, or sell assets. However, there can be no assurance that we will be able to accomplish any of the foregoing or do so on favorable terms. If we are unable to meet our obligations when they become due over the next twelve months through our available capital resources, or obtain new sources of capital when needed, we may have to delay expenditures, reduce the scope of our manufacturing operations, reduce or eliminate one or more of our development programs, make significant changes to our operating plan, or cease our operations.
Additionally, we are subject to certain financial covenants under the terms of the Amended Credit Agreement. These financial covenants include (i) a trailing twelve months minimum net revenue covenant that we must meet each calendar month, and (ii) a requirement to maintain a minimum level of cash at all times through the term of the Amended Credit Agreement. To the extent we continue to experience unfavorable market conditions, like other companies in our industry, we may be unable to comply with the monthly revenue covenant during the twelve-month period following the date on which the financial statements are available for issuance. Failing to comply with the monthly revenue covenant would be an event of default under the Amended Credit Agreement and the lender would have the right, but not the obligation, to accelerate our obligations to pay the outstanding balance due and payable under the Term Loan. If we violate one or more of our covenants under the Amended Credit Agreement and are not able to obtain a waiver from or agree to an accommodation with the lender with respect to any such violation, we could be required to pay all or a portion of the outstanding amount under the Term Loan. In that event, we may need to seek other sources of capital and there can be no assurances that we would be able to do so on acceptable terms.
The uncertainty regarding our ability to continue as a going concern could materially adversely affect our share price and our ability to service our indebtedness, raise new capital or enter into commercial transactions. To address these matters, the Company may take actions that materially and adversely affect our business, including significant reductions in administrative, and commercial activities, reduction of our employee base, and ultimately
52
curtailing or ceasing operations, any of which could materially adversely affect our business, financial condition, results of operations, and share price.
Fluctuations in our effective tax rate may adversely affect our results of operations and cash flows.
We are subject to a variety of tax liabilities, including federal, state, and other taxes such as income, sales/use, payroll, withholding, and ad valorem taxes. Changes in tax laws or their interpretations could decrease our net income, the value of any tax loss carryforwards, the value of any tax credits recorded on our balance sheet, and our cash flows, and accordingly could have a material adverse effect on our business, financial condition, results of operations, cash flows, and prospects. In addition, some of our tax liabilities are subject to periodic audits by the relevant taxing authority, which could increase our tax liabilities.
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of our income or other tax returns could adversely affect our operating results and financial condition.
We are currently subject to income taxes in the U.S. only, but our future tax liabilities may be subject to the allocation of expenses in other jurisdictions. Our future effective tax rates could be subject to volatility or adversely affected by a number of factors, including:
In addition, we may be subject to audits of our income, sales, and other transaction taxes. Outcomes from these audits could have an adverse effect on our operating results and financial condition.
If our estimates or judgments relating to our critical accounting policies are based on assumptions that change or prove to be incorrect, our operating results could fall below the expectations of securities analysts and investors, resulting in a decline in the market price of our common stock.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets, liabilities, equity, revenue, and expenses that are not readily apparent from other sources. It is possible that interpretation, industry practice, and guidance may evolve over time. If our assumptions change or if actual circumstances differ from our assumptions, our operating results may be adversely affected and could fall below the expectations of securities analysts and investors, resulting in a decline in the market price of our common stock.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. We have designed our disclosure controls and procedures to provide reasonable assurance that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
53
Our employees, consultants, distributors, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, consultants, distributors, and commercial partners. Misconduct by these parties could include intentional failures to comply with the applicable laws and regulations in the U.S. and abroad, report financial information or data accurately or disclose unauthorized activities to us. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, and other business arrangements. Such misconduct could result in legal or regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees or others, and any precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant civil, criminal, and administrative penalties, which could have a significant impact on our business. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and could distract management in defending ourselves against any of these claims or investigations.
Our operating results and stock price may be volatile. Market volatility may affect the value of an investment in our common stock and could subject us to litigation.
Our quarterly operating results are likely to fluctuate in the future. In addition, securities markets worldwide have experienced, and may again experience, significant price and volume fluctuations for reasons that may be unrelated to our operating performance. Market volatility, as well as general economic, market, or political conditions, could subject the market price of our common stock to wide fluctuations regardless of our operating performance. Our operating results and the trading price of our common stock may fluctuate in response to various factors, including:
These and other factors, many of which are beyond our control, may cause our operating results and the market price and demand for our common stock to fluctuate substantially. Fluctuations in our quarterly operating results could limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the market price and liquidity of our shares of common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management from our business, which could significantly harm our profitability and reputation.
54
If securities or industry analysts do not publish research or reports about our business, if our results of operations do not meet their expectations, if they publish unfavorable research or reports, adversely change their recommendations regarding our common stock or cease coverage of us, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts publish about us or our business. We do not have any control over any securities or industry analyst coverage.
Analysts may develop and publish their own projections of our business, and may form a consensus about our future performance. Our actual business results may vary significantly from that consensus or other guidance or expectations due to a number of factors, many of which are outside of our control and could adversely affect our business and future operating results. In addition, if our publicly announced guidance or other expectations of future operating results fail to meet the expectations of securities analysts, investors, or other interested parties, the price of our common stock could decline.
Moreover, if any of the analysts who cover us provide inaccurate or unfavorable research, issue an adverse opinion regarding our stock price, cease coverage of us, or fail to publish reports on us regularly, we could lose visibility in the financial markets, and our stock price or trading volume could decline.
We may become the subject of various claims, litigation or investigations which could have a material adverse effect on our business, financial condition, results of operations, or stock price.
We may become subject to various claims, litigation, or investigations, such as commercial disputes, employment-related claims, or “whistleblower” complaints, and we may become involved in governmental or regulatory investigations or similar matters. Any claims asserted against us or our management, regardless of merit or eventual outcome, could harm our reputation, distract our management, and have an adverse impact on our relationship with our current and prospective employees, customers, and other third parties and could lead to additional related claims. Furthermore, there is no guarantee that we will be successful in defending ourselves against claims, litigation, or investigations, or that any insurance policies that we may maintain would cover any or all of our liabilities arising from claims, litigation, or investigations. Any judgments or settlements in any future claims, litigation, or investigation could have a material adverse effect on our business, financial condition, results of operations, and price of our common stock.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity.
Our board of directors and management exercise oversight over the Company’s cybersecurity program, which represents an important component of the Company’s overall approach to enterprise risk management.
Governance
Teknova’s Vice President of Information Systems and Architecture (VP of IT) manages a team responsible for leading enterprise-wide strategy, policy, standards, architecture, processes, and risk assessment related to information security and data protection, including data privacy and network security (our Cybersecurity Program). The VP of IT has served in various roles in information technology and information security, along with other members of the IT department, and holds relevant and applicable certifications. The VP of IT reports directly to the Company’s Chief Executive Officer and provides periodic reporting on our Cybersecurity Program to our senior management team, our board of directors, and the audit committee of our board of directors.
Our board of directors, in coordination with our audit committee, oversees our management of cybersecurity risk, with the audit committee reviewing and discussing with management matters related to our Cybersecurity Program, including as it relates to financial reporting. The board of directors and audit committee receive periodic reports about the prevention, detection, mitigation, and remediation of cybersecurity incidents, including material security risks and information security vulnerabilities. Additionally, risks associated with the Cybersecurity Program
55
are integrated into the Company’s enterprise risk management assessment and reported to our Board as needed. We also share the key results of third-party assessments with our board of directors and audit committee.
Risk Management and Strategy
Technical Safeguards
As part of our Cybersecurity Program, the Company deploys technical safeguards that are designed to protect our information systems from cybersecurity threats, which are evaluated and improved through vulnerability assessments and cybersecurity threat intelligence.
Risk Assessment
Our Cybersecurity Program also includes a periodic risk assessment, which is based generally on frameworks established by the National Institute of Standards and Technology (NIST).
Third-Party Risk Management
We also maintain procedures designed to identify and mitigate cybersecurity threats related to our use of material third-party vendors. This includes reviewing the internal controls of certain third-party service providers to assess their procedures to mitigate material cybersecurity risks, among other risks.
Incident Response and Recovery Planning
We have an information security incident response process to prevent, detect, mitigate, and remediate cybersecurity incidents and threats. This process includes controls and procedures that provide for the prompt escalation of certain cybersecurity incidents so that decisions regarding public disclosure and reporting of such incidents can be made by management in a timely manner, with appropriate involvement by our board of directors.
External Assessments
We obtain periodic assessments by third party experts of our vulnerability management and security controls and to assist us in identifying and mitigating security risks.
Education and Awareness
We provide periodic cybersecurity training for all officers and employees as well as periodic additional training for senior management through our cyber insurance carrier.
As of the date of this Annual Report on Form 10-K, we are not aware of any risks from cybersecurity threats that have materially affected the Company, including our business strategy, results of operations, or financial condition. For information regarding cybersecurity risks that may materially affect our Company, see the risk factor titled “Our internal computer systems, or those of our suppliers, customers, or contractors, have been and may in the future be subject to cyberattacks or security breaches, which could result in a material disruption of our business or otherwise adversely affect our business, financial condition, results of operations, cash flows, and prospects.” under “Risk Factors” in Part I, Item 1A. to this Annual Report on Form 10-K.
Item 2. Properties.
See Item 1. "Business – Facilities" for specific information about our commercial, office, manufacturing, and warehouse space.
Item 3. Legal Proceedings.
We are not a party to any material legal proceedings at this time. From time to time, we may become involved in various legal proceedings that arise in the ordinary course of business. For example, we may in the future become involved in legal proceedings relating to customers, employees, suppliers, competitors, government agencies, or others. We will evaluate any claims and lawsuits with respect to their potential merits, our potential defenses and counter claims, and the expected effect on us of defending the claims and a potential adverse result. However, the results of any litigation, investigation, or other legal proceedings are inherently unpredictable and potentially expensive. Any claims against us, whether meritorious or not, could be time consuming, result in costly litigation, damage our reputation, require significant amounts of management time, and divert significant resources. If any
56
legal proceedings were to be determined adversely to us, or we were to enter into a settlement arrangement, we could be exposed to monetary damages or limits on our ability to operate our business, which could have an adverse effect on our business, financial condition, and operating results. Information pertaining to loss contingencies, including those arising out of potential legal liabilities and related matters, are described in Item 8, "Financial Statements and Supplementary Data - Note 16. Contingencies," and incorporated by reference herein.
Item 4. Mine Safety Disclosures.
Not applicable.
57
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information for Common Stock
Our common stock is listed on the Nasdaq Global Market under the symbol “TKNO”.
Holders
On March 22, 2024, we had 16 holders of record of our common stock.
Dividends
We have not paid any dividends since our inception. We currently intend to retain all available funds and any future earnings to support operations and to finance the growth and development of our business. We do not currently intend to declare or pay any cash dividends on our common stock in the foreseeable future. Any future determination to pay dividends on our capital stock will be at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, and other factors that our board of directors considers relevant. In particular, unless waived, the terms of the Amended Credit Agreement prohibit us from paying dividends, other than dividends payable in our stock, without the prior written consent of the lender. Our future ability to pay cash dividends on our common stock may also be limited by the terms of any future debt or preferred securities we may issue or any future credit facilities we may enter into.
Securities Authorized for Issuance Under Equity Compensation Plans
See Item 12. of Part III for information regarding securities authorized for issuance under our equity compensation plans.
Stock Performance Graph
We are a smaller reporting company, as defined by Rule 12b-2 of the Exchange Act and are not required to provide a performance graph.
Issuer Repurchases of Equity Securities
Not applicable.
Item 6. [Reserved].
58
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of Alpha Teknova, Inc.’s financial condition and results of operations should be read in conjunction with the financial statements and the notes thereto contained elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements reflecting our current expectations, estimates, and assumptions concerning events and financial trends that may affect its future operating results or financial position. Actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the sections titled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” appearing elsewhere in this Annual Report on Form 10-K. Unless the context otherwise requires, references in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” to "the Company," “Teknova,” we,” “us,” and “our” are intended to mean the business and operations of Alpha Teknova, Inc.
Overview
Since our founding in 1996, we have been producing critical reagents for the discovery, development, and commercialization of novel therapies, vaccines, and molecular diagnostics. Our more than 2,500 active customers span the entire continuum of the life sciences market, including leading pharmaceutical and biotechnology companies, contract development and manufacturing organizations, in vitro diagnostics franchises, and academic and government research institutions. Our Company is built around our knowledge, methods, and know-how in our proprietary manufacturing processes, which are highly adaptable and configurable. These proprietary processes enable us to manufacture and deliver high-quality, custom, made-to-order products with short turnaround times and at scale, across all stages of our customers’ product development, from early research through commercialization.
We have two primary product categories: Lab Essentials and Clinical Solutions. Our products cross all stages of development, from early research through commercialization. We offer three primary product types: (i) pre-poured media plates for cell growth and cloning; (ii) liquid cell culture media and supplements for cellular expansion; and (iii) molecular biology reagents for sample manipulation, resuspension, and purification. Our liquid cell culture media and supplements and molecular biology reagents are available in both of our two primary product categories; pre-poured media plates are available in our Lab Essentials category only.
We are ISO 13485:2016 certified, enabling us to manufacture products for use in diagnostic and therapeutic applications. Our certification allows us to offer solutions across the entire customer product development workflow, supporting our customers' need for materials in greater volume and that meet increasingly stringent quality requirements as they scale from research to commercialization.
We manufacture our products at our Hollister, California headquarters and stock inventory of raw materials, components, and finished goods at that campus. We rely on a limited number of suppliers for certain raw materials, and we have no long-term supply arrangements with our suppliers, as we order on a purchase order basis. We ship our products directly from our warehouse in Hollister, California, to our customers and distributors, generally pursuant to purchase orders. We typically recognize revenue when products are shipped.
We generated revenue of $36.7 million in 2023, which represents a decrease of $4.7 million as compared to 2022. In 2023 and 2022, only 4.6% and 3.2%, respectively, of our revenue was generated from customers located outside of the U.S. Our sales outside of the U.S. are denominated in U.S. dollars. We primarily generate sales through direct channels and a small salesforce, however, some of our sales are generated through distributors.
We had an operating loss of $35.6 million in 2023 compared to $49.7 million in 2022. Excluding non-recurring charges, we had an operating loss of $29.8 million in 2023 (excluding the following non-recurring charges: $0.7 million related to the reduction in workforce, $2.2 million tradename impairment, $2.2 million long-lived asset impairment, $0.4 million write-off of ATM Facility costs, and $0.3 million loss contingency) compared to an operating loss of $28.9 million in 2022 (excluding the goodwill impairment of $16.6 million and long-lived asset impairment of $4.2 million). While our expenses may fluctuate over the short term, we expect our expenses will continue to increase in future periods, but at a slower rate, in connection with our ongoing activities as we:
59
Key Developments
60
Impact of Broader Economic Trends on Our Business
We are closely monitoring economic uncertainty in the U.S. and abroad. General inflation in the U.S. has risen to levels not experienced in recent decades. General inflation, including rising prices for our raw materials and other inputs, as well as rising salaries and other expenses, negatively impact our business by increasing our cost of sales and operating expenses. In addition, the U.S. Federal Reserve has raised, and may again raise, interest rates in response to concerns about inflation. Inflation, together with increased interest rates, may cause our customers to reduce, delay, or cancel orders for our goods and services thereby causing a decrease in or change in timing of sales of our products and services. We cannot predict the impact of future inflation and interest rate increases on the results of our operations. For further information regarding the impact of these economic factors on the Company, please see Item 1A., "Risk Factors" in this report, which is incorporated herein by reference.
Results of Operations
The following tables set forth our results of operations for the years ended December 31, 2023 and 2022 (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
Revenue |
|
$ |
36,684 |
|
|
$ |
41,420 |
|
|
$ |
(4,736 |
) |
|
|
(11.4 |
)% |
Cost of sales |
|
|
26,388 |
|
|
|
23,944 |
|
|
|
2,444 |
|
|
|
10.2 |
% |
Gross profit |
|
|
10,296 |
|
|
|
17,476 |
|
|
|
(7,180 |
) |
|
|
(41.1 |
)% |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
5,567 |
|
|
|
7,737 |
|
|
|
(2,170 |
) |
|
|
(28.0 |
)% |
Sales and marketing |
|
|
9,330 |
|
|
|
9,151 |
|
|
|
179 |
|
|
|
2.0 |
% |
General and administrative |
|
|
25,450 |
|
|
|
28,298 |
|
|
|
(2,848 |
) |
|
|
(10.1 |
)% |
Amortization of intangible assets |
|
|
1,148 |
|
|
|
1,148 |
|
|
|
— |
|
|
|
— |
|
Goodwill impairment |
|
|
— |
|
|
|
16,613 |
|
|
|
(16,613 |
) |
|
|
(100.0 |
)% |
Tradename impairment |
|
|
2,169 |
|
|
|
— |
|
|
|
2,169 |
|
|
|
100.0 |
% |
Long-lived assets impairment |
|
|
2,195 |
|
|
|
4,188 |
|
|
|
(1,993 |
) |
|
|
(47.6 |
)% |
Total operating expenses |
|
|
45,859 |
|
|
|
67,135 |
|
|
|
(21,276 |
) |
|
|
(31.7 |
)% |
Loss from operations |
|
|
(35,563 |
) |
|
|
(49,659 |
) |
|
|
14,096 |
|
|
|
(28.4 |
)% |
Other (expenses) income, net |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest (expense) income, net |
|
|
(833 |
) |
|
|
213 |
|
|
|
(1,046 |
) |
|
|
(491.1 |
)% |
Other income, net |
|
|
142 |
|
|
|
55 |
|
|
|
87 |
|
|
|
158.2 |
% |
Loss on extinguishment of debt |
|
|
(824 |
) |
|
|
— |
|
|
|
(824 |
) |
|
|
(100.0 |
)% |
Total other (expenses) income, net |
|
|
(1,515 |
) |
|
|
268 |
|
|
|
(1,783 |
) |
|
|
(665.3 |
)% |
Loss before income taxes |
|
|
(37,078 |
) |
|
|
(49,391 |
) |
|
|
12,313 |
|
|
|
(24.9 |
)% |
Benefit from income taxes |
|
|
(298 |
) |
|
|
(1,923 |
) |
|
|
1,625 |
|
|
|
(84.5 |
)% |
Net loss |
|
$ |
(36,780 |
) |
|
$ |
(47,468 |
) |
|
$ |
10,688 |
|
|
|
(22.5 |
)% |
61
Revenue
Our revenue disaggregated by product category, for the years ended December 31, 2023 and 2022 was as follows (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
Lab Essentials |
|
$ |
28,800 |
|
|
$ |
31,772 |
|
|
$ |
(2,972 |
) |
|
|
(9.4 |
)% |
Clinical Solutions |
|
|
6,738 |
|
|
|
8,445 |
|
|
|
(1,707 |
) |
|
|
(20.2 |
)% |
Other |
|
|
1,146 |
|
|
|
1,203 |
|
|
|
(57 |
) |
|
|
(4.7 |
)% |
Total revenue |
|
$ |
36,684 |
|
|
$ |
41,420 |
|
|
$ |
(4,736 |
) |
|
|
(11.4 |
)% |
Total revenue was $36.7 million in 2023, a decrease of $4.7 million, or 11.4%, compared with $41.4 million in 2022.
Lab Essentials revenue was $28.8 million in 2023, a decrease of $3.0 million, or 9.4%, compared with $31.8 million in 2022. The decrease in Lab Essentials revenue was attributable to a decreased number of customers, partially offset by higher average revenue per customer.
Clinical Solutions revenue was $6.7 million in 2023, a decrease of $1.7 million, or 20.2%, compared with $8.4 million in 2022. The decrease in Clinical Solutions revenue was attributable to lower average revenue per customer, partially offset by an increased number of customers.
Our revenue disaggregated by geographic region for the years ended December 31, 2023 and 2022, was as follows (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
United States |
|
$ |
35,000 |
|
|
$ |
40,103 |
|
|
$ |
(5,103 |
) |
|
|
(12.7 |
)% |
International |
|
|
1,684 |
|
|
|
1,317 |
|
|
|
367 |
|
|
|
27.9 |
% |
Total revenue |
|
$ |
36,684 |
|
|
$ |
41,420 |
|
|
$ |
(4,736 |
) |
|
|
(11.4 |
)% |
Revenue from sales to customers in the United States was $35.0 million in 2023, and $40.1 million in 2022. Revenue from U.S. sales was consistent year over year, representing 95.4% and 96.8% of our total revenue in 2023 and 2022, respectively.
Revenue from sales to customers in markets outside of the U.S. was $1.7 million in 2023, and $1.3 million in 2022. Revenue from international sales was also consistent year over year, representing 4.6% and 3.2% of our total revenue in 2023 and 2022, respectively.
Gross profit
Our gross profit for the years ended December 31, 2023 and 2022 was as follows (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
Cost of sales |
|
$ |
26,388 |
|
|
$ |
23,944 |
|
|
$ |
2,444 |
|
|
|
10.2 |
% |
Gross profit |
|
|
10,296 |
|
|
|
17,476 |
|
|
|
(7,180 |
) |
|
|
(41.1 |
)% |
Gross profit % |
|
|
28.1 |
% |
|
|
42.2 |
% |
|
|
|
|
|
|
||
Gross profit percentage was 28.1% in 2023, and 42.2% in 2022. The decrease in gross profit percentage was primarily driven by the decrease in revenue and the associated lower absorption of fixed manufacturing costs, and to a lesser extent increased overhead costs that were partially offset by reduced headcount.
62
Operating expenses
Our operating expenses for the years ended December 31, 2023 and 2022 were as follows (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
Research and development |
|
$ |
5,567 |
|
|
$ |
7,737 |
|
|
$ |
(2,170 |
) |
|
|
(28.0 |
)% |
Sales and marketing |
|
|
9,330 |
|
|
|
9,151 |
|
|
|
179 |
|
|
|
2.0 |
% |
General and administrative |
|
|
25,450 |
|
|
|
28,298 |
|
|
|
(2,848 |
) |
|
|
(10.1 |
)% |
Amortization of intangible assets |
|
|
1,148 |
|
|
|
1,148 |
|
|
|
— |
|
|
|
— |
|
Goodwill impairment |
|
|
— |
|
|
|
16,613 |
|
|
|
(16,613 |
) |
|
|
(100.0 |
)% |
Tradename impairment |
|
|
2,169 |
|
|
|
— |
|
|
|
2,169 |
|
|
|
100.0 |
% |
Long-lived assets impairment |
|
|
2,195 |
|
|
|
4,188 |
|
|
|
(1,993 |
) |
|
|
(47.6 |
)% |
Total operating expenses |
|
$ |
45,859 |
|
|
$ |
67,135 |
|
|
$ |
(21,276 |
) |
|
|
(31.7 |
)% |
Research and development expenses were $5.6 million in 2023 and $7.7 million in 2022. The decrease was primarily driven by reduced headcount and professional fees as well as reduced supplies and equipment expenses.
Sales and marketing expenses were $9.3 million in 2023 and $9.2 million in 2022. The increase was primarily driven by higher labor and benefit costs as well as stock-based compensation, partially offset by lower marketing expenses.
General and administrative expenses were $25.5 million in 2023 and $28.3 million in 2022. Excluding the one-time, non-recurring charges related to the reduction in workforce of $0.7 million, the $0.4 million write off of ATM Facility costs, and $0.3 million accrual for legal claim recorded in 2023, general and administrative expenses decreased $4.3 million compared to 2022. The decrease was driven by reduced spending, primarily in professional fees and occupancy costs, partially offset by higher stock-based compensation expense.
Amortization of intangible assets was consistent in 2023 and 2022, at $1.1 million.
We incurred a $16.6 million goodwill impairment charge in 2022, with no comparable charges in 2023. Refer to the “Notes to Financial Statements—Note 8. Intangible Assets, Net” in our financial statements for details regarding the goodwill impairment.
We incurred a $2.2 million tradename impairment charge in 2023, with no comparable charges in 2022. Refer to the “Notes to Financial Statements—Note 8. Intangible Assets, Net” in our financial statements for details regarding the tradename impairment.
We recorded long-lived asset impairment charges of $2.2 million in 2023 and $4.2 million in 2022. Refer to “Notes to Financial Statements—Note 6. Property, Plant, and Equipment, Net,” in our financial statements for details regarding the long-lived asset impairment.
Other (expenses) income, net
Other (expenses) income, net for the years ended December 31, 2023 and 2022 was as follows (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
Interest (expense) income, net |
|
$ |
(833 |
) |
|
$ |
213 |
|
|
$ |
(1,046 |
) |
|
|
(491.1 |
)% |
Other income, net |
|
|
142 |
|
|
|
55 |
|
|
|
87 |
|
|
|
158.2 |
% |
Loss on extinguishment of debt |
|
|
(824 |
) |
|
|
— |
|
|
|
(824 |
) |
|
|
(100.0 |
)% |
Total other (expenses) income, net |
|
$ |
(1,515 |
) |
|
$ |
268 |
|
|
$ |
(1,783 |
) |
|
|
(665.3 |
)% |
63
Total other expenses, net was $1.5 million in 2023, compared to total other income, net of $0.3 million in 2022. The increase in total other expenses, net was attributable to higher interest expense primarily driven by an increase in our average debt balance coupled with higher interest rates, as well as by a $0.8 million loss on extinguishment of debt related to the partial repayment on the Term Loan. We capitalized a portion of the interest on funds borrowed to finance certain of our capital expenditures. Capitalized interest costs were $0.9 million and $1.6 million in 2023 and 2022, respectively. Offsetting these other expenses is other income, which increased due to higher interest rates and thus income earned on short-term liquid investments.
Benefit from income taxes
Our benefit from income taxes for the years ended December 31, 2023 and 2022 was as follows (dollars in thousands):
|
|
For the Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
$ Change |
|
|
% Change |
|
||||
Benefit from income taxes |
|
$ |
(298 |
) |
|
$ |
(1,923 |
) |
|
$ |
1,625 |
|
|
|
(84.5 |
)% |
Effective tax rate |
|
|
0.8 |
% |
|
|
3.9 |
% |
|
|
|
|
|
|
||
Our benefit from income taxes was $0.3 million in 2023, compared to a $1.9 million in 2022. The decrease in our benefit from income taxes was attributable to operating losses not expected to produce a benefit.
Liquidity and Capital Resources
The primary sources of financing for our operations were our (i) initial public offering, which we completed in June 2021 (IPO) and resulted in net proceeds to us of $99.1 million, after deducting underwriting discounts and commissions of $7.7 million and offering expenses of $3.6 million, and (ii) registered direct offering and concurrent private placement (collectively, the Offerings), which we completed in September 2023 and which resulted in aggregate gross proceeds of $22.915 million before deducting offering expenses of $0.4 million and the prepayment of $10.0 million owed under the Term Loan as discussed below.
To facilitate our expected growth, we have used our sources of liquidity to make investments to expand our operations and increase capacity, and may continue to do so in the future. In particular, we have completed the build out of our new manufacturing facility and have made improvements to our warehouse and distribution facilities, all located in Hollister, California.
Our principal liquidity requirements are to fund our operations and capital expenditures. As of December 31, 2023, we have limited capital resources to fund ongoing operations. During the year ended December 31, 2023, we incurred net losses of $36.8 million. In addition, as of December 31, 2023, we had an accumulated deficit of $91.8 million and borrowings outstanding under our Term Loan (defined below). As of December 31, 2023, we had $36.8 million of working capital, which included $28.5 million in cash and cash equivalents. Our available capital resources may not be sufficient for us to continue to meet our obligations as they become due over the next twelve months if we cannot improve our operating results or increase our operating cash inflows. If these capital resources are not sufficient, we may need to raise additional capital through the sale of equity or debt securities, enter into strategic business collaboration agreements with other companies, seek other funding sources, or sell assets. However, there can be no assurance that we will be able to accomplish any of the foregoing or do so on favorable terms. If we are unable to meet our obligations when they become due over the next twelve months through our available capital resources, or obtain new sources of capital when needed, we may have to delay expenditures, reduce the scope of our manufacturing operations, reduce or eliminate one or more of our development programs, make significant changes to our operating plan, or cease our operations.
As of December 31, 2023, we had an outstanding principal amount of $12.1 million under a senior secured term loan (the Term Loan) pursuant to Amendment No. 4 to our Credit Agreement with MidCap Financial Trust (MidCap). On March 8, 2024, we entered into limited waivers and amendments (collectively Amendment No. 5, or, as amended, the Amended Credit Agreement) which includes a waiver from MidCap of the revenue covenant
64
violations for each of the periods ending November 30, 2023 and January 31, 2024. Amendment No. 5 also reduced these requirements for future periods up to and including for the twelve months ending December 31, 2024, from $42 million to $34 million. Amendment No. 5 also removed those requirements for the periods ending January 31, 2025 through December 31, 2025, instead requiring that for each applicable twelve-month period ending after December 31, 2024, the Company’s minimum net revenue requirement will be determined by MidCap in its reasonable discretion in consultation with the Company’s senior management and based on financial statements and projections delivered to MidCap in accordance with the financial reporting requirements in the Amended Credit Agreement, so long as the minimum net revenue requirements for those periods shall not be less than the greater of (x) the applicable minimum net revenue requirement for the twelve-month period ending on the last day of the immediately preceding month and (y) $34.0 million. In addition, Amendment No. 5 also removed the advance rate for finished goods inventory in the determination of the borrowing base for the Revolving Loan and increased the minimum cash requirement from $9.0 million to $10.0 million. Finally, Amendment No. 5 conditions the next borrowing under the Revolving Loan on the Company achieving net revenue for the preceding twelve-month period of at least $38.0 million down from $45.0 million. See “Notes to Financial Statements—Note 17. Subsequent Events” for a more detailed discussion of the material terms of our Amended Credit Agreement.
To the extent we continue to experience unfavorable market conditions, like other companies in our industry, we may be unable to comply with the monthly revenue covenant during the twelve-month period following the date on which the financial statements are available for issuance. Failing to comply with the monthly revenue covenant would be an event of default under the Amended Credit Agreement and the lender would have the right, but not the obligation, to accelerate our obligations to pay the outstanding balance due and payable under the Term Loan. If we violate one or more of our covenants under the Amended Credit Agreement and are not able to obtain a waiver from or agree to an accommodation with the lender with respect to any such violation, we could be required to pay all or a portion of the outstanding amount under the Term Loan. In that event, we may need to seek other sources of capital and there can be no assurances that we would be able to do so on acceptable terms.
We also have an ATM Facility under which we may offer and sell, from time to time, shares of our common stock having aggregate gross proceeds of up to $50.0 million. We will pay a commission of up to 3.0% of gross sales proceeds of any common stock sold under the ATM Facility. The aggregate market value of shares eligible for sale under the ATM Facility will be subject to the limitations of General Instruction I.B.6 of Form S-3, to the extent required under such instruction. See “Notes to Financial Statements—Note 2. Basis of Presentation and Summary of Significant Accounting Policies,” for a more detailed discussion of the material terms of our ATM Facility.
As of December 31, 2023, our material cash requirements from known contractual obligations and commitments relate primarily to operating leases for our office, manufacturing, warehouse, and distribution facilities. See “Notes to Financial Statements—Note 7. Leases,” for a discussion of our lease obligations reflected on our balance sheet.
Accounting Standards Codification (ASC) 205-40, Presentation of Financial Statements—Going Concern, requires us to evaluate our ability to continue as a going concern for the twelve-month period following the date on which the financial statements are available for issuance. We performed an assessment to determine whether there were conditions or events that, considered individually and in the aggregate, raised substantial doubt about our ability to continue as a going concern for the twelve-month period following the date on which our financial statements are being issued. This assessment indicated certain negative conditions and events, described further above related to our availability of capital resources and ability to meet the monthly revenue covenant under our Amended Credit Agreement, that raise substantial doubt about our ability to continue as a going concern.
The accompanying financial statements included in Part II, Item 8 of this Annual Report on Form 10-K, have been prepared assuming we will continue as a going concern, which contemplates continuity of operations, realization of assets, and the satisfaction of liabilities in the normal course of business for one year following the issuance of the audited financial statements. As such, the accompanying financial statements do not include any adjustments relating to the recoverability and classification of assets and their carrying amounts, or the amount and classification of liabilities that may result should we be unable to continue as a going concern.
The following table sets forth, for the periods indicated, net cash flows used in operating activities, used in investing activities and provided by financing activities (in thousands):
65
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net cash used in operating activities |
|
$ |
(18,814 |
) |
|
$ |
(27,400 |
) |
Net cash used in investing activities |
|
|
(7,737 |
) |
|
|
(28,149 |
) |
Net cash provided by financing activities |
|
|
12,799 |
|
|
|
10,267 |
|
Net decrease in cash and cash equivalents |
|
$ |
(13,752 |
) |
|
$ |
(45,282 |
) |
Operating Activities
Net cash used in operating activities consists primarily of net loss adjusted for certain non-cash items (including depreciation and amortization, bad debt expense, deferred taxes, loss on disposal of property, plant, and equipment, inventory reserve, amortization of debt issuance costs, and stock-based compensation expense), and the effect of changes in working capital and other operating activities.
Net cash used in operating activities was $18.8 million in 2023, which primarily consisted of net loss of $36.8 million plus net adjustments for non-cash charges of $15.7 million and net changes in operating assets and liabilities of $2.2 million. The primary non-cash adjustments to net loss included $5.7 million of depreciation and amortization, $4.1 million of stock-based compensation, a $2.2 million impairment charge related to long-lived assets, a $2.2 million impairment charge related to the Teknova tradename, a $0.8 million loss on extinguishment of debt, $0.5 million in amortization of debt financing costs, and a $0.3 million provision related to our inventory reserve. The main drivers of the changes in operating assets and liabilities were a $1.9 million increase in accrued liabilities, a $0.4 million decrease in other non-current assets, a $0.3 million decrease in inventories, and a $0.3 million decrease in accounts receivable, partially offset by a $0.8 million decrease in accounts payable.
Net cash used in operating activities was $27.4 million in 2022, which primarily consisted of net loss of $47.5 million plus net adjustments for non-cash charges of $27.4 million, offset by net changes in operating assets and liabilities of $7.3 million. The primary non-cash adjustments to net loss included a $16.6 million goodwill impairment charge, a $4.2 million impairment charge related to long-lived assets, $3.7 million of stock-based compensation, $3.2 million of depreciation and amortization, a $0.7 million provision related to our inventory reserve, partially offset by $1.9 million in deferred taxes. The main drivers of the changes in operating assets and liabilities were a $7.6 million increase in inventories and $2.1 million increase in other non-current assets, partially offset by a $1.2 million decrease in income taxes receivable, $0.6 million increase in accounts payable, and $0.4 million decrease in accounts receivable.
Investing Activities
Net cash used in investing activities relates primarily to capital expenditures, partially offset by proceeds from the sale of certain long-lived assets.
Net cash used in investing activities was $7.7 million in 2023, which consisted of purchases of property, plant, and equipment of $7.9 million, partially offset by proceeds from the sale of certain long-lived assets of $0.2 million.
Net cash used in investing activities was $28.1 million in 2022, which consisted of purchases of property, plant, and equipment.
Financing Activities
Net cash provided by financing activities primarily relates to proceeds from our Offerings, proceeds and payments related to our long-term debt, the exercise of stock options, issuance of common stock under our employee stock purchase plan, and other financing activities.
Net cash provided by financing activities was $12.8 million in 2023, which was primarily attributable to net proceeds from the Offerings of $22.5 million and proceeds from financed insurance premiums of $1.0 million, partially offset by repayment of long-term debt of $10.0 million, repayment of financed insurance premiums of $0.6 million, and payment of offering costs of $0.4 million related to the ATM Facility. We also received proceeds of $0.1 million from the exercise of stock options and $0.3 million from issuance of common stock under our employee stock purchase plan.
66
Net cash provided by financing activities was $10.3 million in 2022, which was primarily attributable to proceeds from long-term debt of $10.1 million, partially offset by payment of debt issuance costs of $0.2 million and payment of exit fee costs of $0.1 million related to our debt refinancing. We also received proceeds of $0.1 million from the exercise of stock options and $0.3 million from issuance of common stock under our employee stock purchase plan.
Critical Accounting Policies and Estimates
Our financial statements have been prepared in accordance with GAAP. The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and contingencies as of the date of the financial statements and reported amounts of revenues and expenses during the reporting periods. We evaluate our estimates on an ongoing basis. We base our estimates on historical experience and on other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. However, future events may cause us to change our assumptions and estimates, which may require adjustment. Actual results could differ from these estimates.
We believe the following critical accounting policies involve significant areas where management applies judgments and estimates in the preparation of our financial statements.
Revenue Recognition
We account for revenue in accordance with ASC 606. This process involves identifying the contract with a customer, identifying the performance obligations in the contract, determining the transaction price, allocating the transaction price to the performance obligations in the contract, and recognizing revenue when or as we satisfy performance obligations.
We recognize revenue from the sale of manufactured products and services when control of promised goods or services is transferred to customers in an amount that reflects the consideration we expect to be entitled to in exchange for those goods or services. Control is transferred when the customer has the ability to direct the use of and obtain benefits from the goods or services. The majority of our sales agreements contain performance obligations satisfied at a point in time when control is transferred to the customer.
Occasionally, we offer rebates, discounts, and returns on our products, however, returns and refunds occur rarely. We record rebates, discounts, and returns at the time they occur. The difference between recording these as they occur and estimating the amount of consideration in exchange for the transfer of promised goods would not have a material impact on the financial statements.
Intangible Assets and Other Long-Lived Assets
We review our definite-lived intangible assets and other long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of these assets may not be recoverable. Recoverability is measured by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated. Significant judgment is required to estimate the amount and timing of future cash flows and the relative risk of achieving those cash flows. The key assumptions that we use in our discounted cash flow model are the amount and timing of estimated future cash flows to be generated by the asset over an extended period of time and a rate of return that considers the relative risk of achieving the cash flows, the time value of money, and other factors that a willing market participant would consider.
Indefinite lived intangible assets are also subject to an impairment test at least annually, as of October 1, or more frequently if events or circumstances indicate that it is more likely than not that the asset is impaired. If the fair value of the asset is less than the carrying amount, an impairment loss would be recognized in an amount equal to the difference between the carrying amount and the fair value. We determined that as of December 31, 2023, the fair value of our indefinite lived intangible assets was less than the carrying amount. As a result we recorded a $2.2 million impairment charge during the year ended December 31, 2023 related to our tradename, with no comparable
67
charges in 2022. Refer to “Notes to Financial Statements—Note 8. Intangible Assets, Net,” in our financial statements for details regarding the impairment.
Assumptions and estimates about future values and remaining useful lives are complex and often subjective. They can be affected by a variety of factors, including external factors such as industry and economic trends, and internal factors such as changes in our business strategy and our internal forecasts.
Income Taxes
The asset and liability method is used in accounting for deferred income taxes. Under this method, deferred income taxes are provided for differences between the carrying amounts of assets and liabilities for financial reporting and tax purposes using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Accordingly, our tax provision contemplates tax rates currently in effect to determine our current tax provision as well as enacted tax rates expected to apply to taxable income in the fiscal years in which those temporary differences are expected to be recovered or settled to determine our deferred tax provision. Any significant fluctuation in rates or changes in tax laws could lead to either increases or decreases in our effective tax rate.
Our provision for income taxes, deferred tax assets and liabilities, and reserves for unrecognized tax benefits reflect our best assessment of estimated future taxes to be paid. Significant judgments and estimates based on interpretations of existing tax laws or regulations are required in determining our provision for income taxes. Changes in tax laws, statutory tax rates, and estimates of our future taxable income could impact the deferred tax assets and liabilities provided for in the financial statements and would require an adjustment to the provision for income taxes.
Stock-Based Compensation
Stock-based compensation expense is recognized based on the fair value and is expensed on a straight-line basis over the requisite service periods of the award, which generally represents the scheduled vesting period. Forfeitures are recognized as they occur. We account for stock-based compensation expense based on the estimated grant date fair value, using the Black-Scholes option-pricing model which requires us to make a number of assumptions, including expected volatility, the expected risk-free interest rate, the expected term and the expected dividend.
These inputs are subjective and generally require significant analysis and judgment to develop. Changes in the assumptions can materially affect the fair value and ultimately how much stock-based compensation is recognized.
68
Accounting Pronouncements Not Yet Adopted
In November 2023, the Financial Accounting Standards Board (FASB) issued Accounting Standard Update (ASU) 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which expands public entities’ segment disclosures by requiring disclosure of significant segment expenses that are regularly provided to the CODM and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. Additionally, all disclosure requirements under the guidance are also required for public entities with a single reportable segment. The amendments are effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The amendments should be applied retrospectively to all prior periods presented in the financial statements. We are currently evaluating the impact of this standard to determine its impact on our disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires disclosure in the rate reconciliation table additional categories of information about federal, state and foreign income taxes and to provide more details about the reconciliation items in some categories if the items meet a quantitative threshold. The guidance also requires disclosure of income taxes paid, net of refunds, disaggregated by federal (national), state and foreign taxes for annual periods and to disaggregate the information by jurisdiction based on a quantitative threshold. The guidance is effective for fiscal years beginning after December 15, 2024. We are currently evaluating the impact of this standard to determine its impact on our disclosures.
Recent Securities and Exchange Commission (SEC) Final Rules Not Yet Adopted
In March 2024, the SEC adopted final rules under SEC Release No. 33-11275, The Enhancement and Standardization of Climate-Related Disclosures for Investors, which requires registrants to provide certain climate-related information in their registration statements and annual reports. The rules require information about a registrant’s climate-related risks that have materially impacted, or are reasonably likely to have a material impact on its business, results of operations, or financial condition. In addition, certain disclosures related to severe weather events and other natural conditions will be required in the registrant’s audited financial statements. Disclosure requirements will begin phasing in for fiscal years beginning on or after January 1, 2025. We are currently evaluating the impact of these new final rules on our financial statements and disclosures.
Emerging Growth Company and Smaller Reporting Company
We qualify as an “emerging growth company” as defined in the JOBS Act. As long as we qualify as an emerging growth company, we may take advantage of certain exemptions from various reporting requirements and other burdens that are otherwise applicable generally to public companies. These provisions include, but are not limited to:
In addition, under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to avail ourselves of this exemption from adopting new or revised accounting standards, and, therefore, we will not be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies or that have opted out of using such extended transition period, which may make comparison of our financial statements with those of other public companies more difficult. We may take advantage of these reporting exemptions until we no longer qualify as an emerging growth company, or, with respect to adoption of certain new or revised accounting standards, until we irrevocably elect to opt out of using the extended transition period.
69
Under the JOBS Act, we will remain an emerging growth company until the earliest to occur of:
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company, as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, for this reporting period and are not required to provide the information required under this item.
Item 8. Financial Statements and Supplementary Data.
The information required by this item is set forth at the pages indicated in Part IV, Item 15(a)(1) and 15(a)(2), respectively, of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (CEO) and Chief Financial Officer (CFO) (our principal executive officer and principal financial officer, respectively), evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act means controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Based upon our evaluation of the Company’s disclosure controls and procedures, as of December 31, 2023, the CEO and the CFO concluded that the disclosure controls are effective to provide reasonable assurance that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is accumulated and communicated to management, including the CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure and are effective to provide reasonable assurance that such information is recorded, processed, summarized, and reported within the time periods specified by the SEC’s rules and forms.
70
Internal Control Over Financial Reporting
Management's Report on Internal Controls Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15(d)-15(f) under the Exchange Act). Our management conducted an assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that assessment, our management has concluded that our internal control over financial reporting was effective as of December 31, 2023.
Attestation of Independent Registered Public Accounting Firm
Our independent registered public accounting firm is not required to attest to the effectiveness of our internal control over financial reporting for as long as we are an “emerging growth company” pursuant to the provisions of the JOBS Act.
Changes in Internal Control Over Financial Reporting
Except for the remediation of material weakness described below, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarter ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Remediation of the Prior Year Material Weakness
During the audit of our financial statements, for the fiscal years ended December 31, 2022, we and our independent registered public accounting firm identified a material weakness related to our accounting for income taxes due to errors identified and resulting adjustments recorded in the same period. Specifically, the Company did not have the appropriate complement of tax resources commensurate with the nature and complexity associated with the Company’s income tax accounting process which lead to adjustments to correct errors in our accounting for income taxes during the same period. Our audited financial statements presented income taxes in accordance with GAAP, however, such adjustments amounted to a material weakness. The material weakness remained un-remediated as of December 31, 2022.
As a result of this material weakness, we engaged a new accounting firm with specific income tax accounting experience necessary to assist with our accounting for income taxes as well as implementing and adopting additional controls and procedures. These changes and the remediation of this material weaknesses identified, were completed during the quarter ended December 31, 2023.
Item 9B. Other Information.
Not applicable.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
71
PART III
Item 10. Directors, Executive Officers, and Corporate Governance.
The information required by this Item will be contained in our definitive proxy statement on Schedule 14A to be filed with the SEC in connection with our 2023 Annual Meeting of the Stockholders (the Proxy Statement), which we expect to file not later than 120 days after the end of our fiscal year ended December 31, 2023, and is incorporated in this Annual Report on Form 10-K by reference.
Item 11. Executive Compensation.
The information required by this Item will be contained in our Proxy Statement, which we expect to file not later than 120 days after the end of our fiscal year ended December 31, 2023, and is incorporated in this report by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this Item will be contained in our Proxy Statement, which we expect to file not later than 120 days after the end of our fiscal year ended December 31, 2023, and is incorporated in this report by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this Item will be contained in our Proxy Statement, which we expect to file not later than 120 days after the end of our fiscal year ended December 31, 2023, and is incorporated in this report by reference.
Item 14. Principal Accounting Fees and Services.
The information required by this Item will be contained in our Proxy Statement, which we expect to file not later than 120 days after the end of our fiscal year ended December 31, 2023, and is incorporated in this report by reference.
72
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a) The following documents are filed as a part of this report:
(1) Financial Statements
Our Financial Statements are listed in the “Index to Financial Statements” of Alpha Teknova, Inc. beginning on page F-1 immediately following the signature pages of this Annual Report on Form 10-K.
(2) Financial Statement Schedules
All financial statement schedules called for under Regulation S-X are omitted because either they are not applicable or are not required under the related instructions, or because the required information is included either in the Financial Statements or Notes thereto included elsewhere in this Annual Report on Form 10-K.
(3) Exhibits
The following exhibits are incorporated by reference or filed with this Annual Report on Form 10-K, in each case as indicated therein (numbered in accordance with Item 601 of Regulation S-K).
73
Exhibit Index
Exhibit Number |
|
Description |
1.1 |
|
|
3.1 |
|
|
3.2 |
|
|
4.1 |
|
|
4.2 |
|
|
4.3 |
|
|
4.4 |
|
|
10.1 |
+ |
|
10.2 |
+ |
|
10.3 |
+ |
|
10.4 |
+ |
|
10.5 |
+ |
|
10.6 |
+ |
|
10.7 |
+ |
|
10.8 |
+ |
|
10.9 |
+# |
|
10.10 |
+ |
|
10.11 |
+ |
74
10.12 |
+ |
|
10.13 |
+ |
|
10.14 |
+ |
|
10.15 |
+* |
|
10.16 |
|
|
10.17 |
|
|
10.18 |
|
|
10.19 |
|
|
10.20 |
|
|
10.21 |
|
|
10.22 |
+ |
|
10.23 |
§ |
|
10.24 |
§ |
|
10.25 |
§ |
|
10.26 |
§ |
|
10.27 |
§ |
75
|
|
|
10.28 |
§ |
|
10.29 |
§ |
|
10.30 |
§ |
|
10.31 |
§ |
|
10.32 |
§ |
|
10.33 |
§ |
|
10.34 |
§ |
|
10.35 |
§ |
|
10.36 |
§ |
|
10.37 |
|
|
10.38 |
* |
Summary of Teknova's Non-Employee Director Compensation Policy. |
23.1 |
* |
Consent of Ernst & Young, LLP, Independent Registered Public Accounting Firm. |
24.1 |
* |
Power of Attorney (see page 78 of this Annual Report on Form 10-K). |
31.1 |
* |
|
31.2 |
* |
76
32.1 |
* |
|
97 |
* |
Policy Relating to Recovery of Erroneously Awarded Compensation |
101.INS |
|
Inline XBRL Instance Document |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
104 |
|
Cover Page Interactive Data File, formatted in Inline XBRL |
* |
Filed herewith. |
+ |
Management contract or compensatory plan or arrangement. |
# |
Certain confidential information contained in this Exhibit has been omitted because it is both (i) not material and (ii) of the type that the Registrant treats as private or confidential. |
§ |
Non-material schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Registrant hereby undertakes to furnish supplemental copies of any of the omitted Schedules and exhibits upon request by the SEC. |
(c) Financial Statement Schedules
No financial statement schedules are provided because the information called for is not applicable.
Item 16. Form 10-K Summary
None.
77
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Stephen Gunstream and Matt Lowell, and each of them, his or her true and lawful attorneys-in-fact, each with full power of substitution, for him or her in any and all capacities, to sign any amendments to this Annual Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact or their substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
Alpha Teknova, Inc. |
|
|
|
|
|
Date: March 26, 2024 |
|
By: |
/s/ Stephen Gunstream |
|
|
|
Stephen Gunstream |
|
|
|
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
Name |
|
Title |
|
Date |
|
|
|
|
|
/s/ Stephen Gunstream |
|
President, Chief Executive Officer, and Director (Principal Executive Officer) |
|
March 26, 2024 |
Stephen Gunstream |
|
|
|
|
|
|
|
|
|
/s/ Matt Lowell |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 26, 2024 |
Matt Lowell |
|
|
|
|
|
|
|
|
|
/s/ Paul Grossman |
|
Chairman of the Board |
|
March 26, 2024 |
Paul Grossman |
|
|
|
|
|
|
|
|
|
/s/ Irene Davis |
|
Director |
|
March 26, 2024 |
Irene Davis |
|
|
|
|
|
|
|
|
|
/s/ Ted Davis |
|
Director |
|
March 26, 2024 |
Ted Davis |
|
|
|
|
|
|
|
|
|
/s/ Martha J. Demski |
|
Director |
|
March 26, 2024 |
Martha J. Demski |
|
|
|
|
|
|
|
|
|
/s/ Alexander Herzick |
|
Director |
|
March 26, 2024 |
Alexander Herzick |
|
|
|
|
|
|
|
|
|
/s/ J. Matthew Mackowski |
|
Director |
|
March 26, 2024 |
J. Matthew Mackowski |
|
|
|
|
|
|
|
|
|
/s/ Brett Robertson |
|
Director |
|
March 26, 2024 |
Brett Robertson |
|
|
|
|
|
|
|
|
|
/s/ Alexander Vos |
|
Director |
|
March 26, 2024 |
Alexander Vos |
|
|
|
|
78
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Alpha Teknova, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Alpha Teknova, Inc. as of December 31, 2023 and December 31, 2022, the related statements of operations, stockholders’ equity and cash flows for the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and December 31, 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
The Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses from operations, has an accumulated deficit, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/
We have served as the Company’s auditor since 2020.
March 26, 2024
F-2
ALPHA TEKNOVA, INC.
Statements of Operations
(in thousands, except share and per share data)
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Revenue |
|
$ |
|
|
$ |
|
||
Cost of sales |
|
|
|
|
|
|
||
Gross profit |
|
|
|
|
|
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
|
|
|
|
|
||
Sales and marketing |
|
|
|
|
|
|
||
General and administrative |
|
|
|
|
|
|
||
Amortization of intangible assets |
|
|
|
|
|
|
||
Goodwill impairment |
|
|
|
|
|
|
||
Tradename impairment |
|
|
|
|
|
|
||
Long-lived assets impairment |
|
|
|
|
|
|
||
Total operating expenses |
|
|
|
|
|
|
||
Loss from operations |
|
|
( |
) |
|
|
( |
) |
Other (expenses) income, net |
|
|
|
|
|
|
||
Interest (expense) income, net |
|
|
( |
) |
|
|
|
|
Other income, net |
|
|
|
|
|
|
||
Loss on extinguishment of debt |
|
|
( |
) |
|
|
|
|
Total other (expenses) income, net |
|
|
( |
) |
|
|
|
|
Loss before income taxes |
|
|
( |
) |
|
|
( |
) |
Benefit from income taxes |
|
|
( |
) |
|
|
( |
) |
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Net loss per share—basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted average shares used in computing net loss per share—basic and diluted |
|
|
|
|
|
|
||
The accompanying notes are an integral part of these financial statements.
F-3
ALPHA TEKNOVA, INC.
Balance Sheets
(in thousands, except share and per share data)
|
|
As of December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Accounts receivable, net of allowance for doubtful accounts of $ |
|
|
|
|
|
|
||
Inventories, net |
|
|
|
|
|
|
||
Income taxes receivable |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Property, plant, and equipment, net |
|
|
|
|
|
|
||
Operating right-of-use lease assets |
|
|
|
|
|
|
||
Intangible assets, net |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Accrued liabilities |
|
|
|
|
|
|
||
Current portion of operating lease liabilities |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
Deferred tax liabilities |
|
|
|
|
|
|
||
Other accrued liabilities |
|
|
|
|
|
|
||
Long-term debt, net |
|
|
|
|
|
|
||
Long-term operating lease liabilities |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
Stockholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $ |
|
|
|
|
|
|
||
Common stock, $ |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total stockholders’ equity |
|
|
|
|
|
|
||
Total liabilities and stockholders’ equity |
|
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these financial statements.
F-4
ALPHA TEKNOVA, INC.
Statements of Stockholders’ Equity
(in thousands, except share data)
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated |
|
|
Stockholders’ |
|
||||||||
Successor |
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Equity |
|
|||||
Balance at January 1, 2022 |
|
|
|
|
$ |
— |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Issuance of common stock upon exercise of stock options |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Issuance of common stock under employee stock purchase plan |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2022 |
|
|
|
|
|
— |
|
|
|
|
|
|
( |
) |
|
|
|
|||
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Issuance of common stock upon exercise of stock options |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Issuance of common stock under employee stock purchase plan |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|||
Vesting of restricted stock units |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Equity financing, net of issuance costs |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
||||
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance at December 31, 2023 |
|
|
|
|
$ |
— |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
The accompanying notes are an integral part of these financial statements.
F-5
ALPHA TEKNOVA, INC.
Statements of Cash Flows
(in thousands)
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Bad debt expense |
|
|
|
|
|
|
||
Inventory reserve |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
|
|
|
|
||
Stock-based compensation |
|
|
|
|
|
|
||
Deferred taxes |
|
|
( |
) |
|
|
( |
) |
Amortization of debt financing costs |
|
|
|
|
|
|
||
Non-cash lease expense |
|
|
|
|
|
|
||
Loss on disposal of property, plant, and equipment |
|
|
|
|
|
|
||
Goodwill impairment |
|
|
|
|
|
|
||
Tradename impairment |
|
|
|
|
|
|
||
Long-lived assets impairment |
|
|
|
|
|
|
||
Loss on extinguishment of debt |
|
|
|
|
|
|
||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable |
|
|
|
|
|
|
||
Inventories |
|
|
|
|
|
( |
) |
|
Income taxes receivable |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
( |
) |
|
Accounts payable |
|
|
( |
) |
|
|
|
|
Accrued liabilities |
|
|
|
|
|
|
||
Other |
|
|
( |
) |
|
|
( |
) |
Cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
Investing activities: |
|
|
|
|
|
|
||
Proceeds from sale of property, plant, and equipment |
|
|
|
|
|
|
||
Purchases of property, plant, and equipment |
|
|
( |
) |
|
|
( |
) |
Cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
Financing activities: |
|
|
|
|
|
|
||
Proceeds from equity financing, net |
|
|
|
|
|
|
||
Repayment of long-term debt |
|
|
( |
) |
|
|
|
|
Proceeds from financed insurance premiums |
|
|
|
|
|
|
||
Repayment of financed insurance premiums |
|
|
( |
) |
|
|
|
|
Proceeds from long-term debt |
|
|
|
|
|
|
||
Payment of debt issuance costs |
|
|
( |
) |
|
|
( |
) |
Payment of exit fee costs |
|
|
|
|
|
( |
) |
|
Payment of at-the-market facility costs |
|
|
( |
) |
|
|
|
|
Proceeds from exercise of stock options |
|
|
|
|
|
|
||
Proceeds from issuance of common stock under employee stock purchase plan |
|
|
|
|
|
|
||
Cash provided by financing activities |
|
|
|
|
|
|
||
Change in cash and cash equivalents |
|
|
( |
) |
|
|
( |
) |
Cash and cash equivalents at beginning of period |
|
|
|
|
|
|
||
Cash and cash equivalents at end of period |
|
$ |
|
|
$ |
|
||
Supplemental cash flow disclosures: |
|
|
|
|
|
|
||
Income taxes paid |
|
$ |
|
|
$ |
|
||
Interest paid, net of amounts capitalized |
|
$ |
|
|
$ |
|
||
Offering costs included in accounts payable |
|
$ |
|
|
$ |
|
||
Capitalized property, plant, and equipment included in accounts payable and accrued liabilities |
|
$ |
|
|
$ |
|
||
Recognition of operating right-of-use lease asset |
|
$ |
( |
) |
|
$ |
|
|
Recognition of operating lease liabilities |
|
$ |
( |
) |
|
$ |
|
|
The accompanying notes are an integral part of these financial statements.
F-6
ALPHA TEKNOVA, INC.
Notes to Financial Statements
Note 1. Nature of the Business
Alpha Teknova, Inc. (referred to herein as the Company or Teknova), produces critical reagents for the discovery, development, and commercialization of novel therapies, vaccines, and molecular diagnostics. Product offerings include pre-poured media plates for cell growth and cloning; liquid cell culture media and supplements for cellular expansion; and molecular biology reagents for sample manipulation, resuspension, and purification. Teknova supports customers spanning the life sciences market, including pharmaceutical and biotechnology companies, contract development and manufacturing organizations, in vitro diagnostic franchises, and academic and government research institutions, with catalog and custom, made-to-order products.
Teknova manufactures its products at its Hollister, California headquarters and stocks inventory of raw materials, components, and finished goods at that location. The Company ships products directly from its warehouse in Hollister, California.
Concurrent Registered Direct Offering and Private Placements
On September 15, 2023, the Company entered into a securities purchase agreement (the Registered Direct Purchase Agreement) in connection with a registered direct offering (the Registered Direct Offering) with certain accredited investors and qualified institutional buyers. On September 15, 2023, the Company also entered into a securities purchase agreement (the PIPE Purchase Agreement and, together with the Registered Direct Purchase Agreement, the Purchase Agreements) and a registration rights agreement (the Registration Rights Agreement) in connection with a concurrent private placement (the PIPE Private Placement) with certain accredited investors and qualified institutional buyers.
Pursuant to the Registered Direct Purchase Agreement, the Company sold
The Company’s controlling stockholder, Telegraph Hill Partners Management Company LLC, through its affiliates Telegraph Hill Partners IV, L.P. and THP IV Affiliates Fund, LLC, the Company’s President and Chief Executive Officer and a member of its board of directors, Stephen Gunstream, the Company’s Chief Financial Officer, Matthew Lowell, and the Company’s General Counsel and Chief Compliance Officer, Damon Terrill, and the Mackowski Family Trust, which is affiliated with J. Matthew Mackowski, a member of the Company’s board of directors, participated in the PIPE Private Placement and purchased an aggregate of
The Company received aggregate gross proceeds of $
The Offerings closed on September 19, 2023.
Note 2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Accounting, Presentation, and Use of Estimates
The accompanying financial statements and related notes are prepared in accordance with U.S. generally accepted accounting principles (GAAP). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect certain amounts of assets and liabilities, and disclosures of assets and liabilities, at the date of each financial statement, and the reported amount of revenues and expenses
F-7
during the reporting period. Significant items that are subject to such estimates and assumptions include, but are not limited to, the revenue recognition, impairment of long-lived assets and intangible assets, valuation of share-based payment awards, and income taxes. Although management bases its estimates on historical experience and various other assumptions that are believed to be reasonable under the circumstances, actual results could differ significantly from the estimates under different assumptions or conditions.
Going Concern
Accounting Standards Codification (ASC) 205-40, Presentation of Financial Statements—Going Concern, requires management to evaluate an entity’s ability to continue as a going concern for the twelve-month period following the date on which the financial statements are available for issuance. Management performed an assessment to determine whether there were conditions or events that, considered individually and in the aggregate, raised substantial doubt about the Company’s ability to continue as a going concern for the twelve-month period following the date on which the accompanying financial statements are being issued. This assessment indicated certain negative conditions and events, described further below, that raise substantial doubt about the Company’s ability to continue as a going concern.
As of December 31, 2023, the Company had limited capital resources to fund ongoing operations. During the year ended December 31, 2023, Teknova incurred net losses of $
As disclosed in Note. 17. Subsequent Events, the Company is subject to certain financial covenants as set forth in the Amended Credit Agreement (defined in Note 17). These financial covenants include (i) a trailing twelve months minimum net revenue covenant that must be met each calendar month, and (ii) a requirement to maintain a minimum level of cash at all times through the term of the Amended Credit Agreement. To the extent the Company continues to experience unfavorable market conditions, like other companies in the industry, the Company believes it may be unable to comply with the trailing twelve months revenue covenant during the twelve-month period following the date on which the financial statements are available for issuance. Failing to comply with the monthly revenue covenant would be an event of default under the Amended Credit Agreement and the lender would have the right, but not the obligation, to accelerate the Company’s obligations to pay the outstanding balance due and payable under the Term Loan (defined in Note 10). If the Company violates one or more of its covenants under the Amended Credit Agreement and is not able to obtain a waiver from or agree to an accommodation with the lender with respect to any such violation, the Company could be required to pay all or a portion of the outstanding amount under the Term Loan. In that event, the Company may need to seek other sources of capital and there can be no assurances that the Company would be able to do so on acceptable terms.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern, which contemplates continuity of operations, realization of assets, and the satisfaction of liabilities in the normal course of business for one year following the issuance of these audited financial statements. As such, the accompanying financial statements do not include any adjustments relating to the recoverability and classification of assets and their carrying amounts, or the amount and classification of liabilities that may result should the Company be unable to continue as a going concern.
F-8
Segment Reporting
Operating segments are defined as components of an entity for which separate financial information is available and that is regularly reviewed by the Chief Operating Decision Maker (CODM) in deciding how to allocate resources to an individual segment and in assessing performance. Teknova’s CODM is its Chief Executive Officer, currently Stephen Gunstream. Teknova has determined that it operates in one reporting unit,
Concentrations of Credit Risk
Teknova’s financial instruments that are exposed to concentration of credit risk consist primarily of cash and cash equivalents and accounts receivable. The Company places its cash with high-quality banking institutions. At times, the Company’s cash balances may exceed the Federal Deposit Insurance Corporation (FDIC) insurance limit. Teknova’s cash equivalents consist primarily of money market funds and U.S. Treasuries. Teknova extends credit to customers based on its evaluation of the customer’s financial condition and routinely communicates with its customers regarding payments. The Company has a history of limited write-offs, and therefore believes that its accounts receivable credit risk exposure is low. For information regarding the Company’s significant customers and suppliers, see Note 4. Concentrations of Risk.
Cash and Cash Equivalents
Teknova’s cash and cash equivalents include cash on hand, cash held in banks, and highly-liquid investments with maturities of three months or less at the date of acquisition. Teknova maintains its cash in bank deposit accounts in financial institutions that are insured by the FDIC up to a balance of $
Fair Value of Financial Instruments
The carrying amounts of certain of Teknova’s financial instruments, including cash equivalents, accounts receivable, inventories, accounts payable and accrued liabilities, approximate fair value due to their relatively short maturities.
Accounts Receivable
Accounts receivable are stated at invoice value, less estimated allowances for doubtful accounts. Teknova uses the allowance method to account for uncollectible accounts receivable, calculated by management using the historical average of uncollectible accounts. The Company continually monitors its customer payments and maintains an allowance for estimated losses resulting from its customers’ inability to make required payments. Accounts receivable are considered past due once customer payment terms have been exceeded. Receivables are written off when deemed uncollectible. Recoveries of trade receivables previously written off are recorded when received.
Inventories
Inventory, consisting of raw materials, work in process and finished goods, is stated at the lower of cost or net realizable value, on a first-in, first-out basis. Teknova writes down its inventory for estimated obsolescence or inventory in excess of reasonably expected near-term sales or unmarketable inventory, in an amount equal to the difference between the cost of inventory and the estimated net realizable value, based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by Teknova, additional inventory write-downs may be required. Inventory impairment charges establish a new cost basis for inventory, and charges are not reversed subsequently to income, even if circumstances later suggest that increased carrying amounts are recoverable.
F-9
Capitalized Software Implementation Costs
Teknova capitalizes certain implementation costs incurred under a cloud computing hosting arrangement. Costs incurred during the application development stage related to the implementation of the hosting arrangement are capitalized and included within other assets on the accompanying balance sheets. Amortization of capitalized implementation costs is recognized on a straight-line basis over the expected term of the associated hosting arrangement when it is ready for its intended use. Costs related to preliminary project activities and post-implementation activities are expensed as incurred. As of December 31, 2023 and 2022, Teknova had capitalized software implementation costs of $
Property, Plant, and Equipment
Teknova records property, plant, and equipment at fair value when it is acquired in a business combination or at cost for all other purchases of property, plant, and equipment. Property, plant, and equipment is depreciated over the estimated useful lives of the assets, using the straight-line method. Any leasehold improvements are amortized on a straight-line basis over the shorter of the estimated useful life of the asset or the estimated remaining life of the lease. Costs for repairs and maintenance that do not significantly increase the value or estimated lives of property, plant, and equipment are expensed as incurred. Upon retirement or sale, the cost and related accumulated depreciation are removed from the balance sheets, and the resulting gain or loss is reflected in the statements of operations.
The estimated useful lives of the major classes of property and equipment are as follows:
|
|
Estimated Useful Lives |
Machinery and equipment |
|
|
Office furniture and equipment |
|
|
Vehicles |
|
|
Leasehold improvements |
|
Impairment of Long-Lived Assets
Teknova evaluates its long-lived assets for impairment when events or changes in circumstances indicate a possible inability to recover carrying amounts. Recoverability is assessed by comparing the carrying value of the assets to estimated undiscounted future cash flows expected to be generated by the assets. Impairment losses are recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated during the life of those assets are less than the assets’ carrying amounts. If an asset is impaired, the loss is measured as the amount by which the asset’s carrying value exceeds its fair value. For each of the years ended December 31, 2023 and 2022, the Company recorded impairments of its long-lived assets, see Note 6. Property, Plant, and Equipment, Net.
Intangible Assets
Teknova’s intangible assets consist of the Teknova trade name and the Company’s customer relationships.
Indefinite lived intangible assets are not amortized but are tested for impairment at least annually as of October 1, or more frequently if events or circumstances indicate that it is more likely than not that an asset is impaired. If the fair value of the asset is less than its carrying amount, an impairment charge would be recognized in an amount equal to the difference between the carrying amount and the fair value.
Finite-lived intangible assets are amortized over the estimated economic useful lives of the assets, which is the period during which expected cash flows support the fair value of such intangible assets. Teknova reviews finite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets or an asset group may not be recoverable. When such an event occurs, management determines
F-10
whether there has been impairment by comparing the anticipated undiscounted future net cash flows to the related assets’ or asset group’s carrying value.
During the year ended December 31, 2023, the Company impaired its indefinite lived intangible asset. See Note 8. Intangible Assets, Net. During the year ended December 31, 2022, the Company fully impaired its goodwill, see Note 8. Intangible Assets, Net. No other intangible assets were impaired during the year ended December 31, 2023 and 2022.
Leases
The Company determines if an arrangement is an operating lease at a lease’s inception. Leases with an initial term of 12 months or fewer are not recorded on the balance sheet. All other operating leases are recorded on the balance sheet with a corresponding operating lease asset, net, representing the right to use the underlying asset for the lease term and the operating lease liabilities representing the obligation to make lease payments arising from the lease. The Company’s lease agreements do not contain any material residual value guarantees or restrictive covenants.
Operating lease assets and operating lease liabilities are recognized at the commencement date based on the present value of lease payments over the lease term and include options to extend or terminate the lease when such options are reasonably certain to be exercised. The present value of lease payments is determined primarily using the incremental borrowing rate, adjusted for the lease term, based on the information available at the lease commencement or modification date as required. Lease agreements with lease and non-lease components are generally accounted for as a single lease component. The Company’s operating lease expense is recognized on a straight-line basis over the lease term.
Debt Issuance Costs
Debt issuance costs represent legal, consulting, and other financial costs associated with debt financing and are presented on the balance sheets as a direct reduction from the carrying amount of the related debt instrument. Debt issuance costs on the term debt are amortized to interest expense over the term of the applicable debt agreement using the effective interest rate method.
Revenue Recognition
We account for revenue in accordance with ASC 606, Revenue From Contracts With Customers. Teknova recognizes revenue for sales of goods through the following steps:
Teknova recognizes revenue from the sale of manufactured products and services when the Company transfers control of promised goods or services to customers in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods or services. Control is transferred when the customer has the ability to direct the use of and obtain benefits from the goods or services. The majority of the Company’s sales agreements contain performance obligations satisfied at a point in time when control is transferred to the customer.
Teknova’s sales are made directly to customers or through distributors, generally under agreements with payment terms typically shorter than 90 days and, in no case exceeding one year. Therefore, Teknova’s contracts do not contain a significant financing component. Sales, value add, and other taxes collected concurrent with revenue are excluded from sales. The Company records amounts billed to customers for shipping and handling in a sales transaction as revenue. Shipping and handling costs are included in general and administrative expenses as revenue
F-11
is recognized. Shipping and handling costs for the years ended December 31, 2023 and 2022 were $
Occasionally, Teknova offers rebates, discounts, and returns on its products, however returns and refunds occur rarely. The Company records rebates, discounts, and returns at the time they occur. The difference between recording these as they occur and estimating the amount of consideration in exchange for the transfer of promised goods would not have a material impact on the financial statements.
Costs incurred to obtain contracts with customers are expensed immediately, because the amortization period for such costs is one year or less.
Cost of Sales
Cost of sales includes salaries, wages and benefits, raw materials consumption (including direct and indirect material), depreciation, utilities, rent, manufacturing supplies, and other production overhead.
Research and Development Expenses
The Company’s research and development expenses primarily consist of employee-related expenses, including salaries, benefits, and stock-based compensation expense for personnel in process engineering and product development functions, expenses related to occupancy costs, laboratory supplies, consulting fees, and depreciation associated with various assets used in the research and development of the Company’s products and processes.
Sales and Marketing Expenses
The Company’s sales and marketing expenses primarily consist of employee-related expenses, including salaries, benefits, and stock-based compensation expense for sales and marketing employees, expenses related to occupancy costs, brand strategy, website, content, and collateral. The Company expenses advertising costs as incurred. Advertising expenses are included in sales and marketing expenses in the accompanying financial statements. Advertising expenses were $
General and Administrative Expenses
Reduction in Workforce
On February 1, 2023, the Company carried out a reduction in workforce of approximately
At-the-Market Facility
On March 30, 2023, the Company entered into a sales agreement (the ATM Facility) with Cowen and Company, LLC (Cowen), under which the Company may offer and sell, from time to time, shares of its common stock having aggregate gross proceeds of up to $
F-12
Form S-3, to the extent required under such instruction. Following the capital raise through offerings described in Note 1. Nature of the Business, costs capitalized related to the ATM Facility of $
Stock-Based Compensation
Teknova measures and recognizes compensation expense for all stock-based awards, including stock options, restricted stock units, and stock purchase rights granted under the Employee Stock Purchase Plan (ESPP) to employees, based on the estimated fair value of the awards on the date of grant. The fair value of each stock option granted and employee stock purchase rights are estimated using the Black-Scholes option-pricing model, which requires the Company to make a number of assumptions, including expected volatility, the expected risk-free interest rate, the expected term, and the expected dividend. The fair value of each restricted stock unit is based on the fair value of the Company’s common stock on the date of grant. Stock-based compensation expense is recognized over the requisite service period of the award, which generally represents the scheduled vesting period. Forfeitures are recognized as they occur.
Employee Benefit Plans
Teknova has a salary deferral 401(k) plan (the 401(k) Plan) covering substantially all employees. Contributions by the Company to the 401(k) Plan for the years ended December 31, 2023 and 2022 were $
Income Taxes
Teknova uses the asset and liability method in accounting for its deferred income taxes. Under this method, deferred income taxes are provided for differences between the carrying amounts of assets and liabilities for financial reporting and tax purposes, using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets and liabilities are adjusted for the effects of the changes in tax laws and rates on the date of enactment. Deferred tax assets are reduced by a valuation allowance when it is more likely than not that some or all of the deferred tax assets will not be realized.
Teknova accounts for unrecognized tax benefits based upon its assessment of whether tax benefits are more likely than not to be sustained upon examination by tax authorities. The Company reports a liability for unrecognized tax benefits taken, or expected to be taken, in a tax return and recognizes associated interest and penalties, if any, in income tax expense. Unrecognized tax benefits as of December 31, 2023 were $
Net Loss Per Share of Common Stock
Basic net income (loss) per share is computed using the two-class method. Diluted net income (loss) per share is computed using the more dilutive of (i) the treasury stock method or if-converted method, or (ii) the two-class method. The two-class method is an earnings allocation formula that determines net income per share for each class of common stock and participating security according to dividends declared and participation rights in undistributed earnings. The treasury stock method uses the number of new shares that may be created by unexercised in-the-money options, where the exercise price is less than the current share price. The if-converted method calculates the value of convertible securities as if they were converted into new shares. Per share amounts are computed by dividing net income (loss) by the weighted average shares outstanding during each period. The diluted net income (loss) per share is computed by giving effect to all potential dilutive common stock equivalents outstanding for the period.
F-13
Recently Adopted Accounting Pronouncements
Effective January 1, 2023, the Company adopted Accounting Standard Update (ASU) No. 2016-13, Financial Instruments—Credit Losses (Topic 326), which introduced a new model for recognizing credit losses on financial instruments based on an estimate of current expected credit losses and applied to the Company’s accounts receivable. The adoption of this standard did not have a significant impact on the Company’s financial statements.
Accounting Pronouncements Not Yet Adopted
In November 2023, the Financial Accounting Standards Board (FASB) issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which expands public entities’ segment disclosures by requiring disclosure of significant segment expenses that are regularly provided to the CODM and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. Additionally, all disclosure requirements under the guidance are also required for public entities with a single reportable segment. The amendments are effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The amendments should be applied retrospectively to all prior periods presented in the financial statements. The Company is currently evaluating the impact of this standard to determine its impact on the Company’s disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires disclosure in the rate reconciliation table additional categories of information about federal, state and foreign income taxes and to provide more details about the reconciliation items in some categories if the items meet a quantitative threshold. The guidance also requires disclosure of income taxes paid, net of refunds, disaggregated by federal (national), state and foreign taxes for annual periods and to disaggregate the information by jurisdiction based on a quantitative threshold. The guidance is effective for fiscal years beginning after December 15, 2024. The Company is currently evaluating the impact of this standard to determine its impact on the Company’s disclosures.
Recent Securities and Exchange Commission (SEC) Final Rules Not Yet Adopted
In March 2024, the SEC adopted final rules under SEC Release No. 33-11275, The Enhancement and Standardization of Climate-Related Disclosures for Investors, which requires registrants to provide certain climate-related information in their registration statements and annual reports. The rules require information about a registrant’s climate-related risks that have materially impacted, or are reasonably likely to have a material impact on its business, results of operations, or financial condition. In addition, certain disclosures related to severe weather events and other natural conditions will be required in the registrant’s audited financial statements. Disclosure requirements will begin phasing in for fiscal years beginning on or after January 1, 2025. The Company is currently evaluating the impact of these new final rules on its financial statements and disclosures.
Note 3. Revenue Recognition
Teknova has
Lab Essentials
Teknova is a leader in providing highly complex chemical formulations for use in biological research and drug discovery. The Company's core research products consist of commonly used, catalog solutions and customer-specified formulations. During discovery, the Company's products are used regularly in small, bench-scale experiments. As customers optimize their processes and begin to scale up in volume, they tend to order more custom products. The Lab Essentials portion of Teknova's business includes: pre-poured media plates for cell growth and cloning; liquid cell culture media and supplements for cellular expansion; and molecular biology reagents for sample manipulation, resuspension, and purification. Teknova's Lab Essentials products include essential formulations for common research applications and highly customized formulations for customer-specific applications in genomics and bioproduction.
F-14
Clinical Solutions
Teknova is ISO 13485:2016 certified, enabling the Company to meet the quality system regulation of products for use as components in diagnostic and therapeutic products manufactured by the Company's customers. Teknova believes that its Clinical Solutions products are used in the production of protein therapies, gene therapies, mRNA vaccines, and diagnostic kits. The Clinical Solutions portion of our business includes: liquid cell culture media and supplements for cellular expansion and molecular biology reagents for sample manipulation, resuspension, and purification.
Teknova’s revenue, disaggregated by product category, was as follows (in thousands):
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Lab Essentials |
|
$ |
|
|
$ |
|
||
Clinical Solutions |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Total revenue |
|
$ |
|
|
$ |
|
||
Teknova’s revenue, disaggregated by geographic region, was as follows (in thousands):
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
United States |
|
$ |
|
|
$ |
|
||
International |
|
|
|
|
|
|
||
Total revenue |
|
$ |
|
|
$ |
|
||
Note 4. Concentrations of Risk
Customers
Customers who accounted for 10% or more of the Company's revenues and outstanding balance of accounts receivable are presented as follows:
|
|
For the Year Ended December 31, |
|
As of December 31, |
||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
Distributor customer A |
|
|
|
|
||||
Distributor customer B |
|
* |
|
* |
|
* |
|
|
* Represents less than 10%.
The Company's customers that are distributors, as opposed to direct customers, represent highly diversified customer bases.
Suppliers
Suppliers who accounted for 10% or more of the Company's inventory purchases and outstanding balance of accounts payable are presented as follows:
|
|
For the Year Ended December 31, |
|
As of December 31, |
||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
Distributor supplier A |
|
|
|
|
||||
Direct supplier A |
|
* |
|
|
* |
|
* |
|
Direct supplier B |
|
* |
|
|
* |
|
* |
|
Direct supplier C |
|
|
* |
|
* |
|
* |
|
* Represents less than 10%.
F-15
The Company’s suppliers that are distributors, as opposed to direct suppliers, represent highly diversified supplier bases.
Note 5. Inventories, Net
Inventories consist of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Finished goods, net |
|
$ |
|
|
$ |
|
||
Work in process |
|
|
|
|
|
|
||
Raw materials, net |
|
|
|
|
|
|
||
Total inventories, net |
|
$ |
|
|
$ |
|
||
Note 6. Property, Plant, and Equipment, Net
Property, plant, and equipment consist of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Machinery and equipment |
|
$ |
|
|
$ |
|
||
Office furniture and equipment |
|
|
|
|
|
|
||
Vehicles |
|
|
|
|
|
|
||
Leasehold improvements |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Less—Accumulated depreciation |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
||
Construction in progress |
|
|
|
|
|
|
||
Total property, plant, and equipment, net |
|
$ |
|
|
$ |
|
||
Depreciation expense related to property, plant, and equipment recorded for the years ended December 31, 2023 and 2022 was $
Teknova capitalizes interest on funds borrowed to finance certain of its capital expenditures. Capitalized interest is recorded as part of an asset’s cost and depreciated over the asset’s useful life. Capitalized interest costs were $
In June 2023, the Company identified circumstances that indicated certain of its machinery and equipment may not be fully recoverable. Specifically, these circumstances included changes in the market price of the asset group, continued losses, and an expectation that, more likely than not, the long-lived assets in question would be sold or otherwise disposed of significantly before the end of their previously estimated useful life. The Company reviewed the recoverability of the carrying value of these assets and determined that their carrying value exceeded their fair value. The fair value of these assets was measured employing cost and market approaches, using Level 3 inputs under ASC 820, Fair Value Measurement. Unobservable inputs included salvage value estimates, replacement or reproduction cost estimates, as well as consideration of physical deterioration, and functional and economic obsolescence, where measurable. As a result of this fair value analysis, an impairment charge of $
In December 2022, the Company decided to cease further use and development of certain manufacturing machinery and equipment. The Company reviewed the recoverability of the carrying value of these assets and determined that their carrying value exceeded their fair value. The fair value of these assets was measured employing cost and market approaches, using Level 3 inputs under ASC 820, Fair Value Measurement.
F-16
Unobservable inputs included salvage value estimates, replacement or reproduction cost estimates as well as consideration of physical deterioration, functional and economic obsolescence, where measurable. As a result of this fair value analysis, an impairment charge of $
Note 7. Leases
The Company leases office space, warehouse and manufacturing space, and equipment. The Company's lease agreements have remaining lease terms of
Operating lease expense was $
Maturities of operating lease liabilities at December 31, 2023, is as follows (in thousands):
|
|
Amount |
|
|
2024 |
|
$ |
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
2028 |
|
|
|
|
Thereafter |
|
|
|
|
Total lease payments |
|
|
|
|
Less: imputed interest |
|
|
( |
) |
Present value of lease liabilities |
|
$ |
|
|
Note 8. Intangible Assets, Net
The following is a summary of intangible assets with definite and indefinite lives (in thousands):
|
|
Balance at December 31, 2023 |
|
|
Balance at December 31, 2022 |
|
||||||||||||||||||
|
|
Gross |
|
|
Accumulated |
|
|
Net |
|
|
Gross |
|
|
Accumulated |
|
|
Net |
|
||||||
Definite Lived: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Customer relationships |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
Indefinite Lived: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Tradename(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Total intangible assets |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||||
F-17
For each of the years ended December 31, 2023 and 2022 amortization expense was approximately $
The remaining weighted-average useful life of definite lived intangible assets is
|
|
Amount |
|
|
2024 |
|
$ |
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
Estimated future amortization expense of definite-lived intangible assets |
|
$ |
|
|
In connection with the Company’s annual budgeting process, during the fourth quarter of 2023 the Company updated its 2024 budget to lower its financial projections in 2024 and beyond. Given the significance of the downward revisions to the Company’s forecast, primarily resulting from adverse industry and market conditions, an impairment test of Teknova’s indefinite lived tradename asset was performed as of December 31, 2023.
The fair value of the tradename was determined using the relief from royalty method. Significant assumptions under this method include forecasted revenues, royalty rate, discount rate, and terminal value. Fair values were determined using Level 3 inputs under ASC 820, Fair Value Measurement (ASC 820). Based on the impairment test performed, the Company determined that the Teknova tradename was impaired as of December 31, 2023. As a result, the Company recognized a $
There was
During the year ended December 31, 2022 the Company recorded a $
The fair value of the Company was determined using a combination of an income approach and market approach. The income approach was based on the present value of future cash flows, which were derived from financial forecasts, and requires significant assumptions and judgment including, among others, a discount rate and a terminal value. Fair values were based on expected future cash flows using Level 3 inputs under ASC 820. The cash flows are those expected to be generated by the Company, discounted at the weighted average cost of capital. The present value of future cash flows was determined by discounting estimated future cash flows at an
The guideline public company method, a market approach method, was also used to estimate the fair value of the Company. The guideline public company method utilizes the trading multiples of similarly traded public companies. The unobservable inputs used to measure the fair value primarily included projected revenue growth rates and the determination of appropriate market comparison companies. Selected multiples were considered and applied to the trailing-twelve-month and next-twelve-month enterprise value-to-revenue multiples.
The resulting estimated fair value was reconciled to the Company’s market capitalization. The reconciliation included an estimated implied control premium above the Company's market capitalization on September 30, 2022, of approximately
F-18
impaired as of December 31, 2022.
Note 9. Accrued Liabilities
Accrued liabilities were comprised of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Payroll-related |
|
$ |
|
|
$ |
|
||
Property, plant, and equipment |
|
|
|
|
|
|
||
Deferred revenue |
|
|
|
|
|
|
||
Insurance premiums and accrued interest |
|
|
|
|
|
|
||
Loss contingency accrual |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Total current accrued liabilities |
|
$ |
|
|
$ |
|
||
On July 13, 2023, the Company entered into a financing agreement with First Insurance Funding for the financing of the Company's Directors and Officers (D&O) liability insurance and related policies. Under the terms of the financing agreement, the Company agreed to pay a total of $
Note 10. Long-Term Debt, Net
On May 10, 2022, the Company entered into the Amended and Restated Credit and Security Agreement (Term Loan) as borrower, with MidCap Financial Trust (MidCap), as agent and lender, and the additional lenders from time to time party thereto (the Term Loan Credit Agreement) and the Amended and Restated Credit and Security Agreement (Revolving Loan) as borrower, with MidCap as agent and lender, and the additional lenders from time to time party thereto (the Revolving Loan Credit Agreement, together with the Term Loan Credit Agreement, the Credit Agreement).
The Credit Agreement provided for a $
The interest on the Term Loan was based on the annual rate of one-month London Inter-Bank Offered Rate (LIBOR) plus
F-19
The maturity date of the Credit Facility is
On November 8, 2022, the Company entered into Amendment No. 1 to the Credit Agreement (Amendment No. 1) which (i) replaced the LIBOR-based interest rate with a rate equal to the forward-looking one-month term Secured Overnight Financing Rate adjusted upward by
On March 28, 2023, the Company entered into Amendment No. 2 to the Credit Agreement (Amendment No. 2) which (i) increased the applicable margin from
On July 13, 2023, the Company entered into Amendment No. 3 to the Credit Agreement (Amendment No. 3), which amended the definition of Permitted Debt in the Credit Agreement from $
On September 19, 2023, the Company entered into Amendment No. 4 to the Credit Agreement (Amendment No. 4). As previously disclosed in our Form 10-Q for the period ended June 30, 2023, the Company determined that it was not in compliance with the trailing twelve months minimum net revenue covenant contained in the Credit Agreement as of July 31, 2023. Amendment No. 4 includes a waiver from MidCap of the revenue covenant violation for the period ending July 31, 2023. As a condition to the effectiveness of Amendment No. 4, the Company prepaid the principal amount of the Term Loan in an amount equal to $
F-20
Concurrent with Amendment No. 4, the exit fee due on the date of termination of the Term Loan, or the date on which the obligations under the Term Loan become due and payable in full, increased from
Long-term debt, net consists of the following (in thousands):
|
|
As of December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Long-term debt |
|
$ |
|
|
$ |
|
||
Cumulative accretion of exit fee |
|
|
|
|
|
|
||
Unamortized debt discount and debt issuance costs |
|
|
( |
) |
|
|
( |
) |
Long-term debt, net |
|
$ |
|
|
$ |
|
||
At December 31, 2023, the scheduled maturities of the Company's debt obligations were as follows (in thousands):
|
|
Amount |
|
|
2024 |
|
$ |
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
Total |
|
$ |
|
|
As of December 31, 2023, the fair value of the Company's long-term debt approximates its carrying value. The fair value of the Company's long-term debt was based on observable market inputs (Level 2).
Note 11. Stock-Based Compensation
Equity Incentive Plans
Teknova maintains stock incentive plans for the benefit of certain of Teknova's officers, directors, consultants and employees. The Company granted time-based and performance-based options to purchase common shares under both its 2016 Stock Plan, as amended (2016 Plan) and 2020 Equity Incentive Plan, as amended (2020 Plan). At the time the 2020 Plan became effective, no additional stock awards were granted or are able to be granted in the future under the 2016 Plan. In June 2021, the Company’s board of directors and the Company’s stockholders approved the 2021 Equity Incentive Plan (2021 Plan), which became effective in connection with the IPO. From and after the date on which the 2021 Plan became effective, no further grants were made or will be made under the 2020 Plan. At December 31, 2023,
The types of equity-based awards that may be granted under the 2021 Plan include: incentive stock options or nonqualified stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance awards, and other stock-based awards.
F-21
Generally, the number of shares of the Company’s common stock that will be reserved for issuance under the 2021 Plan will automatically increase on January 1 of each year for a period of
Stock Options
The following table summarizes the stock option activity for the year ended December 31, 2023 (in thousands, except share and per share data):
|
|
Number of |
|
|
Weighted |
|
|
Weighted Average |
|
|
Aggregate |
|
||||
Outstanding at January 1, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Granted |
|
|
|
|
$ |
|
|
|
— |
|
|
|
— |
|
||
Exercised |
|
|
( |
) |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
Forfeited |
|
|
( |
) |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
Expired |
|
|
( |
) |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
Outstanding at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Exercisable at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Vested and expected to vest at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
The total intrinsic value of options exercised during the year ended December 31, 2023 and 2022 was $
Restricted Stock Units
The following table summarizes the restricted stock unit activity for the year ended December 31, 2023 (in thousands, except share and per share data):
|
|
Number of |
|
|
Weighted |
|
|
Weighted Average |
|
|
Aggregate |
|
||||
Outstanding at January 1, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Granted |
|
|
|
|
$ |
|
|
|
— |
|
|
|
— |
|
||
Vested |
|
|
( |
) |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
Forfeited |
|
|
( |
) |
|
$ |
|
|
|
— |
|
|
|
— |
|
|
Outstanding at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
Vested and expected to vest at December 31, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
||||
The aggregate grant-date fair value of restricted stock units vested during the year ended December 31, 2023 and 2022, was $
F-22
Employee Stock Purchase Plan
In June 2021, the Company’s board of directors and the Company’s stockholders, respectively, approved the Company’s 2021 Employee Stock Purchase Plan (the ESPP), which became effective in connection with the IPO. At December 31, 2023,
Generally, all regular employees, including executive officers, employed by the Company will be eligible to participate in the ESPP and to contribute, normally through payroll deductions, up to
Valuation of Employee Share-Based Awards
Teknova uses the Black-Scholes option-pricing model to determine the fair value of stock options. The valuation model for stock compensation expense requires the Company to make assumptions and judgments about the variables used in the calculation, including the expected term, expected volatility and fair value of the Company’s common stock, and an assumed risk-free interest rate. The assumptions used in the Black-Scholes option-pricing model were as follows:
Volatility. Since the Company has limited historical data on volatility of its stock, expected volatility is based on the volatility of the stock of similar publicly traded entities. In evaluating similarity, the Company considers factors such as industry, stage of life cycle, size, and financial leverage.
Fair value of underlying common stock. The fair value of the Company’s common stock is determined by the closing price of its common stock as reported on the Nasdaq Global Market on the date of grant.
Risk-free interest rate. The risk-free rate that the Company uses is based on U.S. Treasury zero-coupon issues with remaining terms similar to the expected term on the options.
Expected term. As the Company does not have sufficient historical exercise activity to estimate expected life, the expected life of options granted is determined using the simplified method. The simplified method is based on the vesting period and the contractual term for each grant or for each vesting tranche for awards with graded vesting. The midpoint of the vesting date and the maximum contractual expiration date are used to determine the expected term under this method.
Dividend yield. The Company has never declared or paid any cash dividends and does not plan to pay cash dividends in the foreseeable future. Therefore, the Company uses an expected dividend yield of zero. In addition, the terms of the Amended Credit Agreement prohibit us from paying dividends, other than dividends payable in the Company’s common stock, without the prior consent of the lender.
F-23
The weighted average assumptions used in the Black-Scholes option-pricing model are as follows:
|
|
For the Year Ended December 31, |
|
|||||||||||||
|
|
Employee Stock Option Plans |
|
|
Employee Stock Purchase Plan |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Estimated dividend yield |
|
|
% |
|
|
% |
|
|
% |
|
|
% |
||||
Weighted-average expected stock price volatility |
|
|
% |
|
|
% |
|
|
% |
|
|
% |
||||
Weighted-average risk-free interest rate |
|
|
% |
|
|
% |
|
|
% |
|
|
% |
||||
Expected average term of options (in years) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Weighted-average fair value of common stock |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Weighted-average fair value per option |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Summary of Stock-Based Compensation Expense
Stock-based compensation expense included in the accompanying financial statements was as follows (in thousands):
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Cost of sales |
|
$ |
|
|
$ |
|
||
Research and development |
|
|
|
|
|
|
||
Sales and marketing |
|
|
|
|
|
|
||
General and administrative |
|
|
|
|
|
|
||
Total stock-based compensation expense |
|
$ |
|
|
$ |
|
||
Stock-based compensation expense related to stock options was $
Stock-based compensation expense related to restricted stock units was $
During the year ended December 31, 2021, the Company’s board of directors approved an amendment to the outstanding performance-based option to acquire
Total stock-based compensation expense related to the ESPP was $
F-24
Note 12. Income Taxes
Teknova’s benefit from income taxes consist of the following (in thousands):
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Current: |
|
|
|
|
|
|
||
Federal |
|
$ |
|
|
$ |
|
||
State |
|
|
|
|
|
|
||
Total current |
|
|
|
|
|
|
||
Deferred: |
|
|
|
|
|
|
||
Federal |
|
|
( |
) |
|
|
( |
) |
State |
|
|
( |
) |
|
|
|
|
Total deferred |
|
|
( |
) |
|
|
( |
) |
Income tax benefit |
|
$ |
( |
) |
|
$ |
( |
) |
A reconciliation of the statutory tax rate to the Company’s effective tax rate is as follows:
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Statutory federal income tax rate % |
|
|
% |
|
|
% |
||
State income tax rate |
|
|
|
|
|
|
||
Stock compensation |
|
|
( |
) |
|
|
( |
) |
Research and development credit |
|
|
|
|
|
|
||
Change in valuation allowance |
|
|
( |
) |
|
|
( |
) |
Goodwill impairment |
|
|
|
|
|
( |
) |
|
Other |
|
|
( |
) |
|
|
|
|
Effective tax rate % |
|
|
% |
|
|
% |
||
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due, plus deferred taxes. Deferred taxes are recognized for differences between the basis of assets and liabilities for financial statement and income tax purposes. The deferred tax assets and liabilities represent the future tax return consequences of those differences, which will be either deductible or taxable when the assets and liabilities are recovered or settled.
|
|
As of December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Deferred tax asset |
|
|
|
|
|
|
||
Net operating loss carryforwards |
|
$ |
|
|
$ |
|
||
Accrued compensation |
|
|
|
|
|
|
||
Stock compensation |
|
|
|
|
|
|
||
Tax credit carryforwards |
|
|
|
|
|
|
||
Accruals and other |
|
|
|
|
|
|
||
Operating lease liabilities |
|
|
|
|
|
|
||
Capitalized research and development expenses |
|
|
|
|
|
|
||
Inventory capitalization |
|
|
|
|
|
|
||
Total deferred tax asset |
|
|
|
|
|
|
||
Deferred tax liability |
|
|
|
|
|
|
||
Property, plant, and equipment |
|
|
( |
) |
|
|
( |
) |
Intangibles |
|
|
( |
) |
|
|
( |
) |
Operating right-of-use lease assets |
|
|
( |
) |
|
|
( |
) |
Total deferred tax liability |
|
|
( |
) |
|
|
( |
) |
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
Net deferred tax liability |
|
$ |
( |
) |
|
$ |
( |
) |
F-25
As of the end of December 31, 2023, Teknova has federal and state net operating loss carryforwards (NOLs) of $
For the years ended December 31, 2023 and December 31, 2022, the Company recorded a net increase in valuation allowances of $
The Company had unrecognized tax benefits of $
Teknova files income tax returns in the U.S. federal jurisdiction and various states. The Company is no longer subject to U.S. federal income tax examinations for tax years prior to 2020. The Company is no longer subject to state income tax examinations for tax years prior to 2019. All net operating losses and tax credits generated to date are subject to adjustment for U.S. federal and state income tax purposes. The Company is currently not under examination by the Internal Revenue Service or any other taxing authorities.
Note 13. Net Loss Per Share
Basic net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share is computed by giving effect to all potentially dilutive common stock equivalents to the extent they are dilutive. For purposes of this calculation, stock options, restricted stock units, and employee stock purchase rights are considered to be common stock equivalents but have been excluded from the calculation of diluted net loss per share as their effect is anti-dilutive for all periods presented.
The following table sets forth the computation of basic and diluted net loss per share (in thousands, except share and per share data):
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted average shares used in computing net loss per share—basic and diluted |
|
|
|
|
|
|
||
Net loss per share—basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
The following is a summary of the common stock equivalents for the securities outstanding during the respective periods that have been excluded from the computation of diluted net loss per common share, as their effect would be anti-dilutive:
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Employee share-based awards to purchase common stock |
|
|
|
|
|
|
||
F-26
Note 14. Related Parties
The Company has identified Meeches LLC (Meeches) as a related party through common control. Meeches is controlled by Ted Davis and Irene Davis, founders and current directors, and greater than five percent stockholders of the Company. Prior to May 16, 2023, the Company leased certain real property in Mansfield, Massachusetts, from Meeches. As of December 31, 2023 and 2022 the Company did not have any outstanding balances owed to Meeches. For the years ended December 31, 2023 and 2022, the Company paid Meeches lease payments of $
On April 11, 2023, the Company and Meeches entered into an agreement to terminate the Mansfield lease, which termination occurred on May 16, 2023. Shortly thereafter, Meeches sold the property to a third party. As part of the consideration for the early termination of the Mansfield lease, the Company entered into an escrow agreement with the new owner on May 17, 2023, and placed in escrow an amount equal to five months of base rent plus related expenses assumed by Teknova under the Mansfield lease. Escrow funds were released to the new owner on a pro-rata monthly basis. No amounts remain in escrow at December 31, 2023.
Note 15. Other Financial Information
The change in the allowance for doubtful accounts is as follows:
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Beginning balance |
|
$ |
|
|
$ |
|
||
Provisions (benefits) |
|
|
|
|
|
|
||
Recoveries (write-offs), net |
|
|
( |
) |
|
|
( |
) |
Ending balance |
|
$ |
|
|
$ |
|
||
The change in the inventory reserve is as follows:
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Beginning balance |
|
$ |
|
|
$ |
|
||
Provisions (benefits) |
|
|
|
|
|
|
||
Write-offs and other |
|
|
( |
) |
|
|
( |
) |
Ending balance |
|
$ |
|
|
$ |
|
||
The change in the income tax valuation allowance is as follows:
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Beginning balance |
|
$ |
|
|
$ |
|
||
Additions charged to expense |
|
|
|
|
|
|
||
Reductions charged to other accounts |
|
|
|
|
|
|
||
Ending balance |
|
$ |
|
|
$ |
|
||
F-27
The change in unrecognized tax benefits are as follows:
|
|
For the Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Beginning balance |
|
$ |
|
|
$ |
|
||
Tax positions related to the current year: |
|
|
|
|
|
|
||
Additions |
|
|
|
|
|
|
||
Reductions |
|
|
|
|
|
|
||
Tax positions related to the prior year: |
|
|
|
|
|
|
||
Additions |
|
|
|
|
|
|
||
Reductions |
|
|
|
|
|
|
||
Ending balance |
|
$ |
|
|
$ |
|
||
Note 16. Contingencies
From time to time, we may become involved in lawsuits and other claims arising from our ordinary course of business. The Company regularly evaluates its exposure to threatened or pending litigation and other business contingencies. Because of the uncertainties related to the amount of loss from litigation and other business contingencies, the recording of losses relating to such exposures requires significant judgment about the potential range of outcomes. We establish loss provisions for matters in which losses are probable and can be reasonably estimated. If a loss is not both probable and reasonably estimable, or if an exposure to loss exists in excess of the amount accrued, the Company assesses whether there is at least a reasonable possibility that a loss, or additional loss, may have been incurred. If there is a reasonable possibility that a loss, or additional loss, may have been incurred, the Company will disclose the estimate of the possible loss or range of loss if it is material and an estimate can be made, or disclose that such an estimate cannot be made. The determination as to whether a loss can reasonably be considered to be possible or probable is based on our assessment, together with legal counsel, regarding the ultimate outcome of the matter. As additional information about current or future litigation or other contingencies becomes available, the Company will assess whether adjustments should be made to legal accruals.
In August 2023, a former Teknova employee filed a claim with the Labor and Workforce Development Agency alleging various causes of action under California’s labor, wage, and hour laws. The plaintiff generally alleges that Teknova did not appropriately calculate and pay meal break premiums and otherwise failed to calculate and pay appropriate overtime wages or bonuses to certain of its California non-exempt employees. A mediation has been scheduled for June 6, 2024. As of December 31, 2023, the Company has accrued its best estimate of potential loss related to a possible settlement of the claims of the former employee and other employees who may assert similar claims, in the amount of $
Note 17. Subsequent Events
On January 11, 2024, the Company announced a reduction in its workforce that affected approximately
F-28
On March 8, 2024, the Company entered into limited waivers and amendments (collectively Amendment No. 5, or, as amended, the Amended Credit Agreement) which includes a waiver from MidCap of the revenue covenant violations for each of the periods ending November 30, 2023 and January 31, 2024. Amendment No. 5 also reduced these requirements for future periods up to and including for the twelve months ending December 31, 2024, from $
On March 8, 2024, as a condition to the effectiveness of Amendment No. 5, the Company issued to MidCap Funding XXVII a warrant to purchase up to an aggregate of
F-29