40Use these links to rapidly review the document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||||||
For the fiscal year ended December 31 , 2023
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||||||
Commission File Number 001-33133
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
(Address of principal executive offices) | (Zip Code) | |||||||
(Registrant's telephone number, including area code): (617 ) 583-1700
______________________________________________________________
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
The | ||||||||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act:
| Large accelerated filer | ☐ | Accelerated filer | ☐ | ||||||||
| ☒ | Smaller reporting company | ||||||||||
| Emerging growth company | |||||||||||
If an emerging growth company, indicate by check mark if the registrant elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ý
The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold on the Nasdaq Capital Market on June 30, 2023 was $9,843,350 .
The number of shares outstanding of the registrant's common stock as of March 28, 2024 was 15,401,706 .
DOCUMENTS INCORPORATED BY REFERENCE
Pursuant to General Instruction G to Form 10-K, the information required by Part III, Items 10, 11, 12, 13 and 14 is incorporated herein by reference from the Company's proxy statement for the Annual Meeting of Stockholders to be held on June 20, 2024, which is expected to be filed not later than 120 days after the fiscal year end covered by this Form 10-K.
YIELD10 BIOSCIENCE, INC.
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2023
INDEX
| Page | ||||||||
3
Forward-Looking Statements
This Annual Report on Form 10-K contains "forward-looking statements" within the meaning of 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). These statements relate to our future plans, objectives, expectations and intentions and may be identified by words such as "may," "will," "should," "expects," "plans," "anticipate," "intends," "target," "projects," "contemplates," "believe," "estimates," "predicts," "potential," and "continue," or similar words.
Although we believe that our expectations are based on reasonable assumptions within the limits of our knowledge of our business and operations, the forward-looking statements contained in this document are neither promises nor guarantees. Our business is subject to significant risks and uncertainties and there can be no assurance that our actual results will not differ materially from our expectations. These forward-looking statements include, but are not limited to, statements concerning our business plans and strategies; expected future financial results and cash requirements; plans for obtaining additional funding; plans and expectations that depend on our ability to continue as a going concern; and plans for development and commercialization of our crop yield traits, technologies and intellectual property. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated including, without limitation, risks related to our limited cash resources, uncertainty about our ability to secure additional funding, risks related to the execution of our business plans and strategies, risks associated with the protection and enforcement of our intellectual property rights, as well as other risks and uncertainties set forth below under the caption "Risk Factors" in Part I, Item 1A, of this report.
The forward-looking statements and risk factors presented in this document are made only as of the date hereof and we do not intend to update any of these risk factors or to publicly announce the results of any revisions to any of our forward-looking statements other than as required under the federal securities laws.
Unless the context otherwise requires, all references in this Annual Report on Form 10-K to "Yield10 Bioscience," "Yield10," "we," "our," "us," "our company" or "the company" refer to Yield10 Bioscience, Inc., a Delaware corporation and its subsidiaries.
PART I
(With the exception of stock prices and earnings per share disclosures, all dollar amounts throughout this report are shown in thousands unless otherwise indicated.)
ITEM 1. BUSINESS
Overview
Yield10 Bioscience, Inc. ("Yield10" or the "Company") is an agricultural bioscience company focused on commercializing sustainable products using the oilseed Camelina sativa ("Camelina") as a platform crop.
We are pursuing Camelina seed oil products for two market opportunities and value chains. Each product has its own set of scale requirements, value proposition and challenges. The first product, is seed oil produced by Camelina which has been genetically engineered to enable production of high levels of the omega-3 fatty acids eicosapentanoic acid (EPA) and docosahexanoic acid (DHA). Our development is driven by the growing demand for new sources of omega-3 ingredients and the production constraints and supply volatility of the traditional raw material source fish oil. This growing omega-3 supply gap offers a market opportunity with the potential for revenue and margin growth at acreage levels operationally accessible to Yield10. When commercially available, the Company's omega-3 products will address an unmet need for a reliable, scalable supply of EPA and DHA omega-3 ingredients for aquaculture and pet/animal feed. Multiple opportunities exist for further product development to address higher value markets for omega-3 oils in nutraceuticals and pharmaceuticals. Earlier this year we received regulatory approval from USDA-APHIS for two engineered Camelina lines, the first produces our EPA Omega-3 product and the second produces our EPA+DHA Omega-3 product.
The second product is Camelina seed oil for use as a low-carbon intensity feedstock oil for biofuels, including biodiesel, renewable diesel (“RD”) and sustainable aviation fuel (“SAF”). Markets for biofuels are driven by government policies, have the potential to be very large, and will require the production of tens of millions of acres of new oilseed cover crops like Camelina that do not compete for land with food production.
4
In early 2024, we adjusted our commercial strategy for biofuels to focus on providing research and development services to third parties interested in Camelina for biofuels with the goal of generating service and licensing revenues from our advanced Camelina technology capabilities, varieties and traits. This decision reflects the current excess supply of soybean oil for the biofuels market as well as the challenges the Company faced in the current financing environment as a small cap pre-revenue company. Over the past four years, Yield10 has developed proprietary, engineered spring and winter Camelina varieties, including herbicide tolerant (“HT”) and stacked herbicide tolerant (“stacked HT”) traits which form the genetic foundation for introducing commercial-quality Camelina varieties tailored to address our target markets. Based on this biofuels strategy, we recently signed our first non-exclusive global, commercial license with Vision Bioenergy Oilseeds for certain Camelina lines and herbicide tolerance traits. Going forward, we plan to continue executing partnerships and other types of agreements for the development of Camelina technologies used in the production of biofuels. Yield10 is headquartered in Woburn, Massachusetts and has a Canadian subsidiary, Yield10 Oilseeds Inc., located in Saskatoon, Saskatchewan, Canada.
We selected Camelina, an annual oilseed plant in the mustard family, as our platform crop based on its unique attributes, including its excellent agronomic traits, such as low water and fertilizer input, drought resistance and its short growing cycle. Based on Camelina's flexible agronomic profile, we believe growers have the option of using Camelina as a winter cover crop, as a relay crop with soybean in the U.S. Midwest, and as a spring rotation crop within the U.S. and regions of Canada. Meeting the growing demand for biofuel feedstocks in North America will require tens of millions of acres of new non-food oilseed production. Given today's crop production practices, the best way to access this acreage scale is through double cropping using short season winter oilseeds as cash cover crops integrated into crop rotations in a second growing season with the major food crops corn and soybean. Winter cover crops reduce soil erosion and nutrient runoff, promote soil health and trap subsoil moisture. Camelina is in the same plant family as canola and naturally produces a relatively abundant harvest of oil-containing protein-rich seeds. Camelina has no close plant relatives in North America and as a new non-food crop it is readily segregated from commodity crops making it advantaged for producing novel seed products. This dramatically simplifies the regulatory path for engineered products in North America. Planting, harvesting, storage and transportation of Camelina does not require growers to make capital investments in new equipment. The grain can be processed in soft seed (e.g. canola) crushing facilities, using either cold press or solvent extraction and the residual protein meal can be used in certain animal feed rations in the U.S. and Canada once regulatory approval has been obtained. To unlock this potential and make Camelina an attractive option for farmers, we are developing and plan to commercialize advanced varieties with elite weed control herbicide tolerance traits, improved agronomic performance, and increased crop value.
We learned through our interactions with growers that developing elite weed control technology for Camelina to enable seamless integration into current crop rotations is a critical factor in achieving large-scale adoption of Camelina as a new crop. As a result, we prioritized the development of herbicide tolerant Camelina over the past four years. We have successfully developed commercial-quality Camelina varieties containing an HT trait for glufosinate tolerance alone or as a stacked HT trait for glufosinate tolerance in combination with tolerance to Group 2 herbicides, specifically including tolerance to both imidazolinones (“IMI”) and sulfonylureas (“SU”). The prevalence and persistence of Group 2 soil residues in some production regions limit the amount of land available for planting conventional Camelina. In November of 2023, our HT and stacked HT technologies received regulatory approval from USDA-APHIS indicating that the agency does not consider our Camelina varieties with these traits to be regulated in the United States. In early 2024, over 50 acres of our spring E3902 HT Camelina was harvested from a contra season seed scale-up conducted in Chile. This accomplishment from first field trials of HT Camelina in the spring of 2022 to regulatory approval in Q4 of 2023 and seed bulk up to tonnage scale in Q1, 2024 reflects Yield10's development strengths. We believe this outcome together with the more recent approvals for our Omega-3 Camelina reflects the favorable regulatory path for engineered Camelina in the U.S. and bodes well for the accelerated development of this crop using Yield10s advanced technology, gene traits and capabilities. We plan to continue our development work with spring E3902 stack HT Camelina and winter stack HT Camelina during early 2024, in order to continue our assessment of the efficacy of the traits in-field agronomics, seed yields and oil content. In February of 2024 we signed a non-exclusive commercial license for the HT and stacked HT traits with Vision Bioenergy Oilseeds (“Vision”) to enable Vision to begin scaling up Camelina production for the biofuel market.
In October of 2023, we executed our option with The Rothamsted Institute ("Rothamsted") to acquire an exclusive, global commercial license to advanced Omega-3 Camelina technology. We expect to secure this license in the second quarter of 2024. As a result of industry interest in new sources of omega-3, in October of 2023 we signed a Letter of Intent (“LOI”) with BioMar Group, an industry leader in the production of aquafeed for salmon farming, with the intent of forming a collaboration to commercially produce the oil from Omega-3 Camelina to supply this market. The omega-3 fatty acids EPA and DHA are essential for human health and wellness, and the primary dietary source of these nutrients is ocean
5
harvested fish oil. For clarity, we are using “fish oil” to include oil containing EPA and DHA omega-3 fatty acids extracted from ocean harvested fish and Krill. A third omega-3 fatty acid, alpha-linoleic acid (ALA), is also considered essential for human health and wellness. ALA is readily available from vegetable oils, including canola, soybean, flaxseed and Camelina however, plants on the other hand do not make EPA or DHA. Fish oil containing EPA and DHA purified from harvested fish, such as anchovies, is an important ingredient in the production of feed used in salmon aquafarming. Salmon is a preferred high quality protein source in the human diet and today over 85% of the salmon consumed globally is produced through aquafarming. EPA and DHA are essential feed components for farmed salmon to protect fish health during production and to provide market differentiation of the product as healthier protein source based on its omega-3 content. The growing market for farmed salmon and expanded use of omega-3 fatty acids in human nutrition, nutraceutical, pet food and other markets has placed additional pressure on harvested fish as a source of fish oil. Significant disruptions to omega-3 oil supply occurred in 2023 causing sharp increases in fish oil prices. Industry sources project a deficit in omega-3 supply to meet growing demand of over 500,000 tons per year by 2030.
Under our collaboration, Rothamsted provided us with both the two Omega-3 Camelina varieties that produce seed oil with 16-20% EPA or seed oil with 10% EPA and 10% DHA content. In 2023, the Company filed requests for Regulatory Status Review (“RSRs”) with USDA-APHIS for both Camelina varieties and both of these were approved in March 2024. In 2023, we planted EPA8 Omega-3 Camelina on 50 acres in contra season in Chile. This EPA8 Omega-3 Camelina was harvested in the first quarter of 2024. Going forward in 2024, we plan to continue executing further seed scale-up of EPA8 Omega-3 Camelina to enable production of the first commercial omega-3 product for use in aquafeed. The Company also expects to begin scaling up DHA1 Omega-3 Camelina in Chile during the fourth quarter of 2024.
We plan to bring both Omega-3 Camelina products forward in development with 2026 being the target for the first commercial-scale production of the EPA8 oil. Herbicide tolerance is critical in Camelina for on-farm performance. We are currently breeding HT traits into both current Omega-3 Camelina varieties to create second generation varieties for large acreage production. As a world leader in Camelina seed genetics and advanced trait technology development, Yield10 shares a common goal with the aquaculture industry including feed and salmon producers to establish the commercial production of thousands of tons of Camelina omega-3 oil as a new scalable, cost effective, and sustainable supplement to fish oil. We believe Camelina omega-3 oil production at scale (50,000 tons per year, based on approximately 200,000 acres) can reduce supply and price volatility in aquafeed and farmed salmon production leading to a potential increase the growth rate of the industry.
Over the course of 2022 and 2023, we gained valuable experience in establishing a closed loop value chain, the model we plan to use for the omega-3 business. We engaged growers in contracts to produce Camelina grain (for processing) and Camelina seed (for future planting) having a total acreage of approximately 1,000 acres for each of the winter and spring growing seasons. We also contracted with growers in the U.S., Canada and Chile to grow our E3902 (spring, high oil yield), WDH2 (winter, increased cold tolerance) and WDH3 (winter, early flowering) and other Camelina plant varieties to produce commercial planting seed. This activity is expected to be an essential part of our business model to produce commercial seed inventory for future grower contracts. During the second half of 2023, we took first-time deliveries of harvested Camelina grain produced under our winter and spring season grower contracts in Western Canada and the United States. Although small in scale, these initial growing seasons represented a proof-of-concept for our Camelina to be used in the commercial biofuel feedstock market.
We are building an intellectual property portfolio around our crop technologies and traits. As of December 31, 2023, Yield10 owns or holds exclusive rights to 18 patent families, including 20 issued patents and 30 pending patent applications. In October of 2023, Yield10 exercised its exclusive option to an exclusive global license to two active patent families of Rothamsted Research, including six issued patents and six pending applications which cover the production of our EPA and EPA+DHA products.
Market Opportunities
We believe the market opportunity is significant for omega-3 oil and biofuel feedstock oil produced from our elite Camelina varieties, as well as for our other proprietary seed products in development, including performance traits for use in other crops. We are targeting uses for our Camelina seed products in commercial applications such as: a) omega-3 oils for aquaculture and nutrition based on establishing a closed loop production system, and b) low-carbon feedstock oils for biofuels using an R&D services, partnering and licensing model.
6
Omega-3 (EPA, EPA+DHA) Oils: Long chain omega-3 fatty acids are essential for human nutrition, health and wellness. The three main omega-3 fatty acids are alpha-linolenic acid (“ALA”), eicosapentaenoic acid (“EPA”), and docosahexaenoic acid (“DHA”). EPA and DHA are found primarily in fish and other seafood while ALA is found primarily in plant oils such as flax. Plant oils including canola, soybean, flaxseed and Camelina naturally containing high levels of ALA but plants do not produce EPA or DHA which are found only in marine organisms. The markets and uses for omega-3 fatty acids include aquafeed used for salmon, trout and shrimp feed, pet feed, baby formula, nutraceuticals and pharmaceutical products. Omega-3 fatty acids provide many health benefits, including preventing and managing heart disease. The American Heart Association recommends that everyone eat fish at least twice a week, such as salmon that is high in omega-3 acids, and/or adding fish oils supplements to their diets. In addition, DHA is used as an essential ingredient in baby formula because of its importance in early cognitive development.
The global availability of ocean wild caught fish and krill containing omega-3 oil used in nutritional oils and aquafarming is declining due to overfishing. The primary dietary source of EPA and DHA is the consumption of oily fish or fish oil. Approximately 10% of all fish harvested from the ocean is used to produce fish meal and fish oil with the latter valued primarily as a source of omega-3. Global fish oil production has remained flat at approximately 1 million tons per annum over the last decade. In 2023, there was a significant drop in production of nearly 20% due to the cancellation of the Peru anchovy harvest. Fish oil contains different levels of the omega-3 fatty acids depending on the type of fish and location of the fish harvest. The oil extracted from recent annual anchovy harvests in Peru historically provided almost 20% of global fish oil production and had high levels of EPA (≈ 17%) and DHA (≈ 12%). The higher levels of EPA and DHA in anchovy oil make it a preferred feedstock for producing omega-3 concentrates and derivatives for direct human consumption. Northern hemisphere fish oil contains approximately 12% EPA and 10% DHA. Our vegan EPA and EPA+DHA products produced by Camelina will be attractive clean ingredients for their target markets, free of ocean pollutants including the heavy metals, lead, cadmium and mercury as well as the persistent organic pollutants increasingly prevalent in the oceans. We believe our Camelina oils will provide safety advantages to manufacturers of omega-3 pharmaceuticals and nutraceuticals and these same benefits will also accrue to farmed salmon and other species.
Figure 1. Camelina Omega-3 Oil Production Value Chain
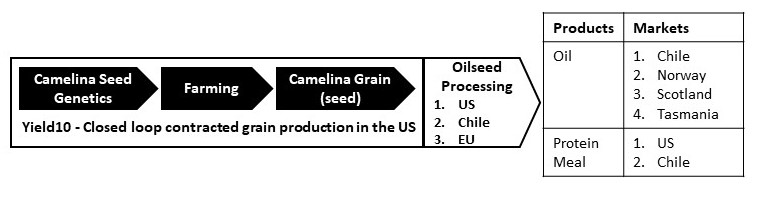
Source: Yield10 Bioscience
The Omega-3 Deficit: Based on a recent analyst report on Nuseed from UBS and our discussions with prospective customers and partners interested in new sources of EPA and DHA, there is a growing disconnect between the constrained supply from ocean harvested fish and krill with demand growing steadily at around 3% per year for aquafeed and 7-8% for other applications mainly human nutrition. This omega-3 deficit is estimated to increase to around 300,000 -600,000 tons of fish oil equivalent per year.
Fish oil for aquafeed is used primarily to supply three main markets, Chile, Norway and Scotland, as shown in Figure 1. The aquaculture product sector will play a major role in meeting the growing global demand for fish, an important high value protein source in human diets. Sustainable land-based sources of key feed ingredients will need to be developed and adopted to support this demand. This includes high value specialty ingredients, and in particular, new sources of EPA and DHA to replace oil from stagnating supplies of ocean-harvested fish used in feed production. Fish oil supplied from ocean-harvested fish is particularly important for farmed salmon. The Atlantic Salmon aquaculture sector is expected to grow at 4% CAGR through 2026, reaching an annual harvest of over 3 million tonnes. The growth of the salmon farming sector along with increasing additional demand from new nutraceutical product markets for direct human consumption are expected to exceed the world's sustainable supply. In 2021, 4.7 million metric tonnes of fish feed was used globally for salmon aquafarming. Although it can vary, fish oil represented up to 10% of fish feed by weight and is the most expensive ingredient. We estimate that there is approximately 500,000 tons of fish oil consumed annually in salmon feed production.
7
Global fish oil production has been relatively flat over the last 10 years and some industry experts predict the demand for omega-3 will increase by more than 7% per year over the next 5 years. The demand from salmon farming alone is expected to grow by approximately 4% per year, according to the 2022 Salmon Farming Industry Handbook. The entire aquaculture industry rests on a shaky sustainability platform as wild caught fish are ground to produce for feed, including omega-3 and fish meal protein. We believe our Camelina omega-3 oils will enable this entire industry to attain a truly sustainable foundation.
Fish oil is also a key raw material source for producing refined and concentrated omega-3 oils and purified omega-3 fatty acid derivative compounds for use in the growing human nutrition, nutraceutical and pharmaceutical markets. One such derivative is ethyl-EPA. There are currently about 20 commercial producers of ethyl-EPA. Currently produced from fish oil, we estimate that ethyl-EPA production is in the range of 10,000 – 14,000 tons per year with a reported market value of approximately $1.6 billion which is predicted to grow to $3.4 billion by 2030. We believe that the EPA oil from our first and second generation EPA8 Omega-3 Camelina may be an advantaged feedstock for this market given the high EPA content and the absence of DHA in those oils.
High Protein Meal: There is a growing global demand for additional protein sources for animal feed and food applications. Camelina seed can be processed using existing oilseed processing facilities to extract the oil, and the residual meal that remains is a high-quality protein. On a dry basis, the meal contains approximately 42% protein with a good amino acid profile for animal feed applications. Camelina meal has been approved for use in some animal feed applications, and we expect that with accelerated breeding using genome editing, the meal quality can be further enhanced to improve its feed value and expand this application.
Incentives to Reduce Carbon Emissions: The global macro driver to reduce greenhouse gas emissions encompasses not only liquid transportation fuels but includes reducing CO2 production from agricultural and aquaculture farming practices. In the case of liquid transportation fuels this has resulted in targeted government policies.
When renewable diesel or sustainable aviation fuel produced primarily from used cooking oil and vegetable oil is used instead of traditional petroleum diesel, it helps reduce carbon emissions. Camelina oil has a particular advantage because of its low carbon footprint. Currently, there are existing regulatory incentives from regional greenhouse gas reduction mandates established for fuel producers. This includes California's Low Carbon Fuel Standards market, which measures the specific carbon index, or CI, of every type of fuel, assigns a credit/deficit for every gallon of fuel produced based on its CI, and requires all fuel producers selling into California to purchase enough credits to keep their portfolio CI score below an established baseline. Biofuel manufacturers are highly motivated to utilize compatible feedstocks with a low-carbon footprint in order to meet the regulatory standards to lower carbon emissions. As a benchmark, petroleum diesel has a reported CI of 100, soybean oil has a CI of 56, and in the case of Camelina oil, Sustainable Oils, a subsidiary of Global Clean Energy Holdings, Inc., has reported a CI of 23.
Similarly, large food companies are working to reduce their greenhouse gas footprint and promoting regenerative agricultural practices to improve their carbon footprint and overall ESG scores, in part to create marketing advantages with consumers. Vegetable oils produced using winter Camelina cover cropping to produce low CI vegetable oils for human consumption may be an attractive option. Similarly, the salmon farming sector is looking specifically to reduce its carbon footprint and transition to more sustainable practices including reducing the use of ocean harvested fish as sources of protein and omega-3 fatty acids for aquafeed.
Feedstock Oil for Biofuels: As part of the energy transition, a substantial increase in renewable diesel capacity in the United States and Canada is currently underway, with proposed and funded renewable diesel facilities having a total capacity of over 6 billion gallons of biofuels per year. Initiatives are also underway in the U.S, targeting 35 billion gallons of annual production as well as in the EU and Japan where incentives are being considered to encourage the use of sustainable aviation fuel (SAF), all of which will further increase the demand for vegetable oil feedstocks. Renewable diesel expansion has surged due to its low carbon footprint, federal and local subsidies, and its ability to be used as a drop-in replacement for petroleum diesel. Renewable diesel feedstock is supplied primarily from used cooking oil, animal fats (e.g., tallow), and vegetable oil, with the former two feedstock sources in short supply due to limited production availability. Numerous studies and regulatory approvals have shown Camelina oil’s usefulness as a low-carbon feedstock oil for renewable diesel and sustainable aviation fuel. Camelina’s low-carbon footprint, and ability to be grown as a cover crop on otherwise fallow land, makes it an attractive choice to fill the biofuel feedstock supply gap. Based on the assumption of 60-100 gallons of Camelina oil per acre, 1 billion gallons of feedstock oil would require 10 to 15 million acres of Camelina production. For comparison purposes, canola is currently grown on approximately 21 million acres per year in Canada.
8
Figure 2. Outlook for Biofuel Demand
Source: IEA.Org
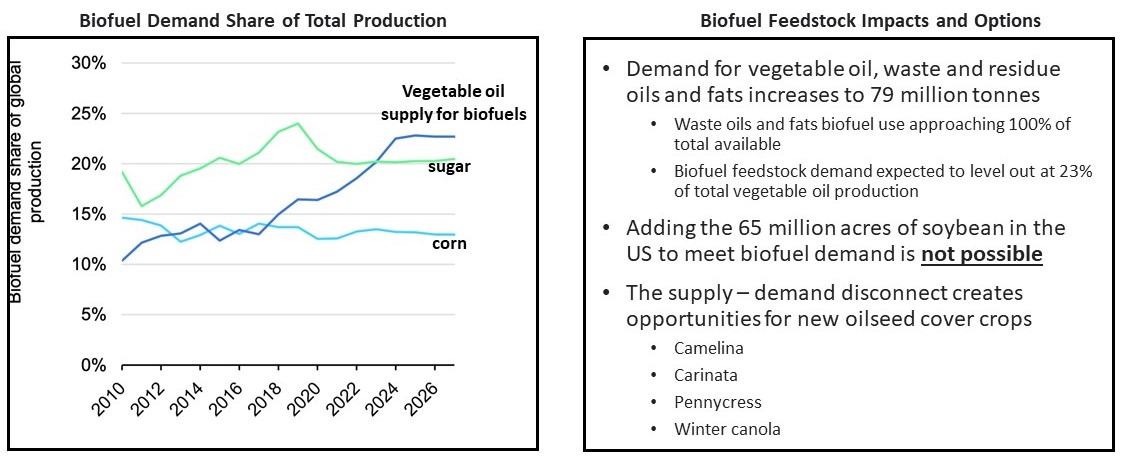
Cover Crops: To meet growing demand for oils and protein, and to mitigate the negative environmental impacts of current farming practices, particularly in the corn belt region, the development of cash cover crops or relay crops is another means to increase land productivity and address growing demand without impacting food production. Cover or relay crops are planted between harvest and sowing of major commodities, such as soybean, in effect increasing the number of harvests per growing season. Yield10 believes that Camelina, with its winter hardiness and short growing season, has considerable potential to be used as a cover crop to reduce soil erosion, improve soil quality, control diseases and pests and nutrient run-off from land that is used for row crop production. Camelina can also be used in crop rotation with other crops such as wheat, cereals, corn, and soybean. Third party estimates indicate that Camelina has up to 30 million acres of potential as a cover crop in the U.S. Midwest. By developing technologies to enable winter cover cropping of Camelina to be seamlessly integrated into major row crop rotations, we are in effect “creating” an additional 30 million acres of productive crop land. We plan to evaluate the CI of Camelina oil from cover cropping for both biofuel feedstock and omega-3 oil production where we anticipate there may be further CI improvements.
Business Strategy
In addition to our Camelina products addressing key sustainability drivers, we believe they should also reward farmers and increase profitability across the value chain with any sustainability benefits providing a marketing advantage for our future customers along with potential upside from any available government credits.
Our business strategy for our omega-3 products is to produce omega-3 oil in a closed loop production system based on the capabilities we established and demonstrated in 2022/2023. We will contract with growers to produce omega-3 Camelina solely for sale to Yield10 and contract crushing to produce the oil. Yield10 plans to sell the EPA or EPA+DHA oil focused initially on the aquafeed market through offtake agreements. Our plan is to generate sales for the first 50,000 tons of omega-3 oil in Chile to serve the demand in that market. We plan to file for regulatory approval for the use of our omega- oils in aquafeed formulations in Chile this year.
In early 2024, we revised our business strategy for biofuels to focus on providing research and development services to third parties better resourced and interested in scaling Camelina production for biofuels with the goal of generating R&D service and licensing revenues. By becoming an R&D partner and/or licensing our technologies on a non-exclusive basis to third parties we hope to enable them to accelerate grower adoption and supporting robust value chains to supply the oil. Provided this strategy is successful, we believe Yield10 will be able to build a royalty revenue stream from trait licensing for Camelina which can grow as the number of acres of Camelina planted increases year over year.
We plan to launch a business focused on the production of EPA and EPA+DHA oils based on the Omega-3 Camelina traits developed over the last 10 years by Rothamsted in the U.K. Yield10 signed an Exclusive Collaboration and Option Agreement for this technology with Rothamsted in November 2020 and this Agreement was extended until December
9
31, 2023. In October of 2023 Yield10 executed its exclusive option and expects to finalize the Exclusive Commercial License in the second quarter of 2024. The Omega-3 Camelina business model is to establish closed loop production of the oil for sale into the different omega-3 markets. For the largest volume opportunity, aquafeed, we are looking to form a partnership with one of the world's major aquafeed producers to facilitate and accelerate our entry into that market. For these reasons we plan to continue developing our grower network by shifting to a traditional seed sales model for some of our Camelina varieties. We have a considerable number of growers interested in the buildup of agronomic experience and the development of other applications for Camelina as a crop. Once we complete the regulatory work for the HT and stacked HT Camelina varieties, we expect to make them available to growers as well. The grower network built up for these activities will form the foundation for the future contracted production of the Omega-3 Camelina that is currently in early commercial development.
Yield10 will continue its focus on developing elite Camelina varieties with herbicide tolerance, disease resistance, higher yield and oil content, with the intent to breed Rothamsted's omega-3 product trait into this germplasm in the future. In the meantime, Yield10 plans to use the existing EPA8 and DHA1 Omega-3 Camelina varieties to begin scaling up omega-3 oils production in the near term.
Target Crop: The Oilseed Camelina
Camelina was grown extensively in Europe, Russia and Central Asia since medieval times for oil and protein but was replaced by cultivation of rapeseed during the 1940s. Camelina has the potential to replicate the development path of modern canola from rapeseed on an accelerated timeline based on modern biotechnologies. Starting in the 1960s, the breeding of canola from rapeseed to the first generation was not completed until 1982 and was based on time consuming traditional breeding through mutagenesis and selection to improve the oil fatty acid profile for human consumption (low erucic acid content) and improving the protein meal (low glucosinolates) for use in animal feed. This was followed by incorporating herbicide tolerance and hybrid technologies in the 1990s. Today, canola is grown on 21 million acres in Canada and is reported by the Canola Council of Canada to generate an estimated $30 billion for the Canadian economy.
Camelina has not been subject to intensive plant breeding efforts or crop production improvements, therefore, the full potential of this crop has not yet been achieved. Initial interest in using Camelina oil in biofuels resulted in additional investment in the development of the crop in North America. This work demonstrated that Camelina has several beneficial attributes; it is amenable to production practices used for canola, grows on marginal lands, has enhanced drought and cold tolerance, demonstrated early maturation and requires fewer inputs than other oilseed crops. Camelina is also naturally resistant to diseases that impact canola and its fast-growing cycle makes this crop suitable for spring and winter planting in the Northwest U.S. and into Western Canada as an alternative rotation crop where the relatively overall short growing season would make double cropping very challenging. Further south, the longer overall growing season in the upper mid-west together with Camelina’s short growing season makes it an attractive winter oilseed for relay or cover cropping in corn and soybean rotations. Although the double cropping scenario is where we see the greatest long-term potential for Camelina, our current varieties and capabilities are better positioned for initial commercial activities in the Northwest U.S. and Western Canada. Camelina is an attractive choice of crop for the following reasons:
•Camelina, as an underdeveloped crop, has high technology upside potential to improve agronomics (including herbicide tolerance), seed yield, and seed value.
•There is a growing demand for crops that diversify the crop landscape, have a lower environmental footprint and have the potential to produce high value secondary products, opening new opportunities for farmers.
•Although it can be planted, harvested, stored and processed using existing farm equipment and infrastructure, Camelina has a small seed size and is readily segregated from other grains.
•Camelina, like soybean and corn is non-native to North America and does not outcross to weedy relatives to form viable plants and is readily engineered using advanced genetic tools, making it an ideal platform for accelerated development and the production of novel seed products using advanced gene technologies.
◦Yield10 has engineered Camelina to incorporate well-proven elite herbicide tolerance trait technologies that are not considered regulated by USDA-APHIS in the U.S. under the SECURE rule.
◦Camelina has been engineered to produce seed oil containing high levels of omega-3 (EPA and DHA+EPA) fatty acids as a drop-in replacement oil for fish oil in aquafeed markets as well as for use in nutraceutical and pharmaceutical applications.
10
Yield10 Technology Advantage and Traits in Development
As our predecessor company, Metabolix, we carried out an in-depth review of the scale potential and positive and negative attributes of several “non-food” alternative oilseed crops, including Crambe, pennycress, and Brassica carinata (a rapeseed relative). As a result, we selected Camelina as offering the best option for developing a new platform crop for accelerated development and producing new seed products. Based on our background in advanced synthetic biology, commitment to deploying GMO traits and over 14 years of R&D investment, we believe we are the world leader in discovering and developing advanced gene technologies for Camelina. In addition to our ongoing spring and winter Camelina breeding programs to select new lines with higher yield, early maturity and fungal disease resistance, Yield10 has established a strong pipeline of gene traits for Camelina including input traits such as herbicide tolerance, performance traits including seed and oil yield and new product traits including EPA and EPA plus DHA omega- 3 oils. Yield10 has also demonstrated the production of PHA bioplastic in Camelina seed, and although this project is on hold due to resource constraints, we view this as potentially an exciting future product.
Figure 3. Approaches to Oilseed Cover Crop Development
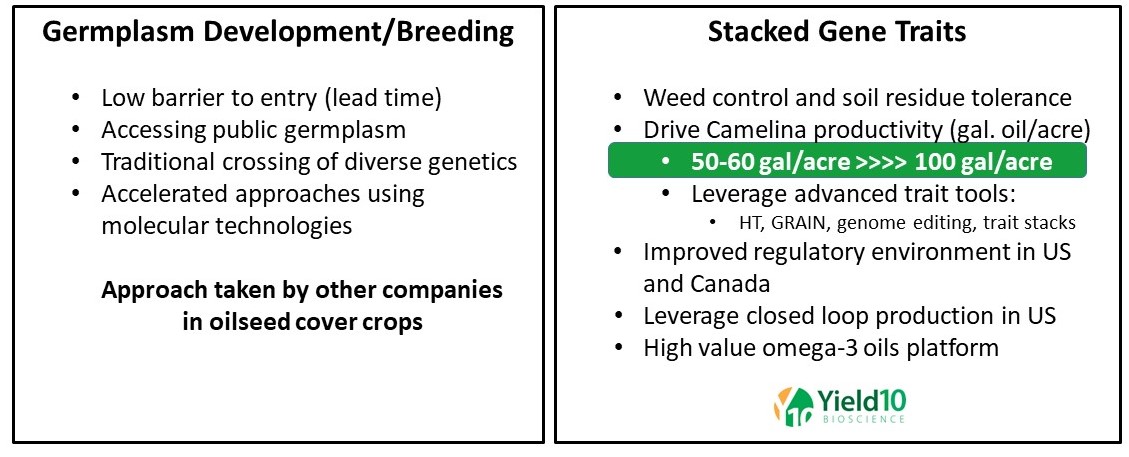
Figure 4. Pipeline of Genetic Traits for Camelina
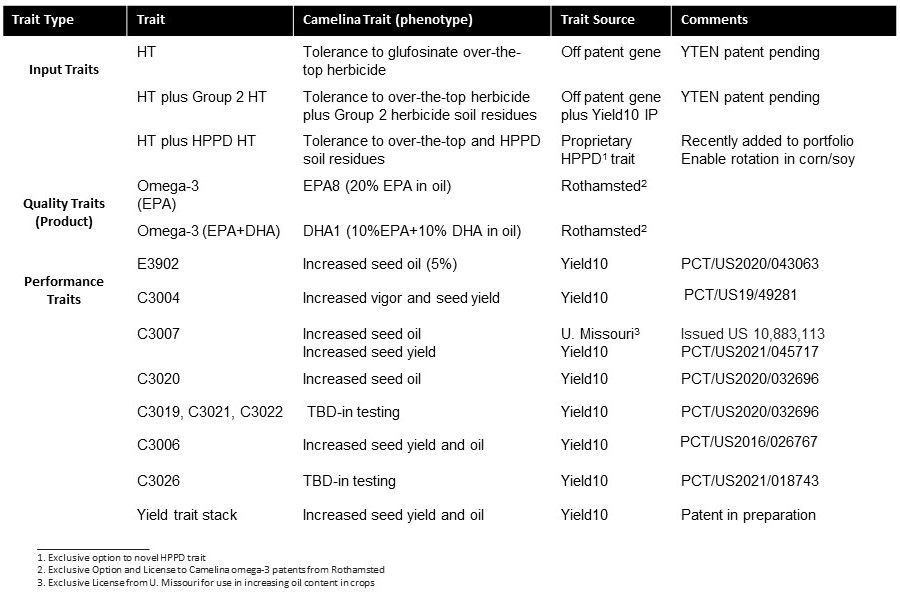
11
INPUT TRAITS
Herbicide Tolerance
We recognized early on the need to develop Camelina lines optimized for seamless integration into both crop and chemical rotations in our target geographies. For the Northwest U.S. and Western Canada, the herbicide tolerance traits selected for accelerated development were chosen to address critical farmer issues in that geography. The first requirement is for broadleaf and grassy weed control. The second requirement is to impart into Camelina tolerance to soil residues of herbicides used in crop rotations. An ideal herbicide trait package in Camelina would combine herbicide tolerance traits to manage grassy weeds, broadleaf weeds, and soil residual herbicides. With respect to managing grassy weeds, Camelina is naturally tolerant to the grass herbicide Clethodim, so there is no need to develop new traits in Camelina to provide tolerance to this chemical.
Our glufosinate herbicide tolerance (“GLU”) trait offers farmers an important tool for fighting broadleaf weeds that, if left uncontrolled, can reduce crop yields significantly and increase weed pressure on that land for subsequent crop plantings. We have field tested spring and winter Camelina engineered with GLU with encouraging results over the past two years.
Camelina is very sensitive to Group 2 herbicide residues which can prevent seed emergence and severely impair seed yields. Group 2 weed control chemistries persist longer in the soil which results in plant back restrictions for the next crop in the rotation and in some cases, this can be up to two years. Development is on track with our stacked HT trait which includes tolerance to GLU in combination with both types of Group 2 herbicides, SU and IMI which are commonly used in the target production region for Camelina. Based on discussions with farmers we believe that the stacked HT trait package will be an important enabler for large acre adoption and particularly for winter Camelina varieties which will be planted in the fall following the harvest of spring crops. These HT and stacked HT traits and the availability of Clethodim for grassy weed control provides Yield10 with an ideal herbicide package for the commercial launch of these varieties in the target regions of North America. Yield10 is executing a plan to complete the development of GLU tolerant Camelina including steps to de-regulate meal for use in animal feed.
Complementing our work in engineering new traits into Camelina we have also established a Camelina breeding program. A key goal of this program is to develop a wide range of traits to improve the agronomic performance of Camelina including developing traits for tolerance to the fungal pathogen downy mildew ("DMR") and other fungal diseases, which can negatively impact Camelina seed yields. In 2021, we acquired the rights to a Camelina line with DMR. We also have two backup Camelina lines demonstrating partial resistance and a funded breeding program ongoing for producing additional DMR lines. As these traits are identified we plan to breed them into our advanced HT Camelina lines.
PRODUCT TRAITS
Omega-3 (EPA, DHA+EPA) oil traits
In October of 2023, we executed our option to a global exclusive License to the Omega-3 Camelina technology from Rothamsted and expect to finalize the Commercial License Agreement in the second quarter of 2024. The EPA8 omega-3 trait enables the accumulation of EPA at up to 20% of the fatty acid composition in the Omega-3 Camelina seed oil. The DHA1 omega-3 trait enables the accumulation of EPA at up to 10% of the fatty acid composition plus DHA at up to 10% of the fatty acid composition in the DHA1 Omega-3 Camelina seed oil. We plan to bring both omega-3 varieties forward in development with 2026 being the target for the first commercial-scale production for EPA8 Omega-3 Camelina. Herbicide tolerance is critical in Camelina for on-farm performance and we are breeding HT traits into the omega-3 Camelina varieties to create second generation varieties for large acreage production.
PERFORMANCE TRAITS
Examples of Seed Oil Enhancing Traits: C3007, C3008, C3009, C3010, C3012 and C3020
Yield10 is progressing a series of novel performance traits targeted at increasing the seed oil content in Camelina. We began the technical work in Camelina during 2016 with our C3008a, C3008b and C3009 traits, which regulate the
12
production and degradation of oils in oilseed crops. The best line containing these modifications is designated as our triple-edited, or C3008a, C3008b and C3009 trait in the spring Camelina line E3902 line which consistently shows an approximately 4.7 percent increase in seed oil content as a percentage of overall seed weight. In 2018, we received confirmation from the U.S. Department of Agriculture - Animal and Plant Health Inspection Service's ("USDA-APHIS") Biotechnology Regulatory Services ("BRS") that Camelina line E3902 is not considered to be regulated articles under 7 CFR part 340. We have also gained additional clearances that allow growth of Camelina line E3902 in Argentina, Chile and Canada.
In 2018, we signed an exclusive global license agreement with the University of Missouri for advanced oilseed technology, including the C3007 trait. We have produced several CRISPR genome edited versions of C3007 in both Camelina and canola. Through a series of submissions to USDA-APHIS, we have developed several lines of Camelina and canola edited in combinations of the C3007 genes that USDA-APHIS BRS does not consider to be regulated under 7 CFR part 340. In addition, in 2021 the Ministry of Agriculture, Livestock and Fisheries in Argentina indicated that two C3007 Camelina lines would not be subject to regulation in that country.
Yield10 researchers achieved proof of concept showing that four novel gene targets identified using our proprietary GRAIN trait gene discovery platform impact seed development and/or oil content. In greenhouse testing in 2020, one of the three targets, C3020, produced a 10% increase in seed oil content when engineered with increased activity in Camelina. Data obtained from increasing activity of the other three targets, C3019, C3021, and C3022 indicates these may represent suitable targets for CRISPR genome-editing.
Regulatory Requirements
Ever since the first successful commercialization of a biotechnology-derived agricultural crop in the 1990s, many new crop varieties have been developed and made available to farmers in the U.S. and worldwide. Farmers in the U.S., Canada, and areas of South America have rapidly adopted many of these new biotechnology-derived varieties. According to the USDA, in 2020 over 90 percent of U.S. corn, upland cotton and soybeans planted in the U.S. were varieties produced through traditional forms of genetic engineering. We have established certain core regulatory capabilities and in key geographies, we believe we are benefiting from a science-driven approach to regulations pertaining to genetically engineered (“GE”) crops based on a 30-year history of biotechnology-derived agricultural crops.
Biotechnology-derived or genetically engineered crops are subject to a significant amount of regulation in the U.S. and worldwide. Field tests and field trials of such crops need to ensure that traits in development do not escape or mix with native plants, and crops that may be used in the human and animal food chains must meet certain safety standards. Government regulations, regulatory systems and the political environment that influence them vary significantly among jurisdictions.
For purposes of this discussion, the term “GE” includes both biotechnology-derived or genetically engineered plants that are modified by the insertion of recombinant DNA (“Traditional Genome Modification”) or genetically engineered plants modified through the application of more modern techniques of genome editing. We have seed traits that fall within each of these two generalized categories of GE plants, as summarized above under the subheading “Traits in Development.”
United States Regulation
The U.S. government agencies primarily responsible for overseeing the products of modern agricultural biotechnology are the USDA, the FDA and the EPA. Depending on its characteristics, a product may be subject to the jurisdiction of one or more of these agencies under the federal government’s 1986 Coordinated Framework for the Regulation of Biotechnology, as updated. Other environmental laws or regulations also apply, depending on the specific product and its potential applications or intended uses.
Our seed traits and any future products that are successfully developed containing our seed traits are subject to USDA, FDA and EPA regulatory requirements. Those requirements will vary depending on the particular seed trait and the type and intended use of any product that will be commercialized. Future products that we plan to produce and sell, for
13
example deployment of herbicide tolerant traits, are likely to have EPA regulatory requirements, and the regulations relating to manufacturing and consumer protection will also need to be addressed.
Within USDA, APHIS administers the regulations in 7 CFR part 340, ‘‘Introduction of organisms and products altered or produced through genetic engineering which are plant pests for which there is reason to believe are plant pests.’’ These regulations govern the introduction (importation, interstate movement, or release into the environment) of certain GE organisms. Along with the EPA and the FDA, APHIS is responsible for the oversight and review of GE organisms.
On May 18, 2020, the USDA updated the biotechnology regulations in the Plant Protection Act (7 C.F.R. Part 340) and set up a new paradigm called the Sustainable, Ecological, Consistent, Uniform, Responsible, Efficient (“SECURE”) rule. This Act establishes updated regulations for importation, interstate movement, and environmental release of GE organisms and products. It provides exemptions for plants if the genetic modification is solely a deletion of any size, or the genetic modification is a single base pair substitution or if the genetic modification is solely introducing nucleic acid sequences from within the plant’s natural gene pool. Exemptions also apply if the modification is from editing nucleic acid sequences in a plant to correspond to a sequence known to occur in that plant’s natural gene pool or if the plant is an offspring of a GE plant and does not retain the genetic modification in the GE plant parent. In addition to the above, § 340.1(c) states that modified plants would not be subject to the regulations if they have plant-trait mechanism of action (“MOA”) combinations that are the same as those of modified plants for which APHIS has conducted a regulatory status review and found not to be subject to the regulations under part 340. The focus rests on the organism itself rather than the methods and technologies used to generate it, which is important given improvements in delivery and genome editing modalities over the past 33 years.
In 2020, the regulatory exemptions and confirmation process under the SECURE rule took effect, representing the first comprehensive revision of APHIS’ biotechnology regulations since 1987. The revisions enable APHIS to regulate organisms developed using genetic engineering for plant pest risk with greater precision and reduces the regulatory burden for developers of organisms that are unlikely to pose plant pest risks. Since the new process was enacted, certain of our CRISPR edited Camelina C3007 lines have been confirmed by APHIS as being exempt from regulation by USDA-APHIS under 7 CFR part 340. In addition, in the fourth quarter of 2023 we received confirmation from USDA that both the HT and Stacked HT Camelina are not considered regulated by USDA-APHIS.
For herbicide-tolerant crops the USDA-APHIS regulates the crops while the EPA regulates the herbicide. The EPA establishes tolerances for the allowable amount of herbicide residues that may remain on the crop. Tolerances, as defined by the EPA, are “the maximum amount of a pesticide allowed to remain in or on a food” as part of the process of regulating pesticides. Yield10 cooperated with one of the major producers of glufosinate herbicides to request EPA approval to add Camelina to the producer's herbicide label for use with our GLU HT Camelina. A response from EPA is pending.
Separate from approval for genetic modifications from USDA-APHIS regulations under 7 CFR part 340, a GE plant also will be regulated by the FDA if it is intended to be used as human food or animal feed. Since 1992, the FDA has had in place a voluntary consultation process for developers of bioengineered food (“Biotechnology Consultations”). Final agency decisions and other information from these Biotechnology Consultations are made publicly available by the FDA. Biotechnology Consultations are data-intensive and examine the new food product’s safety and nutritional profile, among other issues. Generally, the FDA has found that such food products do not pose unique health risks to humans or animals, but if a novel allergen or other distinction from the conventional food is present in the new plant variety, the agency may require specific label statements on the product to ensure that consumers are made aware of material differences between GE and conventional versions.
In October 2018, FDA leadership issued a document entitled the “Plant and Animal Biotechnology Innovation Action Plan” (the “Action Plan”) that identified three key priorities for the agency in this area: 1) advancing human and animal health by promoting product innovation and applying modern, efficient and risk-based regulatory pathways; 2) strengthening public outreach and communication regarding the FDA’s approach to innovative plant and animal biotechnology; and 3) increasing engagement with domestic and international partners on biotechnology issues. The Action Plan also stated that the FDA has reviewed the comments and other information it received in response to the January 2017 request for comments, and that it intends to develop guidance for the industry explaining how the FDA’s existing regulatory policy for foods derived from new plant varieties applies to foods produced using genome editing. The FDA also stated in the Action Plan that it intends to begin updating the existing procedures for voluntary Biotechnology Consultations to reflect the agency’s 25 years of experience with foods derived from biotechnology plants and to incorporate any additional issues
14
related to genome editing of food crops. Such procedural updates are expected to be developed and implemented over the next two years.
For herbicide tolerant and Omega-3 Camelina, field trials and analytical work must be conducted to evaluate the composition of the meal and/or oil to ensure their composition is similar to conventional Camelina and that they are free of potential allergens and toxins. Yield10 is developing regulatory plans to support commercialization of products produced in HT, stacked HT and omega-3 Camelina.
Canadian Regulation
In Canada, GE crops, and the food products into which they are incorporated, are regulated by multiple government agencies under a federal framework for the regulation of biotechnology products that is similar to the U.S. system. First, the CFIA is the lead agency for ensuring that a new agricultural biotechnology crop will not pose new risks to Canadian plants, animals, and other agricultural commodities. The Plant Biosafety Office ("PBO") is responsible for conducting environmental assessments of biotechnology-derived plants, referred to as "plants with novel traits" ("PNT"). Authority for the PBO includes both approving confined field trials with the PNT through permits and authorizing their “unconfined release” as a first step towards commercialization. PNTs are defined in the Canadian Seeds Regulations (i) as plants into which a trait or traits have been intentionally introduced, and (ii) where the trait is new in Canada and has the potential to impact the environment. The CFIA also has in place a remutation policy, whereby plants containing the same mutation as a previously authorized plant of the same species are included in the authorization of the original PNT and are therefore subject to the same conditions.
In 2023, the CFIA adopted new regulation and guidelines on determining whether a plant is regulated under Part V of the Seeds Regulations and specifically indicating that genome editing technologies as utilized in crops do not present any unique or specifically identifiable environmental or human health safety concerns as compared to other technologies of plant development. In early 2024, the PBO of the CFIA reviewed information on the Company’s E3902 Camelina and determined that E3902 is not a PNT and is not subject to a pre-market notification under Part V of the Seeds Regulations.
Under the Food and Drugs Act and related regulations, Health Canada is responsible for reviewing a pre-market safety assessment that must be submitted by the manufacturer or importer of a “novel food,” a term of art that includes any PNT or other biotechnology-derived foods. The safety assessment should provide assurances that the novel food is safe when prepared or consumed according to its intended use before it enters the Canadian market and food system. A multi-disciplinary team of experts from Health Canada evaluates the data and information about the novel food and make a determination regarding whether it is safe and nutritious before it can be sold in Canada, as well as whether any restrictions are warranted under applicable law or the product’s safety profile. Health Canada’s final decision documents regarding the safety of these novel foods are made available to the public by the government. As in the U.S., approval of a PNT or a novel food product does not take into account the method with which such product was produced. Rather, Health Canada employs a product-based (as opposed to a process-based) approach to its regulatory oversight of such emerging foods and food ingredients.
As the lead agency for public health and safety, Health Canada also works in conjunction with the CFIA on food labeling oversight when it identifies a potential health or safety issue with a food that could be mitigated through labeling or other disclosures. For example, if the biotechnology-derived food contains a new allergen that is otherwise not present in the conventional version of the food, then specific label statements are required to alert consumers to that important health information. However, the CFIA has primary oversight over non-health issues related to food labeling, packaging, and advertising. Accordingly, the CFIA is the lead agency for ensuring that food labeling and advertising meet the legal requirements of the Food and Drugs Act, and that labeling representations do not create a potential risk of fraud or consumer confusion and are compliant with Canada’s voluntary disclosure standard for GE food ingredients.
Environment Canada is also available to serve as a regulatory “safety net” if a novel product does not naturally fall within the jurisdiction of the CFIA, Health Canada, or the Pest Management Regulatory Agency that oversees pesticide products.
Our work involving the development, greenhouse testing, and field testing of novel yield trait genes in crop plants requires certain government and municipal permits, and we must ensure compliance with all applicable regulations, including regulations relating to GE crops. With laboratories and greenhouses in both the U.S. and Canada, we are also subject to regulations governing the shipment of seeds and other plant material (including GE seeds and GE plant material) between our
15
facilities in the U.S. and Canada, including USDA-APHIS and CFIA permits for the import and phytosanitary certificates for the export of plant materials that could pose a risk to domestic agriculture.
Having deployed our own research and development operations in Saskatoon, Canada in 2010, we have been conducting field studies of various yield traits in that country since 2016 under PNT permits issued by Canadian regulators.
Regulation in Other Jurisdictions
Other jurisdictions and governmental authorities, including in South America and Asia, are increasingly taking an interest in regulating agricultural products of biotechnology. Regulatory approaches vary by jurisdiction, including the existing public health framework and phytosanitary laws in the country, and other less tangible factors such as cultural and religious norms that may have an impact on individual country risk assessments and decision-making. We cannot predict future changes in the global regulatory landscape regarding GE plants subjected to Traditional Genome Modification or GE plants subjected to genome editing. Further, although U.S. and Canadian regulatory authorities have taken similar approaches to overseeing both traditional biotechnology-derived plants and genome edited plants under their national plant health and biosafety laws, regulation of all GE plants in the EU is significantly more stringent than in North America. U.S. and Canadian regulators have also determined that genome edited GE plants pose fewer risks that those subjected to Traditional Genome Modification. A July 2018, Court of Justice of the European Union legal ruling indicates that the existing European regulations for GE plants modified by the insertion of recombinant DNA should be strictly applied to genome edited plants as well. There is thus a sharp distinction between how European and North American regulatory agencies oversee novel seed traits, including those that are generated using the more modern techniques of genome editing. Although we are not currently targeting European markets for the development or commercialization of our products, the EU approach to regulating GE plants without regard to the scientific distinctions between Traditional Genome Modification and directed genome editing could be adopted by emerging oversight regimes for GE products in other jurisdictions. However, an increasing number of countries that dominate GE crop production such as Argentina, Brazil and China are adopting a more favorable regulatory approach towards genome edited plants that do not contain foreign DNA by equating the crops to conventionally bred varieties. This approach first implemented by Argentina, followed by many other countries, demonstrates the evolving landscape for GE crops informed by over 25 years of regulation and GE crop production.
In 2020, Japan published final guidelines for genome edited plants and food that state that these can be sold to the public, without the need for pre-market authorization provided they meet the criteria of being similar to products of traditional breeding.
In 2022, the Chinese Ministry of Agriculture and Rural Affairs published new guidelines for the review and approval of genome edited crops and products paving the way for faster commercialization in that country.
In-License Agreements
Exclusive Collaboration Agreement with Rothamsted Research
On November 12, 2020, Yield10 signed an exclusive collaboration agreement with Rothamsted to support Rothamsted’s Flagship Program to develop omega-3 oils in Camelina. As part of the agreement, Yield10 received an exclusive two-year option to sign a global, exclusive or non-exclusive license agreement to the technology. In November 2022, Yield10 and Rothamsted agreed to extend the collaboration agreement, including the license option, without additional funding support, through December 31, 2023. In October of 2023 Yield10 exercised its exclusive option and expects to execute the global Exclusive License Agreement for this technology with Rothamsted in the second quarter of 2024.
License Agreement with the University of Missouri
Pursuant to a license agreement with the University of Missouri (“UM”) dated as of May 17, 2018, we have an exclusive, worldwide license to novel gene technologies including the C3007 trait to boost oil content in crops. We are obligated to pay UM a license execution payment, milestone payments relating to any regulatory filings and approvals covered by the license agreement, royalties on any sales of licensed products following regulatory approval, as well as a percentage of any sublicense royalties related to the licensed products.
16
We may terminate the license agreement at any time upon 90 days’ prior written notice to UM. Either party may terminate the license agreement upon written notice for a breach that is not cured within 30 days after receiving written notice of the breach. In addition, UM may terminate the license agreement with respect to certain patent rights immediately upon written notice in the event we contest the validity or enforceability of such patent rights.
Competitive Landscape for our Business
Camelina Oilseed and Alternative Cover Crops: Camelina, because it is not currently a major food crop, has been of recent interest in North America for large-scale production of feedstock oil for biofuels, both renewable diesel and sustainable aviation fuel. We believe that there is a growing interest among oilseed crushers and energy companies in sourcing non-food, low CI feedstock oil for supplying the biofuel market. Camelina is attractive, not only as a spring rotation crop, but also as a winter cover crop, enabling a second oil harvest annually for each acre planted. The general interest in cover crops has been steadily increasing over the last several years. The companies focusing on Camelina include Sustainable Oils, S&W Seed, and Smart Earth. There have also been recent investments in oilseed cover crop alternatives to Camelina including the development of carinata by Nuseed and the efforts by CoverCress Inc. over the last several years to develop pennycress as a cover crop for the mid-west corn and soybean belt. In 2023, Chevron/Bunge acquired Chacraservicios S.r.l., based in Argentina from the Italian-based Adamant Group. This latest investment in novel seeds adds a new oil source in Bunge and Chevron’s global supply chains and will help both companies meet the growing demand for lower carbon renewable feedstocks. In 2023 Vision Bioenergy Oilseeds was formed as a joint venture owned by Shell and S&W Seed to develop and commercialize novel plant genetics for oil seed cover crops for biofuel production. Earlier this year, we signed a non-exclusive License Agreement with Vision for the use of our HT and stacked HT Camelina technologies.
Omega-3 Oil: The growing demand for alternative sustainable sources of EPA and DHA omega-3 fatty acids for human nutrition including food/beverage, pharmaceutical, and aquafeed and animal feed applications has made this an attractive area for investment by several companies. Alternative sources include algal fermentation processes commercialized by Veramaris (the joint venture between Evonic and DSM, with a production facility in Blair, Nebraska), Corbion and by Archer Daniels Midland Co. (with a production facility in Clinton, Iowa). We believe that crop based production systems for producing omega-3 oils will always be lower cost to produce and scale than algal fermentations and for this reason we have focused on competing crop based solutions. On the crop-based production side, two different genetically engineered varieties of the oilseed canola have been developed and approved by USDA-APHIS to address this growing demand. Following their acquisition of Bayer’s canola seed business, BASF stopped commercial development of their EPA producing canola. The BASF canola variety produced mostly EPA. Nuseed, a subsidiary of NuFarm is making good progress with the commercial production of its DHA canola oil which has around 10% DHA and a small amount of EPA and recently received regulatory approval for this oil in Norway. This is a major positive development for the use of GMO ingredients in aquaculture feed in the world’s largest farmed salmon production region. We believe the Camelina technology, which enables production of omega-3 EPA, and EPA+DHA oils, have high potential as a drop-in replacement for fish oil in aquafeed. Feed producers in general will blend ingredients from different suppliers to meet their own final product specifications and manage costs. Our Camelina EPA oil has potential use in feed in combinations with other sources of DHA such as algae or the Nuseed DHA oil product.
Intellectual Property
Our continued success depends in large part on our proprietary technology. As of December 31, 2023, Yield10 owns or holds exclusive rights to 18 patent families, including 20 issued patents and 30 pending patent applications. In October of 2023 Yield10 exercised its exclusive option to an exclusive global license to two active patent families of Rothamsted Research, RR213 and RR305, including six issued patents and six pending applications which cover the production of EPA and EPA plus DHA oils in Camelina. We expect to finalize the Commercial License Agreement in the second quarter of 2024.
We continue to seek, develop and evaluate new technologies and related intellectual property that might enhance our business strategy, industry position or deployment options.
Human Capital Resources
As of December 31, 2023, we had 29 full-time employees. Of those employees, 24 were in research and development. Among our staff, 13 hold Ph.D.’s and 13 hold masters’ or bachelors’ degrees in their respective disciplines. Our technical staff has expertise in the following areas: plant genetics, plant biology, plant breeding, microbial genetics,
17
bioinformatics, metabolic engineering and systems biology. Our headquarters is located in Massachusetts, and our wholly-owned subsidiary, Yield10 Oilseeds Inc. ("YOI"), maintains a research and development facility, including greenhouse facilities, in Saskatoon, Canada. None of our employees are subject to a collective bargaining agreement and we consider our relationship with our employees to be good.
Talent Acquisition and Retention
We recognize that our employees largely contribute to our success. To this end, we support business growth by seeking to attract and retain best-in-class talent. We use internal and external resources to recruit highly skilled candidates for open positions. We believe that we are able to attract and retain superior talent as measured by our minimal turnover rate and high employee service tenure.
Total Rewards
Our total rewards philosophy has been to create investment in our workforce by offering a competitive compensation and benefits package for the two geographies in which we have offices. We provide employees with compensation packages that include base salary, annual incentive bonuses, and long-term equity incentive awards. We also offer comprehensive employee benefits, such as life, disability, and health insurance as well as flexible spending accounts, paid time off, and a 401(k) plan. It is our expressed intent to be an employer of choice in our industry by providing a market-competitive compensation and benefits package.
Health, Safety, and Wellness
The health, safety, and wellness of our employees is a priority in which we have always invested and will continue to do so. We provide our employees with access to a variety of innovative, flexible, and convenient health and wellness programs. Program benefits are intended to provide protection and security, so employees can have peace of mind concerning events that may require time away from work or that may impact their financial well-being.
Diversity, Equity, and Inclusion
We believe a diverse workforce is critical to our success. Our mission is to value differences in races, ethnicities, religions, nationalities, genders, ages, and sexual orientations, as well as education, skill sets and experience. We are focused on inclusive hiring practices, fair and equitable treatment, organizational flexibility, and training and resources.
Corporate History and Investor Information
In 1992, our Company was incorporated in Massachusetts under the name Metabolix, Inc. In September 1998, we reincorporated in Delaware and in January 2017, we changed our name to Yield10 Bioscience, Inc. to reflect our change in mission around innovations in agricultural biotechnology focused on developing disruptive technologies for step-change improvements in crop yield. Financial and other information about our Company is available on our website at www.yield10bio.com.
We make available on our website, free of charge, copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the Securities and Exchange Commission (the "SEC"). In addition, the SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Our filings with the SEC may be accessed through the SEC's website at http://www.sec.gov.
ITEM 1A. RISK FACTORS
Risk Factor Summary
Our business is subject to numerous risks. We encourage you to carefully review the full risk factors contained in this Annual Report on Form 10-K. Some of the principal risk factors are summarized below:
•We have a history of net losses and our future profitability is uncertain.
18
•We will need to secure additional funding to finance our operations and may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us.
•Our seed products and crop science technologies are at an early stage of development. We may never commercialize a technology or product that will generate meaningful, or any, revenues.
•There can be no assurance that we will be able to comply with the continued listing standards of The Nasdaq Capital Market.
•The impact of global geopolitical conflicts, including the Russian invasion of Ukraine and the Israel-Hamas war may adversely affect our business, financial condition or results of operations.
•The crop science product development cycle is lengthy and uncertain, and our progress will depend on our ability to attract third-party investment in research under license agreements and on our ability to establish collaborative partnerships to develop and commercialize our innovations.
•Any potential collaborative partnerships that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our innovations.
•Our crop science program may not be successful in developing commercial products or if our future collaborators are successful in developing commercial products that incorporate our traits, such products may not achieve commercial success.
•Our estimates of market opportunity and forecasts of market growth may prove to be inaccurate, and even if the markets in which we may compete in the future achieve growth, our business could fail to achieve the same growth rates as others in the industry.
•If ongoing or future field trials conducted by us or our future collaborators are unsuccessful, we may be unable to complete the regulatory process for, or commercialize, our products in development on a timely basis.
•Adverse weather conditions, natural disasters, crop disease, pests and other natural conditions can impose significant costs and losses on our business.
•Competition in the market for traits and seeds is intense and requires continuous technological development, and, if we are unable to compete effectively, our financial results will suffer.
•Our business is subject to various government regulations in the United States and Canada; the regulatory requirements for our future products in development are evolving and are subject to change, and if there are adverse changes to the current regulatory framework, our or our future collaborators’ ability to market our traits could be delayed, prevented or limited.
•If we or our future collaborators are unable to comply with and timely complete the regulatory process in the United States and Canada for our future products in development, our or our future collaborators’ ability to market our traits could be delayed, prevented or limited.
•The regulatory environment for genetically engineered crops in jurisdictions outside the United States and Canada varies greatly, and some jurisdictions have more restrictive regulations that could delay, prevent or limit our or our future collaborators’ ability to market our traits.
•Consumer resistance to genetically engineered crops may negatively affect the ability to commercialize future crops containing our traits, as well as our public image, and may reduce any future sales of seeds containing our yield traits.
•Government policies and regulations, particularly those affecting the agricultural sector and related industries, could adversely affect our operations and our ability to generate future revenues and to achieve profitability.
•The products of third parties, or the environment itself, may be negatively affected by the unintended appearance of our trait genes, novel seed compositions and novel seed products.
•Loss of or damage to our elite novel trait events and plant lines would significantly slow our product development efforts.
•Our insurance coverage may be inadequate to cover all the liabilities we may incur.
19
•We rely on third parties to conduct, monitor, support, and oversee field trials and, in some cases, to maintain regulatory files for those products in development, and any performance issues by third parties, or our inability to engage third parties on acceptable terms, may impact our ability to complete the regulatory process for or commercialize such products.
•If we lose key personnel or are unable to attract and retain necessary talent, we may be unable to develop or commercialize our products under development.
•Our business and operations would suffer in the event of system failures.
•Patent protection for our technologies is both important and uncertain.
•Third parties may claim that we infringe their intellectual property, and we could suffer significant litigation or licensing expense as a result.
•Portions of our crop science technology are owned by or subject to retained rights of third parties.
•We may not be successful in obtaining necessary rights to additional technologies for the development of our products through acquisitions and in-licenses.
•Our license agreements include milestone and royalty payments that we are required to make to third parties.
•The intellectual property landscape around genome editing technology, such as CRISPR, is highly dynamic and uncertain, and any resolution of this uncertainty could have a material adverse effect on our business.
•We rely in part on trade secrets to protect our technology, and our failure to obtain or maintain trade secret protection could harm our business.
•Raising additional funds may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies.
•Trading volume in our stock can fluctuate and an active trading market for our common stock may not be available on a consistent basis to provide stockholders with adequate liquidity. Our stock price may be volatile, and our stockholders could lose a significant part of their investment.
•Provisions in our certificate of incorporation and by-laws and Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
•Concentration of ownership among our officers, directors and principal stockholders may prevent other stockholders from influencing significant corporate decisions and depress our stock price.
We caution you that the following important factors, among others, could cause our actual results to differ materially from those expressed in forward-looking statements made by us or on our behalf in filings with the SEC, press releases, communications with investors and oral statements. Any or all of our forward-looking statements contained in this Annual Report on Form 10-K and in any other public statements we make may turn out to be wrong. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. Many factors mentioned in the discussion below will be important in determining future results. Consequently, no forward-looking statement can be guaranteed. Actual future results may differ materially from those anticipated in forward-looking statements. We undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised, however, to consult any further disclosure we make in our reports filed with the SEC.
Risks Relating to our Financial Position
We have a history of net losses and our future profitability is uncertain.
We have recorded losses every year since our inception, with the exception of 2012. As of December 31, 2023, our accumulated deficit was $414,152. Since 1992, we have been engaged primarily in research and development and early-stage commercial activities. Because our crop science technology is at an early stage of development, we cannot be certain that our business will generate sufficient revenue to become profitable. We expect to continue to have significant losses and negative cash flow for at least the next several years, as we incur additional costs and expenses for the continued development of our
20
technology, including the ongoing expenses of research, development, commercialization and administration. The amount of money we spend will impact our need for capital resources as well as our ability to become profitable and this will depend, in part, on the number of new technologies that we attempt to develop. We may not achieve any or all of these goals and, thus, we cannot provide assurances that we will ever be profitable or achieve significant, or any, product revenues.
If we are unable to raise substantial additional funds in the near term, we will be unable to continue to operate our business and remain a going concern.
We have identified conditions and events that raise substantial doubt about our ability to continue as a going concern. We had cash and cash equivalents of $1,068 as of December 31, 2023, which we believe will only provide funding for our operations into the second quarter of 2024. Our management is urgently evaluating and pursuing different strategies to obtain the required funding for our operations in the near term. These strategies may include, but are not limited to: public and private placements of equity and/or debt, licensing and/or collaboration arrangements and strategic alternatives with third parties, or other funding from the government or third parties. There can be no assurance that these funding efforts will be successful. The sale of equity and convertible debt securities would result in dilution to our stockholders and, in the case of preferred equity securities or convertible debt, those securities could provide for rights, preferences or privileges senior to those of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products or grant licenses on terms that are not favorable to us. Additional capital may not be available on reasonable terms, or at all. If we are unable to obtain funds when needed or on acceptable terms, we may be required to curtail our current development programs, cut operating costs, forego future development and other opportunities or even terminate our operations, which may involve seeking bankruptcy protection.
We will need to secure additional funding to finance our operations and may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us.
As of December 31, 2023, we had unrestricted cash, cash equivalents and short-term investments of $1,068. We estimate that our cash resources will be sufficient to fund operations and meet our obligations into the second quarter of 2024. We follow the guidance of ASC Topic 205-40, Presentation of Financial Statements-Going Concern, in order to determine whether there is substantial doubt about our ability to continue as a going concern for one year after the date our financial statements are issued. Based on our current cash forecast, we expect that our present capital resources will not be sufficient to fund our planned operations for that period of time, which raises substantial doubt as to the Company's ability to continue as a going concern. This forecast of cash resources is forward-looking information that involves risks and uncertainties, and the actual amount of expenses could vary materially and adversely as a result of a number of factors. Our ability to continue operations after our current cash resources are exhausted will depend upon our ability to obtain additional financing through, among other sources, public or private equity financing, secured or unsecured debt financing, equity or debt bridge financing, warrant holders' ability and willingness to exercise the Company's outstanding warrants, additional research grants or collaborative arrangements with third parties, as to which no assurances can be given. We do not know whether additional financing will be available on terms favorable or acceptable to us when needed, if at all. If additional funds are not available when required, we will be forced to curtail our research efforts, explore strategic alternatives and/or wind down our operations and pursue options for liquidating our remaining assets, including intellectual property and equipment.
We continue to face significant challenges and uncertainties and, as a result, our available capital resources may be consumed more rapidly than currently expected due to any or all of the following:
•lower than expected revenues from grants and licenses related to our technologies;
•changes we may make to the business that affect ongoing operating expenses;
•further changes we may make to our business strategy;
•changes in our research and development spending plans; and
•other items affecting our forecasted level of expenditures and use of cash resources.
We will require additional capital resources to support the implementation of our business strategy. There can be no assurance that our financing efforts will be successful.
21
If we issue equity or debt securities to raise additional funds in the future, we may incur fees associated with such issuances, our existing stockholders may experience dilution from the issuance of new equity securities, we may incur ongoing interest expense and be required to grant a security interest in our assets in connection with any debt issuance, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, utilization of our net operating loss and research and development credit carryforwards may be subject to significant annual limitations under Section 382 of the Internal Revenue Code of 1986, as amended, due to ownership changes resulting from equity financing transactions. If we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our potential products or proprietary technologies or grant licenses on terms that are not favorable to us.
Our seed products and crop science technologies are at an early stage of development. We may never commercialize a technology or product that will generate meaningful, or any, revenues.
The crop science products and technologies we are currently developing are at an early stage of development, and the process of developing them is lengthy and uncertain. If we fail to introduce and commercialize a seed product that meets customers’ expectations, our growth prospects may be materially and adversely affected. In addition, our current management has limited experience in developing technologies for the crop science industry and has never commercialized a product or technology in this industry. We may never reach a point at which our efforts result in products that allow us to achieve revenue from their license or sale.
There can be no assurance that we will be able to comply with the continued listing standards of The Nasdaq Capital Market.
We cannot assure you that we will be able to comply with the standards that we are required to meet in order to maintain a listing of our common stock on Nasdaq. Nasdaq listing rules require us to maintain certain closing bid price, stockholders’ equity and other financial metric criteria in order for our common stock to continue trading on Nasdaq. For example, Nasdaq Listing Rule 5550(a)(4) requires companies to maintain a minimum of 500,000 publicly held shares. Nasdaq Listing Rule 5550(a)(2) requires listed securities to maintain a minimum bid price of $1.00 per share, and Listing Rule 5810(c)(3)(A) provides that a failure to meet the minimum bid price requirement exists if the deficiency continues for a period of 30 consecutive business days.
Minimum Stockholders' Equity Deficiency
On May 18, 2023, the Nasdaq Listing Qualifications Department (the “Staff”), informed us that the Company did not comply with the minimum stockholders’ equity requirement of at least $2,500,000 pursuant to Nasdaq Listing Rule 5550(b)(1) (“Rule 5550(b)(1)”). The Staff granted the Company’s request for an extension until September 30, 2023, which was subsequently extended until November 14, 2023, to comply with Rule 5550(b)(1).
On November 15, 2023, we received a notice from Nasdaq of the Staff’s determination that the Company had not met the terms of such extension. The Company requested an appeal of the Staff’s determination and submitted a hearing request to the Nasdaq Hearings Panel (the “Panel”), which request stayed any delisting action by the Staff until the hearing process was concluded. Yield10 participated in a hearing before the Panel on February 6, 2024 and on February 13, 2024, we were notified by the Panel that the Company had been granted an additional extension to remain listed on The Nasdaq Capital Market until May 13, 2024, subject to certain conditions. These conditions include that the Company provide a written update on the status of its plans to obtain financing and strengthen its balance sheet by March 15, 2024, as well as provide prompt notification of any significant events that may occur during the period of extension that may affect the Company's compliance with Nasdaq requirements. We provided the Panel with the requested update on March 14, 2024.
Minimum Bid Price Deficiency
On September 25, 2023, we received a separate notice from the Staff notifying the Company that it was not in compliance with the requirement to maintain a minimum bid price of at least $1.00 per share pursuant to Nasdaq Listing Rule 5550(a)(2). The second notice stated that pursuant to Nasdaq Listing Rule 5810(c)(3)(A), the Company would be afforded 180 calendar days, or until March 25, 2024, to regain compliance with the minimum bid price requirement. In order to regain compliance, shares of the Company’s common stock must maintain a minimum bid closing price of at least $1.00 per share for a minimum of ten consecutive business days. On March 26, 2024, the Company received another notice from the Staff stating that the Company is not eligible for a second 180 day compliance period for the Minimum Bid Price Rule deficiency
22
because the Company does not comply with the $5,000,000 minimum stockholders’ equity initial listing requirement for The Nasdaq Capital Market. The Notice indicates that the Company must present its views with respect to this deficiency to the Panel in writing no later than April 2, 2024, which it intends to do. Among other measures, the Company intends to schedule a special meeting of its shareholders for April 26, 2024 to approve a reverse stock split, in order to increase the price of its common stock to a level that will satisfy the Minimum Bid Price Rule.
Delisting from Nasdaq could make trading our common stock more difficult for investors, potentially leading to further declines in our share price and liquidity. If our common stock is delisted by Nasdaq, our common stock may be eligible to trade on an over-the-counter quotation system, where an investor may find it more difficult to sell our stock or obtain accurate quotations as to the market value of our common stock. We cannot assure you that our common stock, if delisted from Nasdaq, will be listed on another national securities exchange or quoted on an over-the counter quotation system.
The impact of global geopolitical conflicts, including the Russian invasion of Ukraine and the Israel-Hamas war may adversely affect our business, financial condition or results of operations.
The short and long-term implications of the ongoing war between Russia and Ukraine are difficult to predict at this time. The escalation in October 2023 of the conflict between Israel and Hamas also could cause disruptions to global economic conditions. It is unknown how long any of these disruptions will continue and whether such disruptions will become more severe.
To date, we have not experienced any material interruptions in our infrastructure, supplies, technology systems, or networks needed to support our operations. However, Ukraine, and the Black Sea region in general, are a major exporter of wheat and corn to the world, and the disruption of supply could cause volatility in prices and margins of these commodities and related products. Ukraine is also the largest supplier of sun seed and sun oil in the world, which cannot be completely replaced from other geographic regions. The conflict in Ukraine has created disruptions in global supply chains and is expected to create dislocations of key agricultural commodities. While Yield10 has no direct business operations or assets within either Ukraine or Russia, the Company's plans and operating results could be adversely affected by a number of factors, including the crop growing decisions made by farmers in the U.S., Canada, and South America as a consequence of supply shortages and rising commodity prices. These rising prices may negatively impact our ability to contract suitable acreage for future crop trials or could delay our plans to commercialize Yield10's first Camelina plant varieties.
The risk of cybersecurity incidents has increased in connection with the ongoing war in Ukraine. For example, the war has been accompanied by cyberattacks against the Ukrainian government and other countries in the region. The proliferation of malware from the war into systems unrelated to the war, or cyberattacks against U.S. companies in retaliation for U.S. sanctions against Russia or U.S. support of Ukraine, could also adversely affect our operations. In accordance with industry standards, we insure ourselves against many types of risks, including cybersecurity risks. While this insurance may mitigate certain of the risks associated with the ongoing Ukraine-Russia war, our level of insurance may not cover all losses we could incur. The potential effects of these conditions could have a material adverse effect on our business, results of operations and financial condition.
We will continue to monitor this fluid situation and develop contingencies as necessary to address any disruptions to our business operations as they develop.
Risks Relating to our Yield10 Bioscience Crop Science Program
The crop science product development cycle is lengthy and uncertain, and our progress will depend significantly on our ability to attract third-party investment in research under license agreements and on our ability to establish collaborative partnerships to develop and commercialize our innovations.
The technology and processes used in our crop science program and the application of our technology to develop Camelina as a platform crop for large scale production of low-carbon sustainable seed products to address applications in petroleum replacement, food and nutrition markets, are at an early stage of development. Research and development in the seed, agricultural biotechnology, and larger agriculture industries is expensive and prolonged and entails considerable uncertainty. Completion of development work with respect to our products will require a significant investment of both time and money, if it can be completed at all. We expect that collaborations with established agricultural industry companies may be required to successfully develop and commercialize our innovations. We may not be successful in establishing or
23
maintaining suitable additional relationships with established agricultural industry companies for research licenses in the future, and there can be no assurance that any such relationships will result in future collaboration agreements to develop and commercialize our innovations, with terms that are satisfactory to us or at all. In addition, industry collaborators have significant resources and development capabilities and may develop products and technologies that compete with or negatively impact the development and commercialization of our technologies.
Any potential collaborative partnerships that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our innovations.
We expect that collaborations with established agricultural industry companies may be required for us to successfully develop and commercialize our innovations. The agriculture industry is highly concentrated and dominated by a small number of large companies, which could impact efforts to form the collaborations that we will need in order to complete the development of our products. To the extent that we pursue such arrangements, we will face significant competition in seeking appropriate partners. Moreover, such arrangements are complex and time-consuming to negotiate, document, implement and maintain. We may not be successful in establishing or implementing such arrangements. The terms of any partnerships, joint ventures or other collaborative arrangements that we may establish may not be favorable to us.
The success of any future collaborative partnerships is uncertain and will depend heavily on the efforts and activities of our potential partners. Such arrangements are subject to numerous risks, including the risks that:
•our partners may have significant discretion in determining the efforts and resources that they will apply to the arrangement;
•our partners may not pursue the development and commercialization of our product candidates based on trial results, changes in their strategic focus, competing priorities, availability of funding, or other external factors;
•our partners may delay or abandon field trials, fail to conduct field trials that produce sufficient conclusory data, provide insufficient funding for field trials, or repeat or conduct new field trials;
•partners who have marketing, manufacturing and distribution rights with respect to a product may not commit sufficient resources to, or otherwise may not perform satisfactorily in carrying out, these activities;
•to the extent that such arrangements provide for exclusive rights, we may be precluded from collaborating with others;
•our partners may not properly maintain or defend our intellectual property rights, or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
•disputes may arise between us and a partner that causes the delay or termination of the research, development or commercialization of our current or future products, or that results in costly litigation or arbitration that diverts management attention and resources;
•such arrangements may be terminated, and, if terminated, may result in a need for additional capital for our independent pursuit of matters previously covered by such arrangement;
•our partners may own or co-own intellectual property that results from our arrangement; and
•a partner’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings.
Our crop science program may not be successful in developing commercial products.
We and our potential future collaborators may spend many years and dedicate significant financial and other resources developing traits or other seed products that will never be commercialized. Seeds containing the traits that we develop may never become commercialized for any of the following reasons:
24
•our traits may not be successfully validated in the target crops;
•our traits may not achieve our targeted yield improvements;
•we may not be able to secure sufficient funding to progress our traits through development and commercial validation;
•our traits may not have the desired effects sought by future collaborators for the relevant crops;
•development and validation of traits, particularly during field trials, may be adversely affected by environmental or other circumstances beyond our control;
•we or our future collaborators may be unable to obtain the requisite regulatory approvals for the seeds containing our traits, to the extent regulatory approvals are required;
•competitors may launch competing or more effective seed traits or seeds;
•a market may not exist for seeds containing our traits or such seeds may not be commercially successful;
•future collaborators may be unable to fully develop and commercialize products containing our seed traits or may decide, for whatever reason, not to commercialize such products;
•we may be unable to patent our traits in the necessary jurisdictions; and
•our efforts to develop niche crop products based on our Camelina platform, including specialty oils and PHB biomaterials, are in the early stages and may not be successful.
If any of these things were to occur, it could have a material adverse effect on our business and our results of operations. Research and development in the crop science industry is expensive and prolonged and entails considerable uncertainty. Because of the stringent product performance and safety criteria applied in development of crop science products, products currently under development may not survive the development process or may ultimately not receive requisite regulatory approvals that may be needed to market such products. Even when such approvals are obtained, there can be no assurance that a new product will be commercially successful. In addition, research undertaken by competitors may lead to the launch of competing or improved products, which may affect sales of any products that we are able to develop.
Even if we or our future collaborators are successful in developing commercial products that incorporate our traits, such products may not achieve commercial success.
Our strategy depends upon our or our future collaborators’ ability to incorporate our traits into a wide range of crops in significant markets and geographies. Even if we or our future collaborators are able to develop commercial products that incorporate our traits, any such products may not achieve commercial success for one or more of the following reasons, among others:
•products may fail to be effective in particular crops, geographies, or circumstances, limiting their commercialization potential;
•our competitors, or competitors of our collaborators, may launch competing or more effective traits or products;
•significant fluctuations in market prices for agricultural inputs and crops could have an adverse effect on the value of our traits;
•farmers are generally cautious in their adoption of new products and technologies, with conservative initial purchases and proof of product required prior to widespread deployment, and accordingly, it may take several growing seasons for farmers to adopt our or our collaborators’ products on a large scale;
•we may not be able to produce high-quality seeds in sufficient amounts to meet demand; and
•we may not be able to secure the financial or other resources needed to achieve commercial success.
25
Our financial condition and results of operations could be materially and adversely affected if any of the above were to occur.
Our estimates of market opportunity and forecasts of market growth may prove to be inaccurate, and even if the markets in which we may compete in the future achieve growth, our business could fail to achieve the same growth rates as others in the industry.
Market opportunity estimates and market growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. Our estimates and forecasts relating to the size and expected growth of the global seed industry and the biotechnology seeds market, and the market size for any products that we may develop in our Camelina products business, such as Omega-3 Camelina oil, and the estimated ranges of incremental value increase that a novel, newly developed crop trait may produce, may prove to be inaccurate. Even if the markets in which we may compete in the future achieve these opportunity estimates and market growth forecasts, our business could fail to grow at similar rates, if at all.
If ongoing or future field trials conducted by us or our future collaborators are unsuccessful, we may be unable to complete the regulatory process for, or commercialize, our products in development on a timely basis.
The successful completion of multi-year, multi-site field trials is critical to the success of product development and marketing efforts for products containing our traits. If our ongoing or future field trials, or those of our future collaborators, are unsuccessful or produce inconsistent results or unanticipated adverse effects on crops, or if we or our collaborators are unable to collect reliable data, regulatory review of products in development containing our traits could be delayed or commercialization of products in development containing our traits may not be possible. In addition, more than one growing season may be required to collect sufficient data to develop or market a product containing our traits, and it may be necessary to collect data from different geographies to prove performance for customer adoption. Even in cases where field trials are successful, we cannot be certain that additional field trials conducted on a greater number of acres, or in different crops or geographies, will be successful. Generally, we or our research licensees conduct these field trials, or we pay third parties, such as farmers, consultants, contractors, and universities, to conduct field trials on our behalf. Poor trial execution or data collection, failure to follow required agronomic practices, regulatory requirements, or mishandling of products in development by our collaborators or these third parties could impair the success of these field trials.
Adverse weather conditions, natural disasters, crop disease, pests and other natural conditions can impose significant costs and losses on our business.
Many factors that may adversely affect the success of our field trials, seed inventory and seed production are beyond our control, including weather and climatic variations, such as drought or floods, severe heat or frost, hail, tornadoes and hurricanes, uncommon or unanticipated pests and diseases, or acts of protest or vandalism. For example, if there were a prolonged or permanent disruption to the electricity, climate control, or water supply operating systems in our greenhouses or laboratories, the crops in which we or our collaborators are testing our traits and the samples we or our collaborators store in freezers, both of which are essential to our research and development activities including field tests, could be severely damaged or destroyed, adversely affecting these activities and thereby our business and results of operations. Unfavorable weather conditions including drought or excessive rain, or fluctuations in temperature, which we have experienced from time to time in our field trials, can also reduce both acreages planted and incidence, or timing of, certain crop diseases or pest infestations, each of which may halt or delay our field trials. Any field test failure we may experience may not be covered by insurance and, therefore, could result in increased cost for the field trials and development of our traits, which may negatively impact our business, results of operations, and ability to secure financing. Such factors outside of our control can create substantial volatility relating to our business and results of operations.
In addition, seed crops are vulnerable to crop disease and to pests, which may vary in severity and effect, depending on the stage of production at the time of infection or infestation, the type of treatment applied and climatic conditions. Unfavorable growing conditions can reduce both crop size and quality and may reduce our available inventory.
Competition in the market for traits and seeds is intense and requires continuous technological development, and, if we are unable to compete effectively, our financial results will suffer.
26
We face significant competition in the markets in which we operate. The markets for traits and agricultural biotechnology products are intensely competitive and rapidly changing. In most segments of the seed and agricultural biotechnology market, the number of products available to consumers is steadily increasing as new products are introduced. At the same time, the expiration of patents covering existing products reduce the barriers to entry for competitors. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for any products that we or our future collaborators commercialize containing our traits. In addition, most of our competitors have substantially greater financial, marketing, sales, distribution, research and development, and technical resources than we have, and some of our potential future collaborators have more experience in research and development, regulatory matters, manufacturing, and marketing. We anticipate increased competition in the future as new companies enter the market and new technologies become available. Our technologies may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our traits being developed.
Our business is subject to various government regulations in the United States and Canada, the regulatory requirements for our future products in development are evolving and are subject to change, and if there are adverse changes to the current regulatory framework, our or our future collaborators’ ability to market our traits could be delayed, prevented or limited.
In the United States and Canada, where our seed traits and biotechnology-derived plant lines are developed and field tested, changes in regulatory requirements applicable to our seed traits or future products in development containing our traits could result in a substantial increase in the time and costs associated with developing and commercializing future products containing our traits, and could materially affect our ability to meet our desired development timelines or to develop and commercialize a future product containing our traits at all.
In the United States, our seed traits and any future products that are successfully developed containing our seed traits are or will be subject to USDA and FDA regulatory requirements. The USDA and FDA requirements will vary depending on the particular seed trait and the intended use of any product that will be commercialized. Our business strategy is focused on crop yield traits.
Within USDA, the APHIS is responsible for protecting agricultural plants under the Plant Protection Act. USDA-APHIS regulates organisms and products that are known or are suspected to be plant pests or to pose a plant pest risk, including those that have been altered or produced through various genetic engineering techniques. The USDA-APHIS has proposed regulations that could impact our business. For example, in recent years, we and others have submitted various petitions to USDA-APHIS to determine whether particular biotechnology-derived plants developed through the use of different genome editing techniques may be considered to be not regulated under the framework administered by the agency.
The USDA also announced in March 2018 that it would not require an assessment on products that used modern forms of mutagenesis if it was clear these outcomes could occur in nature. The USDA stated at that time that it did not “have any plans to regulate plants that could otherwise have been developed through traditional breeding techniques as long as they are developed without the use of a plant pest as the donor or vector and they are not themselves plant pests.” This USDA policy statement applies to genetic deletions of any size, which would include genome editing through CRISPR-Cas9 and other emerging technologies, although it remains to be seen how this policy announcement will be implemented by USDA-APHIS and what practical effect that may have on seed trait developers like us and our competitors.
There can be no guarantee that the USDA-APHIS governing regulations and policies will not change again in the future. We cannot predict whether advocacy groups will challenge existing regulations and USDA determinations, whether the USDA will alter its interpretations of existing regulations, modify existing regulations or promulgate new regulations, or whether additional laws will come into effect. If these or other developments resulted in adverse changes to the current regulatory framework, our seed traits or future products in development containing our traits could be subjected to more burdensome regulatory standards, thereby substantially increasing the time and costs associated with developing and commercializing any future products. Moreover, we cannot assure you that USDA-APHIS will analyze any of our future yield traits or products in development containing our traits in a manner consistent with its analysis of our genome edited yield traits to date. Complying with the USDA’s plant pest regulations for traits that are classified as “regulated articles,” including the permitting requirements for field testing and environmental release, is a costly, time-consuming process and
27
could substantially delay or prevent the commercialization of any future products containing traits that we expected to be deemed non-regulated by USDA-APHIS under 7 CFR part 340.
In addition to USDA-APHIS regulation of plant breeding and planting, a biotechnology-derived plant also will be regulated by the FDA if it is intended to be used as human food or animal feed. The FDA regulates the safety of food for humans and animals, and foods derived from novel plant varieties must meet the same food safety requirements as foods derived from traditionally bred plants (also called conventional foods). Since 1992, the FDA has had in place a voluntary consultation process for developers of bioengineered food (“Biotechnology Consultations”).
We have not participated in any Biotechnology Consultations or engaged in any informal discussions with the FDA about our novel yield traits, whether those traits have been developed using genome editing or traditional genome modification using the insertion of recombinant DNA. Any delay in the regulatory consultation process, or a determination by the FDA that future product candidates containing our traits raise different safety issues than the relevant conventional crop and therefore must be approved by the agency as a new food additive through an intensive premarket safety review process, could increase the costs associated with or delay or prevent the commercialization of the future product candidate. Such delays may lead to reduced acceptance by farmers, food manufacturers or the public and an increase in competitor products that may directly compete with ours. Further, if the FDA enacts new regulations or policies with respect to genome edited plants in particular, such policies could result in additional compliance costs or delay or prevent the commercialization of any potential commercial products containing our seed traits, which could adversely affect our ability to generate revenues and to achieve profitability.
In Canada, genetically engineered crops and the food products into which they are incorporated are regulated by multiple government agencies under a federal framework for the regulation of biotechnology products that is similar to the U.S. system. Any commercialization of our yield crops in Canada is expected to be done by a third-party collaborator or other partner and complying with Health Canada’s pre-market notification requirement and safety assessment for novel foods would be the obligation of that third-party collaborator.
Complying with the Canadian regulations is a costly, time-consuming process and could substantially delay or prevent the commercialization of our products. In addition, we cannot assure that CFIA and Health Canada regulations or the agencies’ implementation of those regulations will not change, or that the legislative framework in Canada for biotechnology-derived crops, whether for genome edited plants or plants modified using the insertion of recombinant DNA, will not be amended or otherwise changed in a manner that could result in additional compliance costs or delay, or prevent the commercialization of any potential commercial products containing our seed traits, which could adversely affect our ability to generate revenues and to achieve profitability.
Failure to comply with applicable regulatory requirements may, among other things, result in fines, suspensions of regulatory approvals, product recalls, product seizures, operating restrictions and criminal prosecution.
If we or our future collaborators are unable to comply with and timely complete the regulatory process in the United States and Canada for our future products in development, our or our future collaborators’ ability to market our traits could be delayed, prevented or limited.
We apply for and maintain the regulatory permits in the United States and Canada necessary for our operations, particularly those covering our field trials. We anticipate that we or our future collaborators will apply for and maintain regulatory approvals, if any, necessary for the commercialization of any future products containing our seed traits. Even if we and our collaborators make timely and appropriate applications for regulatory permits for our field trials, government delays in issuing such permits can significantly affect the development timelines for our traits, particularly if the planting period for a crop growing season expires before the necessary permits are obtained.
The regulatory process is expensive and time-consuming, and the time required to complete the process is difficult to predict and depends upon numerous factors, including the substantial discretion of the regulatory authorities. We have not completed all phases of the regulatory process for any of our traits in development. Our traits could require a significantly longer time to complete the regulatory process than expected, or may never gain approval, even if we and our collaborators expend substantial time and resources seeking such approval. The time required for regulatory approval, or any delay or denial of such approval, could negatively impact our ability to generate revenues and to achieve profitability and finance our ongoing operations. In addition, changes in regulatory review policies during the development period of any of our traits,
28
changes in, or the enactment of, additional regulations or statutes, or changes in regulatory review practices for a submitted product application may cause a delay in obtaining approval or result in the rejection of an application for regulatory approval. Regulatory approval, if obtained, may be made subject to limitations on the intended uses for which we or our collaborators may market a future product containing our traits. These limitations could adversely affect our potential revenues.
The regulatory environment for genetically engineered crops in jurisdictions outside the United States and Canada varies greatly, and some jurisdictions have more restrictive regulations that could delay, prevent or limit our or our future collaborators’ ability to market our traits.
Other jurisdictions and governmental authorities, including in South America and Asia, are increasingly taking an interest in regulating agricultural products of biotechnology. Regulatory approaches vary by jurisdiction as a result of the existing public health frameworks and phytosanitary laws, as well as other less tangible factors such as cultural and religious norms that may have an impact on individual country risk assessments and decision-making. Each jurisdiction may have its own regulatory framework, which may include restrictions and regulations on planting and growing genetically engineered plants, import of grain and other plant products, and in the consumption and labeling of feed and foods derived from such novel plants, and which may apply to future products containing our traits. We cannot predict future changes in the global regulatory landscape regarding genetically engineered plants or commercial products incorporating such novel plant varieties. The regulatory environment for such plants is greatly uncertain outside of the U.S. and Canada, and some jurisdictions have more restrictive regulations that could delay, prevent or limit our or our future collaborators’ ability to market our traits.
For example, regulation of all genetically engineered plants in the European Union ("EU") is far more stringent than in the U.S. and Canada. U.S. and Canadian regulators have determined that genome edited plants pose fewer risks than traditional biotechnology-derived plants subjected to modification through the insertion of recombinant DNA. In contrast, a recent EU legal ruling indicated that the existing EU regulations for genetically engineered plants modified by the insertion of recombinant DNA, which were already more stringent than corresponding U.S. and Canadian regulations, should be strictly applied to genome edited plants as well. As a result, there is a sharp distinction between how EU and U.S. and Canadian regulatory agencies oversee novel seed traits, and in particular those that are generated using the more modern techniques of genome editing.
Although we are not currently targeting EU markets for the development or commercialization of future products containing our traits, emerging oversight regimes for genetically engineered products in other jurisdictions may follow the EU approach and impose similarly strict requirements for the release of such products into the environment and their incorporation into human food or other consumer products. Such jurisdictions may also elect to regulate genetically engineered plants without distinguishing between traditional biotechnology-derived plants modified with recombinant DNA and genome edited plants. There is no guarantee that countries for which we may have or may develop future marketing plans would not take a stricter legal and regulatory approach to controlling genetically engineered plants similar to that of the EU, which could increase regulatory costs and delay, prevent or limit our or our future collaborators’ ability to market our traits in such jurisdictions.
Consumer resistance to genetically engineered crops may negatively affect the ability to commercialize future crops containing our traits, as well as our public image, and may reduce any future sales of seeds containing our yield traits.
Food and feed made from genetically engineered seeds and plants are not accepted by some consumers, and in certain countries production of certain genetically engineered crops is effectively prohibited, including throughout the EU, due to concerns over such products’ effects on food safety and the environment. Advocacy groups have engaged in publicity campaigns and filed lawsuits in various countries against companies and regulatory authorities, seeking to halt regulatory approval activities or influence public opinion against genetically engineered and/or genome edited products. Actions by consumer groups and others also may disrupt research and development or production of genetically engineered plants, seeds or food products that incorporate such novel plant varieties. The high public profile of the biotechnology industry in food and feed production, and a lack of consumer acceptance of the types of products to which we have devoted substantial development resources, could have a negative impact on the commercial success of any of products incorporating our traits that may successfully complete the development process, as to which no assurance can be given, and could materially and adversely affect our ability to obtain future collaborations and to finance our crop science program. Further, we could incur substantial liability and/or legal expenses if there are claims that genetically engineered crops damage the environment or
29
contaminate other farm crops. This could distract our management and cause us to spend resources defending against such claims.
Government policies and regulations, particularly those affecting the agricultural sector and related industries, could adversely affect our operations and our ability to generate future revenues and to achieve profitability.
Agricultural production and trade flows are subject to government policies and regulations. Governmental policies and approvals of technologies affecting the agricultural industry, such as taxes, tariffs, duties, subsidies, incentives and import and export restrictions on agricultural commodities and commodity products can influence the planting of certain crops, the location and size of crop production, and the volume and types of imports and exports. Future government policies in the United States, Canada or in other countries could discourage farmers from using any of our products that may successfully complete the development process, as to which no assurance can be given. Similarly, these policies could discourage food processors from purchasing harvested crops containing our traits or could encourage the use of our competitors’ products, which would put us at a commercial disadvantage and could negatively impact our ability to generate any revenues and to achieve profitability.
The products of third parties, or the environment itself, may be negatively affected by the unintended appearance of our trait genes, novel seed compositions and novel seed products.
The potential for unintended but unavoidable trace amounts, sometimes called “adventitious presence,” of trait genes, novel seed compositions and novel seed products in conventional seed, or in the grain or products produced from conventional or organic crops, could affect acceptance by the general public or by the agricultural industry of these traits. Trace amounts of yield trait genes may unintentionally be found outside our containment area in the products of third parties, which may result in negative publicity and claims of liability brought by such third parties against us. Furthermore, in the event of an unintended dissemination of our genetically engineered materials to the environment, we could be subject to claims by multiple parties, including environmental advocacy groups, as well as governmental actions such as mandated crop destruction, product recalls or additional stewardship practices and environmental cleanup or monitoring. The occurrence of any of these events could have a material adverse effect on our business and results of operations.
Loss of or damage to our elite novel trait events and plant lines would significantly slow our product development efforts.
We have a collection of elite novel trait events and plant lines in which we are developing traits for incorporation into elite germplasm and potential seed products. Our elite novel trait events and plant lines are a key strategic asset since they form the basis for the introgression of our traits into plant breeding programs. If we suffer loss or damage to our elite novel trait events and plant lines, our research and development activities could be negatively impacted.
Our insurance coverage may be inadequate to cover all the liabilities we may incur.
We face the risk of exposure to liability claims if any products that are successfully developed containing our seed traits, as to which no assurance can be given, are defective and if any product that we develop or any product that uses our technologies or incorporates any of our traits causes injury. Although we carry insurance at levels customary for companies in our industry, such coverage may become unavailable or be inadequate to cover all liabilities we may incur. There can be no assurance that we will be able to continue to maintain such insurance, or obtain comparable insurance at a reasonable cost, if at all. If we are unable to obtain sufficient insurance coverage at an acceptable cost or otherwise, or if the amount of any claim against us exceeds the coverage under our policies, we may face significant expenses.
We rely on third parties to conduct, monitor, support, and oversee field trials and, in some cases, to maintain regulatory files for those products in development, and any performance issues by third parties, or our inability to engage third parties on acceptable terms, may impact our ability to complete the regulatory process for or commercialize such products.
We rely on third parties to conduct, monitor, support, and oversee field trials. As a result, we have less control over the timing and cost of these trials than if we conducted these trials with our own personnel. If we are unable to maintain or enter into agreements with these third parties on acceptable terms, or if any such engagement is terminated prematurely, we may be unable to conduct and complete our trials in the manner we anticipate. In addition, there is no guarantee that these third parties will devote adequate time and resources to our studies or perform as required by our contract or in accordance
30
with regulatory requirements, including maintenance of field trial information regarding our products in development. If any of these third parties fail to meet expected deadlines, fail to transfer to us any regulatory information in a timely manner, fail to adhere to protocols, or fail to act in accordance with regulatory requirements or our agreements with them, or if they otherwise perform in a substandard manner or in a way that compromises the quality or accuracy of their activities or the data they obtain, then field trials of our traits in development may be extended or delayed with additional costs incurred, or our data may be rejected by the applicable regulatory agencies. Ultimately, we are responsible for ensuring that each of our field trials is conducted in accordance with the applicable protocol and with legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our responsibilities. We could be subject to penalties, fines and liabilities if our third-party contractors fail to perform as required.
If our relationship with any of these third parties is terminated, we may be unable to enter into arrangements with alternative parties on commercially reasonable terms, or at all. Switching or adding service providers can involve substantial cost and require extensive management time and focus. Delays may occur, which can materially impact our ability to meet our desired development timelines. If we are required to seek alternative service arrangements, the resulting delays and potential inability to find a suitable replacement could materially and adversely impact our business.
In addition, there has been an increasing trend towards consolidation in the agricultural biotechnology industry. Consolidation among our competitors and third parties upon whom we rely could lead to changes in the competitive landscape, capabilities, and strategic priorities among potential service providers, which could have an adverse effect on our business and operations.
If we lose key personnel or are unable to attract and retain necessary talent, we may be unable to develop or commercialize our products under development.
We are highly dependent on our key technical and scientific personnel, who possess unique knowledge and skills related to our research and technology. If we were to lose the services of these individuals, we may be unable to readily find suitable replacements with comparable knowledge and the experience necessary to advance the research and development of our products. Because of the unique talents and experience of many of our scientific and technical staff, competition for our personnel is intense. The loss of key personnel or our inability to hire and retain personnel who have the required expertise and skills could have a material adverse effect on our research and development efforts, our business, and our ability to secure additional required financing.
Our business and operations would suffer in the event of system failures.
We utilize information technology systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks and the confidentiality, availability and integrity of our data. There can be no assurance that we will be successful in preventing cyber-attacks or successful in mitigating their efforts.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from such cyber-attacks, including computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such an event could cause interruption of our operations. For example, the loss of data from completed field tests for our yield traits could result in delays in our regulatory approval efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could suffer reputational harm or face litigation, or adverse regulatory action and the development of our product candidates could be delayed.
Risks Relating to Intellectual Property
Patent protection for our technologies is both important and uncertain.
31
Our commercial success may depend in part on our obtaining and maintaining patent protection for our technologies in the United States and other jurisdictions, as well as successfully enforcing and defending this intellectual property against third-party challenges. If we are not able to obtain or defend patent protection for our technologies, then we will not be able to exclude competitors from developing or marketing such technologies, and this could negatively impact our ability to generate sufficient revenues or profits from product sales and/or licensing to justify the cost of development of our technologies and to achieve or maintain profitability. Our currently issued patents include five patents on our C3003 gene in-licensed from the University of Massachusetts, three patents on C4001 and other novel yield traits, and one patent relating to our historical business. Our currently issued patents have expiration dates ranging from 2033 through 2038. New pending patent applications owned by or licensed to us relating to crop yield improvements have earliest effective filing dates ranging from 2013 through 2023 and include a new patent application on a breakthrough technology for producing PHA biomaterials in crops. This patent application would have an expiration date in 2040 if granted, however, we may not be able to obtain sufficiently broad claims to cover the new invention.
Our patent position involves complex legal and factual questions. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. Patents may not be issued for any pending or future pending patent applications owned by or licensed to us, and claims allowed under any issued patent or future issued patent owned or licensed by us may not be valid or sufficiently broad to protect our technologies. Moreover, we may be unable to protect certain of our intellectual property in the United States or in foreign countries. Foreign jurisdictions may not afford the same protections as U.S. law, and we cannot ensure that foreign patent applications will have the same scope as the U.S. patents. There will be many countries in which we will choose not to file or maintain patents because of the costs involved. Competitors may also design around our patents or develop competing technologies.
Additionally, any issued patents owned by or licensed to us now or in the future may be challenged, invalidated, or circumvented. We could incur substantial costs to bring suits or other proceedings in which we may assert or defend our patent rights or challenge the patent rights of third parties. An unfavorable outcome of any such litigation could have a material adverse effect on our business and results of operations.
Third parties may claim that we infringe their intellectual property, and we could suffer significant litigation or licensing expense as a result.
Various U.S. and foreign issued patents and pending patent applications owned by third parties exist in areas relevant to our products and processes. We could incur substantial costs to challenge third-party patents. If third parties assert claims against us or our customers alleging infringement of their patents or other intellectual property rights, we could incur substantial costs and diversion of management resources in defending these claims, and the defense of these claims could have a material adverse effect on our business. In addition, if we are unsuccessful in defending against these claims, these third parties may be awarded substantial damages, as well as injunctive or other equitable relief against us, which could effectively block our ability to make, use, sell, distribute, or market our technologies and services based on our technologies in the United States or abroad. Alternatively, we may seek licenses to such third-party intellectual property. However, we may be unable to obtain these licenses on acceptable terms, if at all. Our failure to obtain the necessary licenses or other rights could prevent the sale, manufacture, or distribution of some of our products based on our technologies and, therefore, could have a material adverse effect on our business.
Portions of our crop science technology are owned by or subject to retained rights of third parties.
We have licensed and optioned from academic institutions certain patent rights that may be necessary or important to the development and commercialization of our crop science technology. These licenses and options may not provide exclusive rights to use such intellectual property in all fields of use in which we may wish to develop or commercialize our technology. If we fail to timely exercise our option rights and/or we are unable to negotiate license agreements for optioned patent rights on acceptable terms, the academic institutions may offer such patent rights to third parties. If we fail to comply with our obligations under these license agreements, or if we are subject to a bankruptcy or insolvency proceeding, the licensor may have the right to terminate the license. In some circumstances, we may not have the right to control the preparation, filing and prosecution of licensed patent applications or the maintenance of the licensed patents. Therefore, we cannot be certain that these patents and applications will be prosecuted, maintained and enforced in a manner consistent with the best interests of our business. Furthermore, the research resulting in certain of our licensed and optioned patent rights
32
was funded by the U.S. government. As a result, the government may have certain rights to such patent rights and technology.
We may not be successful in obtaining necessary rights to additional technologies for the development of our products through acquisitions and in-licenses.
We may be unable to acquire or in-license additional technologies from third parties that we decide we need in order to develop our business. A number of more established companies may also pursue strategies to license or acquire crop science technologies that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater development and commercialization capabilities. Any failure on our part to reach an agreement for any applicable intellectual property could result in a third party acquiring the related rights and thereby harm our business.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire relevant crop science technologies on terms that would allow us to make an appropriate return on our investment.
We expect that competition for acquiring and in-licensing crop science technologies that are attractive to us may increase in the future, which may mean fewer suitable opportunities for us as well as higher acquisition or licensing costs. If we are unable to successfully obtain rights to suitable crop science technologies on reasonable terms, or at all, our business and financial condition could suffer.
Our license agreements include royalty payments that we are required to make to third parties.
We are party to license agreements that require us to remit royalty payments and other payments related to our licensed intellectual property. Under our in-license agreements, we may pay upfront fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, or timing of royalties we may owe in the future. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments.
The intellectual property landscape around genome editing technology, such as CRISPR, is highly dynamic and uncertain, and any resolution of this uncertainty could have a material adverse effect on our business.
The field of genome editing, especially in the area of CRISPR technology, is still in its infancy. Due to the intense research and development that is taking place in this field by several companies, including us and our competitors, the intellectual property landscape is in flux, and it may remain uncertain for the coming years. There has been, and may continue to be, significant intellectual property related litigation and proceedings relating to this area in the future. If it is later determined that any patent rights using the CRISPR technology that we obtained under license are invalid or owned by other parties, this could have a material adverse effect on our business.
We rely in part on trade secrets to protect our technology, and our failure to obtain or maintain trade secret protection could harm our business.
We rely on trade secrets to protect some of our technology and proprietary information, especially where we believe patent protection is not appropriate or obtainable as is the case for our GRAIN trait gene discovery platform. However, trade secrets are difficult to protect. Litigating a claim that a third-party had illegally obtained and was using our trade secrets would be expensive and time consuming, and the outcome would be unpredictable. Moreover, if our competitors independently develop similar knowledge, methods and know-how, it will be difficult for us to enforce our rights and our business could be harmed.
Risks Relating to Owning our Common Stock
Raising additional funds may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies.
33
Execution of our business plan requires additional financing. If we raise additional funds through equity offerings or offerings of equity-linked securities, including warrants or convertible debt securities, we expect that our existing stockholders will experience significant dilution, and the terms of such securities may include liquidation or other preferences that adversely affect the rights of current stockholders. Debt financing, if available, may subject us to restrictive covenants that could limit our flexibility in conducting future business activities, including covenants limiting or restricting our ability to incur additional debt, dispose of assets, or make capital expenditures. We may also incur ongoing interest expense and be required to grant a security interest in our assets in connection with any debt issuance. If we raise additional funds through strategic partnerships or licensing agreements with third parties, we may have to relinquish valuable rights to our technologies or grant licenses on terms that are not favorable to us.
Trading volume in our stock can fluctuate and an active trading market for our common stock may not be available on a consistent basis to provide stockholders with adequate liquidity. Our stock price may be volatile, and our stockholders could lose a significant part of their investment.
The public trading price for our common stock will be affected by a number of factors, including:
•any change in the status of our Nasdaq listing;
•the need for near-term financing to continue operations;
•reported progress in our efforts to develop crop related technologies, relative to investor expectations;
•changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates;
•quarterly variations in our or our competitors’ results of operations;
•general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors;
•future issuances and/or sales of our securities;
•announcements or the absence of announcements by us, or our competitors, regarding acquisitions, new products, regulatory developments, significant contracts, commercial relationships or capital commitments;
•commencement of, or involvement in, litigation;
•any major change in our board of directors or management;
•changes in governmental regulations or in the status of our regulatory approvals;
•announcements related to patents issued to us or our competitors and to litigation involving our intellectual property;
•a lack of, or limited, or negative industry or security analyst coverage;
•uncertainty regarding our ability to secure additional cash resources with which to operate our business;
•a decision by our significant stockholders to increase or decrease their holdings in our common stock;
•short-selling or similar activities by third parties; and
•other factors described elsewhere in these risk factors.
As a result of these factors, our stockholders may not be able to resell their shares at, or above, their purchase price. In addition, the stock prices of many technology companies have experienced wide fluctuations that have often been unrelated to the operating performance of those companies. Any negative change in the public’s perception of the prospects of industrial or agricultural biotechnology companies could depress our stock price regardless of our results of operations. These factors may have a material adverse effect on the market price and liquidity of our common stock and affect our ability to obtain required financing.
34
Provisions in our certificate of incorporation and by-laws and Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions of our certificate of incorporation and by-laws and Delaware law may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares of our common stock. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management.
In addition, Section 203 of the Delaware General Corporation Law ("DGCL") prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, which generally refers to a person which together with its affiliates owns, or within the last three years has owned, 15 percent or more of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our company, thereby reducing the likelihood that our stockholders could receive a premium for their common stock in an acquisition.
Concentration of ownership among our officers, directors and principal stockholders may prevent other stockholders from influencing significant corporate decisions and depress our stock price.
Based on the number of shares outstanding as of March 21, 2024, our officers, directors and stockholders who hold at least 5 percent of our stock beneficially own a combined total of approximately 28.1 percent of our outstanding common stock, including shares of common stock subject to stock options and warrants that are currently exercisable or are exercisable within 60 days after March 21, 2024. If these officers, directors, and principal stockholders or a group of our principal stockholders act together, they will be able to exert a significant degree of influence over our management and affairs and control matters requiring stockholder approval, including the election of directors and approval of mergers, business combinations or other significant transactions. The interests of one or more of these stockholders may not always coincide with our interests or the interests of other stockholders. For instance, officers, directors, and principal stockholders, acting together, could cause us to enter into transactions or agreements that we would not otherwise consider. Similarly, this concentration of ownership may have the effect of delaying or preventing a change in control of our company otherwise favored by our other stockholders. As of March 21, 2024, Jack W. Schuler (and his related entities) beneficially owned approximately 22.7 percent of our common stock. To the extent that this or any other significant stockholders oppose any proposal put forth for stockholder approval by our board of directors, they control a sufficient percentage of our outstanding shares to cause such proposal to either fail or be very difficult to achieve without their support. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our stockholders from realizing a premium over the market price for their shares of common stock. The concentration of ownership also may contribute to the low trading volume and volatility of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
Information technology is important to our business operations, and we are committed to protecting the privacy, security and integrity of the data we use in our business, as well as our employee and research data. The Company has a comprehensive cybersecurity program in place for assessing, identifying and managing cybersecurity risks that is designed to protect its systems and data from unauthorized access, use or other security impact.
Through our third-party IT Service Team ("IT Service Team"), we continuously monitor and update our information technology networks and infrastructure to prevent, detect, address and mitigate risks associated with unauthorized access, misuse, computer viruses and other events that could have a security impact. Our IT Service Team invests in industry standard security technology to protect the Company’s data and business processes against risk of cybersecurity incidents. Our data security management program includes identity, trust, vulnerability and threat management business processes, as well as adoption of standard data protection policies.
35
In terms of governance and oversight, the following is in place to enhance transparency and accountability in cybersecurity management:
Responsibility Assignment
•The Company's Vice President - Planning and Communications (VP P&C)) assumes an oversight role in overseeing the cybersecurity risk management program. The VP P&C collaborates with the Company's leadership team and IT Service Team on the matters of cybersecurity across the Company.
•Cybersecurity risks fall within the purview of the Company's Audit Committee and, ultimately, the Board of Directors. Regular oversight and reviews occur at established intervals. The Audit Committee engages in discussions with the COO and Company management at least once a year, covering various aspects of cybersecurity risk management, including recent developments, evolving standards, vulnerability assessments, and the threat environment.
We measure our data security effectiveness by benchmarking against industry-accepted methods and we work to remediate any significant findings. We maintain and routinely test backup systems and disaster recovery and also have processes in place to prevent disruptions resulting from our implementation of new software and systems.
Our IT Services Team has a comprehensive incident response plan to address cybersecurity incidents. Our incident response plan includes procedures for identifying, containing and responding to cybersecurity incidents and is subject to regular review and assessment to ensure that it is effective in protecting our information technology. To date, we believe that our cybersecurity program has been effective in protecting the confidentiality, integrity, and availability of the Company's information. We cannot guarantee that our cybersecurity program will be successful in preventing all cybersecurity incidents. We currently maintain a cyber insurance policy that provides coverage against losses due to cybersecurity events, however, such insurance may not be sufficient in type or amount to cover us against all claims related to security breaches, cyber-attacks and other related breaches.
We engage external consultants and computer security firms to enhance our cybersecurity oversight. Included within our cybersecurity program is the use of computer security firms to provide annual training to our employees to help them identify and report cybersecurity threats. While we are regularly subject to cybersecurity attacks, ransomware and other security breaches, we have not experienced any material cybersecurity incidents for the year ended December 31, 2023. We do not believe that there are currently any known risks from cybersecurity threats that are reasonably likely to materially affect us or our business strategy, results of operations or financial condition.
ITEM 2. PROPERTIES
We do not own any real property. We are party to a lease agreement pursuant to which we lease 22,213 square feet of office and research and development space located at 19 Presidential Way, Woburn, Massachusetts. This lease began on June 1, 2016 and will end on November 30, 2026, and does not include any options for the early termination or the extension of the lease. Upon signing the lease, we provided the landlord with a security deposit of $229 through the issuance of an irrevocable letter of credit.
In December 2023, we notified the landlord that we were deferring monthly rental payments, beginning with the December 2023 rent, until such time that we are able to raise additional working capital. The landlord immediately notified the Company that it was in payment default under the terms of the lease and has subsequently withdrawn funds held under the irrevocable letter of credit to cover rent for the months of December 2023 through February 2024, leaving a remaining balance in the letter of credit of $12. The Company reinitiated payment of its monthly rent beginning with the month of March 2024, and intends to return the letter of credit back to its $229 balance as available funding permits.
We have a sublease agreement with a subsidiary of CJ CheilJedang Corporation ("CJ") for CJ's sublease of 9,874 square feet of our Woburn facility. The subleased space was determined to be in excess of our needs as a result of our strategic shift and the related restructuring of our operations during 2016. The sublease is coterminous with our master lease. CJ pays rent and operating expenses equal to its pro-rata share of the amounts payable to the landlord by us, as adjusted from
36
time-to-time in accordance with the terms of the master lease. CJ has provided us with a security deposit of $103 in the form of an irrevocable letter of credit. As a result of our payment default, CJ now pays the landlord directly for its sublease.
Our wholly-owned subsidiary, YOI, located in Saskatoon, Saskatchewan, Canada, leases approximately 9,600 square feet of office, laboratory and greenhouse space located within Innovation Place at 410 Downey Road and within the research facility of National Research Council Canada located at 110 Gymnasium Place. These leases do not contain renewal or early termination options. YOI's leases for these facilities generally have terms of one year, and are extended annually through amendment. Most of these leases will expire on various dates through September 30, 2024.
ITEM 3. LEGAL PROCEEDINGS
From time to time, the Company may be subject to legal proceedings and claims in the ordinary course of business. We are not currently aware of any such proceedings or claims that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
INFORMATION ABOUT OUR DIRECTORS AND EXECUTIVE OFFICERS
Directors
Robert L. Van Nostrand
Chairman of the Board of Directors
Oliver P. Peoples, Ph.D.
President and Chief Executive Officer
Sherri M. Brown, Ph.D.
Independent Consultant
Richard W. Hamilton, Ph.D.
Chief Executive Officer and Director at Prosper DNA, Inc.
Willie Loh, Ph.D.
Independent Consultant
Anthony J. Sinskey, Sc.D.
Professor of Microbiology, Massachusetts Institute of Technology
Executive Officers
Oliver P. Peoples, Ph.D.
President and Chief Executive Officer
Lynne H. Brum
Vice President, Planning and Corporate Communications
Charles B. Haaser
Vice President, Finance, Chief Accounting Officer and Treasurer
Kristi D. Snell, Ph.D.
Vice President, Research and Chief Science Officer
37
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on the Nasdaq Capital Market under the symbol "YTEN."
Stockholders
As of March 28, 2024, there were 15,401,706 shares of our common stock outstanding held by 28 stockholders of record.
Unregistered Sales of Securities
On January 9, 2024, we issued 104,538 shares of common stock to participants in our Yield10 Bioscience, Inc. 401(k) Plan as a matching contribution. The issuance of these securities was exempt from registration pursuant to Section 3(a)(2) of the Securities Act.
Issuer Purchases of Equity Securities
During the quarter ended December 31, 2023, there were no repurchases made by us or on our behalf, or by any "affiliated purchasers," of shares of our common stock.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with the Consolidated Financial Statements and Notes thereto included in this Annual Report on Form 10-K. All dollar amounts are stated in thousands.
Overview
Yield10 Bioscience, Inc. ("Yield10" or the "Company") is an agricultural bioscience company focused on commercializing sustainable products using the oilseed Camelina sativa ("Camelina") as a platform crop.
We are pursuing Camelina seed oil products for two market opportunities and value chains. Each product has its own set of scale requirements, value proposition and challenges. The first product, is seed oil produced by Camelina which has been genetically engineered to enable production of high levels of the omega-3 fatty acids eicosapentanoic acid (EPA) and docosahexanoic acid (DHA). Our development is driven by the growing demand for new sources of omega-3 ingredients and the production constraints and supply volatility of the traditional raw material source fish oil. This growing omega-3 supply gap offers a market opportunity with the potential for revenue and margin growth at acreage levels operationally accessible to Yield10. When commercially available, the Company's omega-3 products will address an unmet need for a reliable, scalable supply of EPA and DHA omega-3 ingredients for aquaculture and pet/animal feed. Multiple opportunities exist for further product development to address higher value markets for omega-3 oils in nutraceuticals and pharmaceuticals. Earlier this year we received regulatory approval from USDA-APHIS for two engineered Camelina lines, the first produces our EPA Omega-3 product and the second produces our EPA+DHA Omega-3 product.
The second product is Camelina seed oil for use as a low-carbon intensity feedstock oil for biofuels, including biodiesel, renewable diesel (“RD”) and sustainable aviation fuel (“SAF”). Markets for biofuels are driven by government policies, have the potential to be very large, and will require the production of tens of millions of acres of new oilseed cover crops like Camelina that do not compete for land with food production.
In early 2024, we adjusted our commercial strategy for biofuels to focus on providing research and development services to third parties interested in Camelina for biofuels with the goal of generating service and licensing revenues from our advanced Camelina technology capabilities, varieties and traits. This decision reflects the current excess supply of soybean oil for the biofuels market as well as the challenges the Company faced in the current financing environment as a
38
small cap pre-revenue company. Over the past four years, Yield10 has developed proprietary, engineered spring and winter Camelina varieties, including herbicide tolerant (“HT”) and stacked herbicide tolerant (“stacked HT”) traits which form the genetic foundation for introducing commercial-quality Camelina varieties tailored to address our target markets. Based on this biofuels strategy, we recently signed our first non-exclusive global, commercial license with Vision Bioenergy Oilseeds for certain Camelina lines and herbicide tolerance traits. Going forward, we plan to continue executing partnerships and other types of agreements for the development of Camelina technologies used in the production of biofuels. Yield10 is headquartered in Woburn, Massachusetts and has a Canadian subsidiary, Yield10 Oilseeds Inc., located in Saskatoon, Saskatchewan, Canada.
Government Grants
During 2018, we entered into a sub-award with Michigan State University ("MSU") to support a Department of Energy ("DOE") funded grant entitled "A Systems Approach to Increasing Carbon Flux to Seed Oil." Our participation under this five-year grant has been awarded incrementally on an annual basis with the first year commencing on September 15, 2017. Funding for this sub-award for the full grant amount of $2,957 was appropriated by the U.S. Congress through the contractual year ending in September 2022. We were permitted to extend the term of the award into early 2023 in order to earn and recognize the final $60 under the grant. During the years ended December 31, 2023 and December 31, 2022, we recognized $60 and $450, respectively, from this sub-award and as of December 31, 2023, the DOE sub-award has been completed with no further future amounts to be recognized.
| Program Title | Funding Agency | Total Government Funds | Total revenue recognized through December 31, 2023 | Remaining amount to be recognized as of December 31, 2023 | Contract/Grant Expiration | |||||||||||||||||||||||||||
| Subcontract from Michigan State University project funded by DOE entitled "A Systems Approach to Increasing Carbon Flux to Seed Oil" | Department of Energy | $ | 2,957 | $ | 2,957 | $ | — | September 15, 2023 | ||||||||||||||||||||||||
Critical Accounting Estimates and Judgments
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America ("GAAP"). The preparation of these consolidated financial statements often requires us to make judgments and accounting estimates that can materially affect the amounts reported in the consolidated financial statements and accompanying notes. These judgments and estimates can have a significant effect on the financial statements because they result primarily from estimates about the effects of matters that are inherently uncertain. We make these estimates and judgments based on guidance provided by current GAAP, historical experience and various other assumptions that we believe to be reasonable under the circumstances. Our actual results may differ from these estimates.
39
We believe that the specific accounting policies and significant judgments described below are the most critical to aid in fully understanding and evaluating our consolidated financial condition and results of operations.
Research and Development
All costs associated with internal research and development are expensed as incurred. Research and development expenses include, among others, direct costs for salaries, employee benefits, subcontractors, crop trials, regulatory activities, facility related expenses, depreciation, and stock-based compensation. Costs incurred for seed multiplication and processing and the cost of harvested Camelina grain purchased from growers under grain production contracts are included within research and development expense until the Company completes its transition to established commercial operations, at which time these costs are expected to be recorded within inventory. Costs incurred in connection with government research grants are recorded as research and development expense.
During the year ended December 31, 2023, amounts paid to us for Camelina planting seed delivered to growers and from the Company's grain purchases delivered to its grain offtake partner have been recorded as an offset to research and development expense. We believe that we need to more fully establish our commercial operations, substantiate the profitable economics of our Camelina product as a biofuel feedstock and validate grower acceptance of the crop through larger acreage adoption before we begin to record payments for seed and grain deliveries as product revenue. Until then, we will consider our early, small-scale acreage production of Camelina, such as those completed during 2023, to be an initial proof of concept, or prototype, as defined under ASC 740, Research and Development. We will transition to commercialization and begin to record Camelina seed and grain inventory, cost of goods sold and product sales once we are satisfied the product has met these requirements.
Stock-Based Compensation
The accounting standards for stock-based compensation require that all stock-based awards be recognized as an expense in the consolidated financial statements and that such expense be measured based on the fair value of the award. We use the Black-Scholes option-pricing model to value our service-based option grants and to determine the related compensation expense to be recognized over each award's vesting period. Calculating the fair value of stock-based payment awards using modeling techniques requires the use of assumptions. These assumptions represent our best estimates, but the estimates involve inherent uncertainties and the application of judgment. We adjust our modeling assumptions when valuing new stock awards based on actual experience.
Income Taxes
Due to the Company's history of annual income tax losses, it has never incurred significant income tax expense. We have, however, historically recorded and disclosed in our financial statements significant deferred income tax assets for net operating loss carry forwards and research tax credits that may be available to offset future taxable income. We routinely assess the realizability of the Company's deferred tax assets and have historically concluded that it is unlikely that deferred tax assets derived from our U.S. operations will be realized under current accounting standards and therefore we have consistently maintained a full valuation allowance against these tax assets. Our U.S. deferred tax assets are also subject to substantial annual limitation under Section 382 of the Internal Revenue Code of 1986 due to stock ownership changes that have occurred, primarily as a result of our securities offerings. The calculation of Section 382 limitations is highly judgmental and the calculations are complex. Based on an analysis completed during 2022, we have concluded that all of our historical U.S. deferred tax assets generated through November 31, 2019 are no longer available to us for future use to offset taxable income.
Through December 31, 2022, YOI performed research and development services for Yield10 under a research services agreement subject to intercompany transfer pricing regulations established in the U.S. and Canada. These regulations required that YOI earn an arms-length profit from the research services, calculated in accordance with U.S. and Canadian tax regulations. YOI previously filed for research tax credit carryforwards in the past that have been used to offset its taxable income generated as a result of the intercompany profit. Prior to 2022, these accumulated Canadian research credits were recorded as a deferred tax asset within the Company's consolidated balance sheet based on our judgment that YOI would continue to earn taxable income in the future and the value of deferred tax asset would be realized. However, in 2022, the Company's decline in its cash and short-term investment balances created substantial doubt that the Company would continue as a going concern. We believe that it is more likely than not that the remaining deferred tax assets will not be utilized and, as such, we have recorded a full valuation allowance against the remaining deferred tax asset in Canada.
40
Securities Offerings
We offer our securities for sale to public and private investors from time to time. The structure of these offerings can be relatively straight-forward or they can be highly complex, requiring significant judgment in their accounting treatment and financial reporting. Our historical offerings completed to date have included different classes of securities, including common stock, convertible preferred stock and warrants with various exercise prices and terms. Depending on the facts and circumstances of each offering, including; the offering and market price of our common stock, the amount of cash proceeds received, the fair value determination of each type of security issued, the availability of authorized and unissued common shares to support conversion of preferred shares or the exercise of the warrants, the specific terms of securities purchase agreements and other factors that may come into consideration, the shares of an offering may be recorded as permanent or temporary equity within our balance sheets. The fair value of warrants issued in an offering, under certain situations, may be recorded as a liability and be subject to mark-to-market adjustments on each balance sheet date based on changes in their fair value determined using the Black-Scholes valuation model. We carefully analyze our securities offerings to ensure that we record them in accordance with current accounting guidance.
Lease Accounting
As a lessee, we follow the lease accounting guidance codified in ASC 842. Under this guidance, a lease is classified as a finance lease if any of five criteria described in the guidance apply to the lease. Any lease not classified as a finance lease is classified as an operating lease with expense recognition occurring on a straight-line basis over the term of the lease. The application of this guidance requires judgment. Under ASC 842, a lease liability is recorded on the commencement date of a lease and is calculated as the present value of the remaining lease payments, using the interest rate implicit in the lease, or if that rate is not readily determinable, using the lessee's incremental borrowing rate. A right-of-use asset equal to the lease liability is also recorded with adjustments made, as necessary, for lease prepayments, lease accruals, initial direct costs and lessor lease incentives that may be present within the terms of the lease. If a lease subject to ASC 842 is amended, the right-of-use asset and lease liability are adjusted, if appropriate. These mathematical calculations to comply with ASC 842 can be complex.
Comparison of the Years Ended December 31, 2023 and 2022
Revenue
| Year ended December 31, | |||||||||||||||||||||||
| 2023 | 2022 | Change | |||||||||||||||||||||
| Grant revenue | $ | 60 | $ | 450 | $ | (390) | |||||||||||||||||
Grant revenue was $60 and $450 for the years ended December 31, 2023 and 2022, respectively. All of the grant revenue recorded during the year ended December 31, 2023 was derived from the Company's DOE sub-award with MSU. As of March 31, 2023, our work in support of this research grant was completed with no further grant revenue to be recognized. We currently do not have any active government grants and cannot assess whether additional U.S. or Canadian government research grants will be awarded to us in the future.
During the year ended December 31, 2023, we took first-time deliveries of harvested Camelina grain produced under winter and spring season grower contracts in Western Canada and the United States. Our first two seasons were small in scale, representing a proof-of-concept for our Camelina products to be used in the biofuel feedstock market. We are at an early stage in the commercialization of Camelina and have not yet begun to capitalize product inventory. The costs of producing Camelina planting seed and the purchase cost of grain harvests acquired from our growers during 2023 were recorded to research and development expense. Payments received or due to us from our shipments of seed and harvested grain to growers and offtake partners that were completed during the the year ended December 31, 2023 have been recorded as an offset to research and development expense. This accounting practice will continue until such time that we have more fully validated our markets and believe the value of capitalized inventory can be realized through product sales. The Company's Camelina seed and harvested grain shipments are expected to remain seasonal in the foreseeable future, with acreage planted in the winter and spring growing seasons being harvested and shipped annually to our offtake partners in our third and fourth fiscal quarters.
Operating Expenses
41
| Year ended December 31, | |||||||||||||||||||||||
| 2023 | 2022 | Change | |||||||||||||||||||||
| Research and development expenses | $ | 8,323 | $ | 7,750 | $ | 573 | |||||||||||||||||
| General and administrative expenses | 6,154 | 6,151 | 3 | ||||||||||||||||||||
| Total operating expenses | $ | 14,477 | $ | 13,901 | $ | 576 | |||||||||||||||||
Research and Development Expenses
Research and development expense increased by $573, or 7%, from $7,750 during the year ended December 31, 2022 to $8,323 during the year ended December 31, 2023. The 2023 increase is primarily due to the increased cost of crop trials and commercial seed production partially offset by lower employee compensation and benefits expenses. During the year ended December 31, 2023, crop trial expenses increased by $349 to $1,381 from $1,032 incurred during the year ended December 31, 2022, primarily as a result of our ongoing development of herbicide tolerant Camelina plant varieties. Commercial seed production costs increased by $435 from $226 during the year ended December 31, 2022 to $661 during the year ended December 31, 2023 as we prepare for future growing seasons. Partially offsetting these increases were lower employee compensation and benefits of $136 as a result of reduced payroll and stock compensation costs.
Based on current planning and forecasting, we anticipate that our research and development expenses during the year ended December 31, 2024 will decrease below levels incurred during the year ended December 31, 2023, as we shift our primary focus to the development and commercialization of our Omega-3 Camelina plant varieties for the aquaculture fish feed, nutraceutical and pharmaceutical markets while we negotiate opportunities to partner with other parties for the biofuels market. We are reducing our planned level of expenditure for research and development activities to better match our anticipated cash resources for 2024. Our forecast related to research and development expense is subject to change and may be impacted by our ability to raise additional working capital to support our plans or our entry into third-party collaborations or other business opportunities that could alter our plans.
General and Administrative Expenses
General and administrative expenses remained consistent at $6,154 and $6,151 for the fiscal years ended December 31, 2023 and December 31, 2022, respectively. Although unchanged in total, professional service fees increased by $352 from $973 during the year ended December 31, 2022 to $1,325 during the year ended December 31, 2023. This increase was primarily the result of higher legal and accounting expenses of $345 as a result of securities offerings completed during the year, business development activities and technical accounting analysis. Lower employee compensation and benefit expenses offset the increase in professional service fees, decreasing by $398 year-over-year. This decrease is attributable to lower payroll expenses of $138 as the Company furloughed and reduced employee compensation in order to conserve cash and to lower stock-based compensation expense of $231.
Based on current planning and forecasting, we anticipate that our general and administrative expenses during the year ended December 31, 2024 will remain consistent with levels incurred during the year ended December 31, 2023. We will continue to incur costs related to activities in support of our commercial growth, including increasing legal and accounting expenses, license payments and travel-related expenses in support of business development. Our forecast related to general and administrative expense is subject to change and may be impacted by our ability to raise additional working capital to support our plans or our entry into third-party collaborations or other business opportunities that could alter our plans.
Other Income (Expense), net
| Year ended December 31, | ||||||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||||||
| Other income (expense), net | $ | (38) | $ | 41 | $ | (79) | ||||||||||||||
42
Other Income (Expense), net
Other expense of $38 for the year ended December 31, 2023 was primarily the result of interest accrued on our $1,000 short-term convertible note with Marathon. Other income of $41 during the year ended December 31, 2022 was derived primarily from investment income earned from the Company's cash equivalents and investments offset by interest expense and investment management fees incurred during the year.
Liquidity and Capital Resources
Since our inception, we have incurred significant expenses related to our research, development and commercialization efforts, which in recent years have been focused on Camelina. With the exception of 2012, we have recorded annual losses since the Company's initial founding, including our fiscal year ended December 31, 2023. As of December 31, 2023, we had an accumulated deficit of $414,152. Our total unrestricted cash, cash equivalents and short-term investments as of December 31, 2023, totaled $1,068 as compared to $4,347 at December 31, 2022. As of December 31, 2023, we have a short-term convertible note outstanding of $1,000 with MPC Investment LLC, an affiliate of Marathon. The note and interest charges accruing at 8.0% per annum, is expected to mature on August 24, 2024, unless converted to Yield10 equity or retired earlier.
One of the top priorities of our management is the evaluation and pursuit of different strategies to obtain the required funding for our operations in the very near term. These strategies may include, but are not limited to: public and private placements of equity and/or debt, licensing and/or collaboration arrangements and strategic alternatives with third parties, or other funding from the government or third parties. Our ability to secure funding is subject to numerous risks and uncertainties, including the volatility of capital markets, the impact of geopolitical turmoil and economic uncertainty related to rising inflation and disruptions in the global supply chain. As a result, there can be no assurance that these funding efforts will be successful. If they are not successful, we will be required to scale back, or possibly discontinue, our business operations. The sale of equity and convertible debt securities may result in dilution to our stockholders and, in the case of preferred equity securities or convertible debt, those securities could provide for rights, preferences or privileges senior to those of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products or grant licenses on terms that are not favorable to us. Additional capital may not be available on reasonable terms, in a timely manner, or at all.
In our efforts to obtain additional funding, during February 2024, we granted a global license to Vision Bioenergy Oilseeds, LLC to certain proprietary varieties of Camelina for the production of feedstock oils for biofuels. In consideration for the license and our completion of certain deliverables, Vision is making cash payments to us of $3,000.
Our cash, cash equivalents and short-term investments at December 31, 2023 were held for working capital purposes. As of December 31, 2023, we had restricted cash of $229 held in connection with the lease agreement for our Woburn, Massachusetts facility. In December 2023, we notified the landlord that we were deferring monthly rental payments, beginning with the December 2023 rent, until such time that we are able to raise additional working capital. The landlord immediately notified the Company that it was in payment default under the terms of the lease and during the first quarter of 2024 has subsequently withdrawn funds held under the irrevocable letter of credit to cover rent for the months of December 2023 through February 2024, leaving a remaining balance in the letter of credit of $12. The Company reinitiated payment of its monthly rent beginning with the month of March 2024 and intends to return the letter of credit back to its $229 balance as available funding permits.
Investments are made in accordance with our corporate investment policy, as approved by our Board of Directors. The primary objective of this policy is to preserve principal, and consequently, investments are limited to high quality corporate debt, U.S. Treasury bills and notes, money market funds, bank debt obligations, municipal debt obligations and asset-backed securities. The policy establishes maturity and concentration limits, and liquidity requirements. As of December 31, 2023, we were in compliance with this policy.
Material Cash Requirements
We require cash to fund our working capital needs, to purchase capital assets, to pay our lease obligations and other operating costs. The primary sources of our liquidity have historically included equity financings, government research grants and income earned on cash equivalents and short-term investments.
43
We routinely enter into contractual commitments with third parties to support our operating activities. The more significant of these commitments includes real estate operating leases for our office, laboratory and greenhouse facilities located in the U.S. and Canada. In addition, we typically enter into annual premium funding arrangements through our insurance broker that allows us to spread the payment of our directors' and officers' liability and other business insurance premiums over the terms of the policies. Our material commitments also include arrangements with third party growers located in North and South America for the execution of crop trials and seed scale-up activities to further our trait development goals and to progress the commercial development of our Camelina plant varieties. The aggregate cost of these contracted crop activities is substantial. From time-to-time, we also enter into exclusive research licensing and collaboration arrangements with third parties for the development of intellectual property related to trait development. These long-term agreements typically include initial licensing payments and future contingent milestone payments associated with regulatory filings and approvals as well as potential royalty payments based on future product sales. Generally, these licensing arrangements contain early termination provisions within the terms of the respective agreements.
The Company has no off-balance sheet arrangements as defined in Item 303(b) of Regulation S-K of the Securities Exchange Act of 1934.
Letter of Intent with Marathon Petroleum Corporation
On April 27, 2023, we signed a non-binding letter of intent (“LOI”) with Marathon for a potential investment in Yield10 by Marathon and for an offtake agreement for low-carbon intensity Camelina feedstock oil to be used in renewable fuels production. In connection with signing the LOI, we sold and issued to MPC Investment LLC, an affiliate of Marathon, a senior unsecured convertible note in the original principal amount of $1,000 (the “Convertible Note”) which is convertible into shares of the Company’s common stock at a conversion price equal to $3.07 per share, subject to any mandatory adjustments and certain conditions and limitations set forth in the Convertible Note. If not converted or terminated earlier, all outstanding principal and accrued and unpaid interest on the Convertible Note will be due and payable in full in cash on the Convertible Note's expected maturity date of August 24, 2024. We used the net proceeds of $967, after debt issuance costs of $33, from the Convertible Note for working capital and general corporate purposes.
Public Offering
On August 15, 2023, we closed on a public offering of 5,750,000 units at a public offering price of $0.65 per unit. Each unit consisted of one share of common stock and one warrant to purchase one share of common stock. The warrants, when issued, were immediately exercisable at an exercise price of $0.65 per share and expire five years from the date of issuance. The shares of common stock and accompanying warrants could only be purchased together during the offering, but were immediately separable upon issuance. We received cash proceeds of $3,125, from the offering, net of $613 in issuance costs.
Registered Direct Offering and Concurrent Private Placement
On May 5, 2023, we closed on a $3,000 offering of the Company's common stock (or pre-funded warrants in lieu thereof) in a registered direct offering and warrants in a concurrent private placement with investors. Under the terms of the securities purchase agreement, we agreed to sell 1,006,710 shares of common stock and to issue unregistered warrants to purchase 1,006,710 shares of common stock. The combined effective offering price for one share of common stock (or pre-funded warrant in lieu thereof) and accompanying warrant was $2.98. The Company received proceeds of $2,717 from the offering, net of $283 in issuance costs. The shares underlying the warrants issued in the offering were registered by the Company in July 2023.
At-The-Market ("ATM") Program
On January 24, 2023, the Company entered into an Equity Distribution Agreement (the "Sales Agreement") with Maxim Group LLC ("Maxim") under which the Company could offer and sell shares of its common stock, $0.01 par value per share, having an aggregate offering price of up to $4,200 from time to time through Maxim, acting exclusively as the Company's sales agent. Maxim was entitled to compensation at a fixed commission rate of 2.75% of the gross sales price for each share sold. Effective May 3, 2023, the Company terminated the Sales Agreement after issuing a total of 94,665 shares
44
of common stock, all within the three months ended March 31, 2023, at per share prices between $3.03 and $4.08, resulting in gross proceeds to the Company of $299 before offering costs and sales commissions totaling $196.
Going Concern
We follow the guidance of ASC Topic 205-40, Presentation of Financial Statements-Going Concern, in order to determine whether there is substantial doubt about our ability to continue as a going concern for one year after the date our financial statements are issued. Based on our current cash forecast, we expect that our present capital resources will not be sufficient to fund our planned operations for at least that period of time, which raises substantial doubt as to the Company's ability to continue as a going concern. This forecast of cash resources is forward-looking information that involves risks and uncertainties, and the actual amount of expenses could vary materially and adversely as a result of a number of factors. Our ability to continue operations after our current cash resources are exhausted will depend upon our ability to obtain additional financing through, among other sources, public or private equity financing, secured or unsecured debt financing, equity or debt bridge financing, warrant holders' ability and willingness to exercise the Company's outstanding warrants, and additional government research grants or collaborative arrangements with third parties, as to which no assurances can be given. We do not know whether additional financing will be available on terms favorable or acceptable to us when needed, if at all. If additional funds are not available when required, we will be forced to curtail our research efforts, explore strategic alternatives and/or wind down our operations and pursue options for liquidating our remaining assets, including intellectual property and equipment.
If we issue equity or debt securities to raise additional funds, (i) the Company may incur fees associated with such issuance, (ii) our existing stockholders will experience dilution from the issuance of new equity securities, (iii) the Company may incur ongoing interest expense and be required to grant a security interest in Company assets in connection with any debt issuance, and (iv) the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, utilization of our net operating loss and research and development credit carryforwards may be subject to significant annual limitations under Section 382 of the Internal Revenue Code of 1986 due to ownership changes resulting from future equity financing transactions. If we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our potential products or proprietary technologies or grant licenses on terms that are not favorable to the Company.
Fiscal Year 2023 Cash Usage
Net cash used in operating activities was $10,068 during the year ended December 31, 2023, compared to net cash used by operating activities during 2022 of $11,404. Net cash used by operations during the year ended December 31, 2023 primarily reflects the net loss of $14,455 and our cash payments made to reduce the Company's lease liabilities of $456. Non-cash charges offsetting a portion of the net loss include depreciation and amortization expense of $290, stock-based compensation expense of $1,592, our 401(k) stock matching contribution expense of $108 and non-cash lease expense of $308 resulting from amortization of our right-of-use asset. The net cash usage for operating activities during the year ended December 31, 2022 of $11,404 was primarily the result of the Company's net loss of $13,566, cash payments to reduce the Company's lease liabilities of $520 and our payment of 2021 bonus compensation of $378 during early 2022. Non-cash charges offsetting a portion of the net loss included depreciation and amortization expense of $263, stock-based compensation expense of $1,903, our 401(k) stock matching contribution expense of $133, non-cash lease expense of $393 and a non-cash expense of $165 to establish a full valuation allowance against our Canadian deferred tax assets.
Net cash of $1,945 was provided by investing activities during the year ended December 31, 2023, compared to net cash provided for investing activities during 2022 of $8,522. During the year ended December 31, 2023, $1,991 of our short-term investments matured and converted to cash. During the year ended December 31, 2022, we purchased $2,445 in short-term investments and investments totaling $11,121 matured and converted to cash.
Net cash of $6,871 was provided by financing activities during the year ended December 31, 2023, compared to net cash used in financing activities of $37 during the year ended December 31, 2022. During the year ended December 31, 2023, the Company completed two securities offerings of common stock and warrants, receiving net proceeds of $5,842, after issuance costs of $896.
Related Party Transactions
45
The Company did not engage in any transactions during the years ended December 31, 2023 and December 31, 2022 that qualify as related party transactions.
Recent Accounting Standards Changes
For a discussion of recent accounting standards please read Note 2, Summary of Significant Accounting Policies, to our consolidated financial statements included in this report.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
Not applicable.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The consolidated financial statements and related financial statement schedules required to be filed are indexed on page F-1 and are incorporated herein.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Effectiveness of Disclosure Controls and Procedures
As of the end of the period covered by this Annual Report on Form 10-K, under the supervision of our Chief Executive Officer and our Chief Accounting Officer, we evaluated the effectiveness of our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) and Rule 15d-15(e) under the Exchange Act. Based on this evaluation, our Chief Executive Officer and our Chief Accounting Officer concluded that as of December 31, 2023 our disclosure controls and procedures were effective to provide reasonable assurance that the information we are required to disclose in reports that we file or submit under the Exchange Act (1) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and (2) is accumulated and communicated to our management, including our Chief Executive Officer and our Chief Accounting Officer, as appropriate to allow timely decisions regarding required disclosure. Our disclosure controls and procedures include components of our internal control over financial reporting. Management's assessment of the effectiveness of our internal control over financial reporting is expressed at the level of reasonable assurance because a control system, no matter how well designed and operated, can provide only reasonable, but not absolute, assurance that the control system's objectives will be met.
Management's Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023. In making this assessment, management used the criteria set forth in the 2017 Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
46
Based on its assessment of internal control over financial reporting, management has concluded that, as of December 31, 2023, our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) of the Exchange Act that occurred during our last fiscal quarter in the period covered by this Annual Report on Form 10-K that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Directors and Executive Officers,” “Corporate Governance and Board Matters” and “Code of Business Conduct and Ethics” in our proxy statement for the 2024 annual meeting of stockholders.
ITEM 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Executive Compensation,” “Director Compensation,” “Corporate Governance and Board Matters” and “Compensation Risk Assessment” in our proxy statement for the 2024 annual meeting of stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Security Ownership of Certain Beneficial Owners and Management,” and “Securities Authorized for Issuance under Equity Compensation Plans” in our proxy statement for the 2024 annual meeting of stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Certain Relationships and Related Person Transactions,” “Corporate Governance and Board Matters” and “The Board of Directors and its Committees” in our proxy statement for the 2024 annual meeting of stockholders.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICE
The response to this item is incorporated by reference from the discussion responsive thereto under the caption “Independent Registered Public Accountants” in our proxy statement for the 2024 annual meeting of stockholders.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)The following documents are filed as part of this Report:
47
(1)Financial Statements
See Index to Financial Statements on page F-1.
(2)Supplemental Schedules
All schedules have been omitted because the required information is not present in amounts sufficient to require submission of the schedule, or because the required information is included in the consolidated financial statements or notes thereto.
(3)Exhibits
See Item 15(b) below.
(b)The following exhibits are filed as part of, or incorporated by reference into, this Annual Report on Form 10-K:
| Exhibit Number | Exhibit Description | Filed Herewith | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Reg. Number | |||||||||||||||||||||||||||
| 3.1.1 | Form 10-Q (Exhibit 3.1) | 8/9/2018 | 001-33133 | |||||||||||||||||||||||||||||
| 3.1.2 | Form 8-K (Exhibit 3.1) | 1/15/2020 | 001-33133 | |||||||||||||||||||||||||||||
| 3.1.3 | Form 8-K (Exhibit 3.1) | 11/20/2019 | 001-33133 | |||||||||||||||||||||||||||||
| 3.1.4 | Form 8-K (Exhibit 3.2) | 11/20/2019 | 001-33133 | |||||||||||||||||||||||||||||
| 3.2 | Form 10-Q (Exhibit 3.1) | 11/10/2021 | 001-33133 | |||||||||||||||||||||||||||||
| 4.1 | Form 10-K (Exhibit 4.1) | 3/14/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 4.2 | Form 10-Q (Exhibit 4.1) | 11/12/2020 | 001-33133 | |||||||||||||||||||||||||||||
| 4.3 | Form 8-K (Exhibit 4.1) | 7/5/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 4.4 | Form S-1/A (Exhibit 4.3) | 12/15/2017 | 333-221283 | |||||||||||||||||||||||||||||
| 4.5 | Form 8-K (Exhibit 4.1) | 11/20/2019 | 001-33133 | |||||||||||||||||||||||||||||
| 4.6 | Form 8-K (Exhibit 4.2) | 5/4/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 4.7 | Form S-1/A (Exhibit 4.9) | 8/2/2023 | 333-273070 | |||||||||||||||||||||||||||||
| 4.8 | Form 8-K (Exhibit 4.1) | 5/1/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 10.1† | Form S-1/A (Exhibit 10.3) | 10/20/2006 | 333-135760 | |||||||||||||||||||||||||||||
| 10.1.1† | Form S-1/A (Exhibit 10.3.1) | 10/20/2006 | 333-135760 | |||||||||||||||||||||||||||||
| 10.1.2† | Form S-1/A (Exhibit 10.3.2) | 10/20/2006 | 333-135760 | |||||||||||||||||||||||||||||
| 10.1.3† | Form S-1/A (Exhibit 10.3.3) | 10/20/2006 | 333-135760 | |||||||||||||||||||||||||||||
48
| 10.2† | Form 10-Q (Exhibit 10.1) | 8/13/2015 | 001-33133 | |||||||||||||||||||||||||||||
| 10.2.1† | Form 10-K (Exhibit 10.3.1) | 3/25/2015 | 001-33133 | |||||||||||||||||||||||||||||
| 10.2.2† | Form 10-K (Exhibit 10.3.2) | 3/25/2015 | 001-33133 | |||||||||||||||||||||||||||||
| 10.2.3† | Form 10-K (Exhibit 10.3.3) | 3/25/2015 | 001-33133 | |||||||||||||||||||||||||||||
| 10.3† | Form 8-K (Exhibit 10.1) | 5/30/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 10.3.1† | Form 10-K (Exhibit 10.2.5) | 3/28/2019 | 001-33133 | |||||||||||||||||||||||||||||
| 10.3.2† | Form 10-K (Exhibit 10.2.6) | 3/25/2020 | 001-33133 | |||||||||||||||||||||||||||||
| 10.4† | Form 10-K (Exhibit 10.3) | 3/30/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.5† | X | |||||||||||||||||||||||||||||||
| 10.6† | Form 10-K (Exhibit 10.4) | 3/30/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.7† | X | |||||||||||||||||||||||||||||||
| 10.8† | Form 10-K (Exhibit 10.6) | 3/30/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.9† | X | |||||||||||||||||||||||||||||||
| 10.10 | Form 10-K (Exhibit 10.8) | 3/30/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.11 | X | |||||||||||||||||||||||||||||||
| 10.12† | Form 10-K (Exhibit 10.9) | 3/30/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.13† | Form S/1/A (Exhibit 10.14) | 10/20/2006 | 333-135760 | |||||||||||||||||||||||||||||
| 10.14 | Form 8-K (Exhibit 10.1) | 6/17/2015 | 001-33133 | |||||||||||||||||||||||||||||
| 10.15 | Form 8-K (Exhibit 10.1) | 1/26/2016 | 001-33133 | |||||||||||||||||||||||||||||
| 10.16 | Form 10-K (Exhibit 10.20) | 3/30/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.17 | Form 8-K (Exhibit 10.1) | 7/5/2017 | 001-33133 | |||||||||||||||||||||||||||||
| 10.18@ | Form 10-Q (Exhibit 10.2) | 8/9/2018 | 001-33133 | |||||||||||||||||||||||||||||
49
| 10.19 | Form 8-K (Exhibit 10.1) | 3/15/2019 | 001-33133 | |||||||||||||||||||||||||||||
| 10.20 | Form 8-K (Exhibit 10.1) | 11/20/2019 | 001-33133 | |||||||||||||||||||||||||||||
| 10.21 | Form 8-K (Exhibit 10.1) | 8/25/2020 | 001-33133 | |||||||||||||||||||||||||||||
| 10.22 | Form 8-K (Exhibit 10.1) | 5/4/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 10.23 | Form S/1/A (Exhibit 10.21) | 8/2/2023 | 333-273240 | |||||||||||||||||||||||||||||
| 10.24 | Form 10-K (Exhibit 10.18) | 3/14/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 10.25 | Form 8-K (Exhibit 1.1) | 1/24/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 10.26 | Form 8-K (Exhibit 10.1) | 5/1/2023 | 001-33133 | |||||||||||||||||||||||||||||
| 21.1 | Form 10-K (Exhibit 21.1) | 3/16/2021 | 001-33133 | |||||||||||||||||||||||||||||
| 23.1 | X | |||||||||||||||||||||||||||||||
| 23.2 | X | |||||||||||||||||||||||||||||||
| 31.1 | X | |||||||||||||||||||||||||||||||
| 31.2 | X | |||||||||||||||||||||||||||||||
| 32.1 | X | |||||||||||||||||||||||||||||||
| 101.1 | The following financial information from the Yield10 Bioscience, Inc. Annual Report on Form 10-K for the year ended December 31, 2023 formatted in XBRL; (i) Consolidated Balance Sheets, December 31, 2023 and December 31, 2022; (ii) Consolidated Statements of Operations, Years Ended December 31, 2023 and 2022; (iii) Consolidated Statements of Comprehensive Income (Loss), Years Ended December 31, 2023 and 2022; (iv) Consolidated Statements of Cash Flows, Years Ended December 31, 2023 and 2022; (v) Consolidated Statements of Stockholders' Equity for the Years Ended December 31, 2023 and 2022; and (vi) Notes to Consolidated Financial Statements. | X | ||||||||||||||||||||||||||||||
| 101.INS | XBRL Instance Document. | |||||||||||||||||||||||||||||||
50
| 101.SCH | XBRL Taxonomy Extension Schema. | |||||||||||||||||||||||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase. | |||||||||||||||||||||||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase. | |||||||||||||||||||||||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase. | |||||||||||||||||||||||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase. | |||||||||||||||||||||||||||||||
† | Management contract or compensatory plan or arrangement. | ||||
| @ | Certain confidential portions of this Exhibit were omitted by means of marking such portions with brackets ("[***]") because the identified confidential portions (i) are not material and (ii) is the type of information that the Company treats as private or confidential. | ||||
51
ITEM 16. FORM 10-K SUMMARY
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| YIELD10 BIOSCIENCE, INC. | |||||||||||
| April 1, 2024 | By: | /s/ OLIVER P. PEOPLES | |||||||||
| Oliver P. Peoples, Ph.D. President and Chief Executive Officer (Principal Executive Officer) | |||||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Title | Date | ||||||||||||
| /s/ OLIVER P. PEOPLES | President and Chief Executive Officer and Director (Principal Executive Officer) | April 1, 2024 | ||||||||||||
| Oliver P. Peoples, Ph.D. | ||||||||||||||
| /s/ CHARLES B. HAASER | Vice President, Finance, and Chief Accounting Officer (Principal Financial and Accounting Officer) | April 1, 2024 | ||||||||||||
| Charles B. Haaser | ||||||||||||||
| /s/ SHERRI M. BROWN | Director | April 1, 2024 | ||||||||||||
| Sherri M. Brown | ||||||||||||||
| /s/ RICHARD W. HAMILTON | Director | April 1, 2024 | ||||||||||||
| Richard W. Hamilton, Ph.D. | ||||||||||||||
| /s/ WILLIE LOH | Director | April 1, 2024 | ||||||||||||
| Willie Loh, Ph.D. | ||||||||||||||
| /s/ ANTHONY J. SINSKEY | Director | April 1, 2024 | ||||||||||||
| Anthony J. Sinskey, Sc.D. | ||||||||||||||
| /s/ ROBERT L. VAN NOSTRAND | Chairman | April 1, 2024 | ||||||||||||
| Robert L. Van Nostrand | ||||||||||||||
52
YIELD10 BIOSCIENCE, INC.
Index to Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm (PCAOB ID: | F- # | ||||
Report of Independent Registered Public Accounting Firm (PCAOB ID: 49) | F- 3 | ||||
F- 4 | |||||
F- 5 | |||||
F- 6 | |||||
F- 7 | |||||
F- 8 | |||||
F- 9 | |||||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Yield10 Bioscience, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Yield10 Bioscience, Inc. (“the Company”) as of year ended December 31, 2023, and the related consolidated statements of operations, comprehensive loss, stockholders’ deficit, and cash flows for the year then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Emphasis of Matter – Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters also are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included
F- 1
examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Pre-funded Warrants and Private Placement Warrants:
As discussed in Note 9 to the financial statements, In May and August 2023, the Company closed multiple financing transactions which included the issuance of common stock, pre-funded warrants and private placement warrants (“Warrants”) to purchase its common stock. Upon issuance, the pre-funded warrants and private placement warrants were recorded at their respective allocated fair value as of the grant date as components of stockholders’ deficit within additional paid-in capital in the amount of $2.3 million.
The principal consideration for our determination that the accounting of the transaction date fair value and allocation of proceeds to the common stock, pre-funded warrants and private placements warrants was a critical audit matter is the complexity in assessing the warrants features, which requires management to make significant judgments in the interpretation of the terms of the agreements and in the application of appropriate accounting guidance.
We obtained related agreements and performed the following primary procedures:
–reviewed the key terms and conditions within the agreements associated with the Warrants for balance sheet classification,
–The completeness and accuracy of the Company’s technical accounting analysis,
–Recalculated management's fair values based on the terms in the agreements and
–Application of the relevant accounting guidance.
/s/ | |||||
| We have served as the Company's auditors since 2024. | |||||
| April 1, 2024 | |||||
F- 2
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors
Yield10 Bioscience, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Yield10 Bioscience, Inc. and its subsidiaries (the Company) as of December 31, 2022, the related consolidated statements of operations, comprehensive loss, stockholders' equity and cash flows for the year then ended, and the related notes to the consolidated financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Emphasis of Matter-Going Concern
The accompanying 2022 financial statements were prepared assuming that the Company would continue as a going concern. As discussed in Note 1 to the 2022 financial statements, the Company suffered recurring losses from operations and did not have sufficient liquidity to meet forecasted costs. This raised substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters were also are described in Note 1 to the 2022 financial statements. The 2022 financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis of Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ RSM US LLP
We served as the Company's auditor from 2017 through 2022.
Boston, Massachusetts
March 14, 2023
F- 3
YIELD10 BIOSCIENCE, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| December 31, 2023 | December 31, 2022 | ||||||||||
| Assets | |||||||||||
| Current Assets: | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Short-term investments | |||||||||||
| Unbilled receivables | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Restricted cash | |||||||||||
| Property and equipment, net | |||||||||||
| Right-of-use assets | |||||||||||
| Other assets | |||||||||||
| Total assets | $ | $ | |||||||||
| Liabilities and Stockholders' Equity (Deficit) | |||||||||||
| Current Liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued expenses | |||||||||||
| Lease liabilities | |||||||||||
| Convertible note payable, net of issuance costs (Note12) | |||||||||||
| Total current liabilities | |||||||||||
| Lease liabilities, net of current portion | |||||||||||
| Total liabilities | |||||||||||
| Commitments and contingencies (Note 7) | |||||||||||
| Stockholders' Equity (Deficit): | |||||||||||
Preferred stock ($ | |||||||||||
Common stock ($ | |||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated other comprehensive loss | ( | ( | |||||||||
| Accumulated deficit | ( | ( | |||||||||
| Total stockholders' equity (deficit) | ( | ||||||||||
| Total liabilities and stockholders' equity (deficit) | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F- 4
YIELD10 BIOSCIENCE, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Revenue: | ||||||||||||||
| Grant revenue | $ | $ | ||||||||||||
| Total revenue | ||||||||||||||
| Expenses: | ||||||||||||||
| Research and development | ||||||||||||||
| General and administrative | ||||||||||||||
| Total expenses | ||||||||||||||
| Loss from operations | ( | ( | ||||||||||||
| Other income (expense): | ||||||||||||||
| Other income (expense), net | ( | |||||||||||||
| Total other income (expense) | ( | |||||||||||||
| Loss from operations before income taxes | ( | ( | ||||||||||||
| Income tax provision | ( | |||||||||||||
| Net loss | $ | ( | $ | ( | ||||||||||
| Basic and diluted net loss per share | $ | ( | $ | ( | ||||||||||
| Number of shares used in per share calculations: | ||||||||||||||
| Basic and diluted | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F- 5
YIELD10 BIOSCIENCE, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| Years Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Net loss | $ | ( | $ | ( | ||||||||||
| Other comprehensive loss: | ||||||||||||||
| Change in unrealized gain on investments, net of income tax | ||||||||||||||
| Change in foreign currency translation adjustment, net of income tax | ( | ( | ||||||||||||
| Total other comprehensive loss | ( | ( | ||||||||||||
| Comprehensive loss | $ | ( | $ | ( | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F- 6
YIELD10 BIOSCIENCE, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Years Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Cash flows from operating activities | ||||||||||||||
| Net loss | $ | ( | $ | ( | ||||||||||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||||||||
| Depreciation and amortization | ||||||||||||||
| Expense for 401(k) company common stock match | ||||||||||||||
| Stock-based compensation | ||||||||||||||
| Noncash lease expense | ||||||||||||||
| Deferred tax asset | ||||||||||||||
| Changes in operating assets and liabilities: | ||||||||||||||
| Accounts receivable | ||||||||||||||
| Unbilled receivables | ||||||||||||||
| Prepaid expenses and other assets | ( | |||||||||||||
| Accounts payable | ||||||||||||||
| Accrued expenses | ( | |||||||||||||
| Lease liabilities | ( | ( | ||||||||||||
| Net cash used in operating activities | ( | ( | ||||||||||||
| Cash flows from investing activities | ||||||||||||||
| Purchase of property and equipment | ( | ( | ||||||||||||
| Purchase of investments | ( | |||||||||||||
| Proceeds from sale and maturity of short-term investments | ||||||||||||||
| Net cash provided by investing activities | ||||||||||||||
| Cash flows from financing activities | ||||||||||||||
| Proceeds from the issuance of common stock and warrants in equity offerings, net of issuance costs | ||||||||||||||
| Proceeds from At-the-Market offering, net of issuance costs | ||||||||||||||
| Proceeds from issuance of convertible debt note, net of issuance costs | ||||||||||||||
| Taxes paid on employees' behalf related to vesting of stock awards | ( | ( | ||||||||||||
| Net cash provided by (used in) financing activities | ( | |||||||||||||
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | ( | ( | ||||||||||||
| Net decrease in cash, cash equivalents and restricted cash | ( | ( | ||||||||||||
| Cash, cash equivalents and restricted cash at beginning of year | ||||||||||||||
| Cash, cash equivalents and restricted cash at end of year | $ | $ | ||||||||||||
| Supplemental Cash Flow Disclosure: | ||||||||||||||
| Interest paid | $ | $ | ||||||||||||
| Right-of-use assets acquired in exchange for lease liabilities | $ | $ | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F- 7
YIELD10 BIOSCIENCE, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT)
(In thousands, except share amounts)
| Common Stock | ||||||||||||||||||||||||||||||||||||||
| Shares | Par Value | Additional Paid-In Capital | Accumulated Other Comprehensive Loss | Accumulated Deficit | Total Stockholders' Equity (Deficit) | |||||||||||||||||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | ( | $ | ( | $ | |||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Issuance of common stock for 401(k) match | — | — | — | |||||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Taxes paid on employees' behalf related to vesting of stock awards | — | — | ( | — | — | ( | ||||||||||||||||||||||||||||||||
| Effect of foreign currency translation and unrelated loss on investments | — | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | ( | $ | ( | $ | |||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Issuance of common stock for 401(k) match | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock under At-the-Market offering, net of issuance costs | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Taxes paid on employees' behalf related to vesting of stock awards | — | — | ( | — | — | ( | ||||||||||||||||||||||||||||||||
| Issuance of common stock and warrants in equity offerings, net of issuance costs | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for director compensation | — | — | ||||||||||||||||||||||||||||||||||||
| Effect of foreign currency translation and unrelated loss on investments | — | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | ( | $ | ( | $ | ( | ||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F- 8
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
1. Nature of Business and Basis of Presentation
Yield10 Bioscience, Inc. ("Yield10" or the "Company") is an agricultural bioscience company focused on commercializing sustainable products using the oilseed Camelina sativa ("Camelina") as a platform crop. The features of Camelina, including the availability of winter varieties and a short growth cycle, make it suitable for integration into crop rotations and double cropping on millions of acres in North America. To unlock this potential and make Camelina an attractive option to farmers, the Company is developing and planning to commercialize advanced varieties with elite weed control herbicide tolerance traits, improved agronomic performance, and increased crop value. The Company is pursuing two Camelina seed oil products with different market opportunities, value chains, scale requirements and challenges. The first product, Camelina seed oil is being developed as a low-carbon intensity feedstock oil for biofuels, including biodiesel, renewable diesel (“RD”) and sustainable aviation fuel (“SAF”). The second Camelina product being developed will be seed oil which has been genetically engineered to enable production of high levels of the omega-3 fatty acids eicosapentaenoic acid (“EPA”) and docosahexaenoic acid (“DHA”) in the oil. The Company's development is driven by the growing demand for new sources of omega-3 feedstocks and the production constraints and supply volatility of the traditional raw material source which is fish oil extracted from ocean harvested fish and krill. When commercially available, the Company's omega-3 Camelina will address a need for a reliable, scalable supply of omega-3 oils for aquaculture.
The accompanying consolidated financial statements have been prepared on a basis which assumes that the Company will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. With the exception of a single year, the Company has recorded losses since its initial founding, including its fiscal year ending December 31, 2023. The Company ended 2023 with unrestricted cash, cash equivalents and short-term investments of $1,068 .
The Company follows the guidance of Accounting Standards Codification ("ASC") Topic 205-40, Presentation of Financial Statements-Going Concern, in order to determine whether there is substantial doubt about its ability to continue as a going concern for one year after the date its consolidated financial statements are issued. The Company's ability to continue operations after its current cash resources are exhausted depends on its ability to obtain additional financing through, among other sources, public or private equity financing, secured or unsecured debt financing, equity or debt bridge financing, warrant holders' ability and willingness to exercise the Company's outstanding warrants, additional research grants or collaborative arrangements with third parties, as to which no assurance can be given. Management does not know whether additional financing will be available on terms favorable or acceptable to the Company when needed, if at all. If adequate additional funds are not available when required, management will be forced to curtail the Company's research efforts, explore strategic alternatives and/or wind down the Company's operations and pursue options for liquidating its remaining assets, including intellectual property and equipment. Based on its current cash forecast, management has determined that the Company's present capital resources will not be sufficient to fund its planned operations for at least one year from when these consolidated financial statements are available to be issued, which raises substantial doubt as to the Company's ability to continue as a going concern. This forecast of cash resource is forward-looking information that involves risks and uncertainties, and the actual amount of expenses could vary materially and adversely as a result of a number of factors.
If the Company issues equity or debt securities to raise additional funds, (i) the Company may incur fees associated with such issuance, (ii) its existing stockholders may experience dilution from the issuance of new equity securities, (iii) the Company may incur ongoing interest expense and be required to grant a security interest in Company assets in connection with any debt issuance, and (iv) the new equity or debt securities may have rights, preferences and privileges senior to those of the Company’s existing stockholders. In addition, utilization of the Company’s net operating loss and research and development credit carryforwards may be subject to significant annual limitations under Section 382 of the Internal Revenue Code of 1986, as amended, (the "Internal Revenue Code") due to ownership changes resulting from equity financing transactions. If the Company raises additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to its potential products or proprietary technologies or grant licenses on terms that are not favorable to the Company.
F- 9
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements are presented in U.S. dollars and are prepared in accordance with accounting standards set by the Financial Accounting Standards Board ("FASB"). The FASB sets generally accepted accounting principles ("GAAP") that the Company follows to ensure its financial condition, results of operations, and cash flows are consistently reported. References to GAAP issued by the FASB in these notes to the consolidated financial statements are to the FASB Accounting Standards Codification ("ASC").
Principles of Consolidation
The Company's consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany transactions were eliminated, including transactions with its subsidiaries, Yield10 Oilseeds Inc. and Yield10 Bioscience Securities Corp.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Cash, Cash Equivalents and Restricted Cash
The Company considers all highly liquid investments purchased with an original maturity date of ninety days or less at the date of purchase to be cash equivalents.
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the Company's consolidated balance sheets included herein:
| December 31, 2023 | December 31, 2022 | ||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Restricted cash | |||||||||||
| Total cash, cash equivalents and restricted cash | $ | $ | |||||||||
Amounts included in restricted cash represent those required to be set aside by contractual agreement. Restricted cash of $264 at December 31, 2023 and December 31, 2022, primarily consists of funds held in connection with the Company's lease agreement for its Woburn, Massachusetts facility.
Investments
Investments represent holdings of available-for-sale marketable debt securities acquired in accordance with the Company's investment policy. The Company considers all investments purchased with an original maturity date of ninety days or more at the date of purchase and a maturity date of one year or less at the balance sheet date to be short-term investments. All other investments are classified as long-term. The Company held no no long-term investments at December 31, 2022.
Investments in marketable debt securities are recorded at fair value, with any unrealized gains and losses reported within accumulated other comprehensive income as a separate component of stockholders' equity (deficit) until realized or until a determination is made that an other-than-temporary decline in market value has occurred. Other-than-temporary
F- 10
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
impairments of investments are recognized in the Company's statements of operations if the Company has experienced a credit loss and has the intent to sell the investment or if it is more likely than not that the Company will be required to sell the investment before recovery of the amortized cost basis. Realized gains and losses, dividends, interest income and declines in value judged to be other-than-temporary credit losses are included in other income (expense). The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion together with interest on securities are included in interest income on the Company's consolidated statements of operations. The cost of marketable securities sold is determined based on the specific identification method and any realized gains or losses on the sale of investments are reflected as a component of other income (expense).
Foreign Currency Translation
Foreign denominated assets and liabilities of the Company's wholly-owned foreign subsidiary are translated into U.S. dollars at the prevailing exchange rates in effect on the balance sheet date. Revenues and expenses are translated at average exchange rates prevailing during the period. Any resulting translation gains or losses are recorded in accumulated other comprehensive income (loss) in the consolidated balance sheets. When the Company dissolves, sells or substantially sells all of the assets of a consolidated foreign subsidiary, the cumulative translation gain or loss of that subsidiary is released from comprehensive income (loss) and included within its consolidated statement of operations during the fiscal period when the dissolution or sale occurs.
Comprehensive Loss
Comprehensive loss is comprised of net loss and certain changes in stockholders' equity (deficit) that are excluded from net loss. The Company includes unrealized gains and losses on debt securities and foreign currency translation adjustments in other comprehensive loss.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk primarily consist of cash and cash equivalents, restricted cash, short-term investments and accounts receivable. The Company has historically invested its cash equivalents in highly rated money market funds, corporate debt, federal agency notes and U.S. treasury notes. Investments are acquired in accordance with the Company's investment policy which establishes a concentration limit per issuer. The Company has significant cash balances at financial institutions, which throughout the year, regularly exceed the federally insured limit of $250,000. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company’s financial condition, results of operations, and cash flows.
At December 31, 2022, the Company's unbilled receivables of $30 were due from MSU for support of the DOE grant.
Fair Value Measurements
The carrying amounts of the Company's financial instruments as of December 31, 2023 and December 31, 2022, which include cash equivalents, restricted cash, accounts receivable, unbilled receivables, accounts payable, and accrued expenses, approximate their fair values due to the short-term nature of these instruments. See Note 4 for further discussion on fair value measurements.
Segment Information
The accounting guidance for segment reporting establishes standards for reporting information on operating segments in annual financial statements. The Company is an agricultural bioscience company operating in one segment, which is the development of new technologies to enable step-change increases in crop yield to enhance global food security and production of specialty oils and niche crops. The Company's chief operating decision-maker does not manage any part of the Company separately, and the allocation of resources and assessment of performance are based on the Company's consolidated operating results.
F- 11
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Repairs and maintenance are charged to operating expense as incurred. Depreciation and amortization is computed using the straight-line method over the estimated useful lives of the assets once they are placed in service as follows:
| Asset Description | Estimated Useful Life (years) | |||||||
| Equipment | ||||||||
| Furniture and fixtures | ||||||||
| Software | ||||||||
| Leasehold improvements | Shorter of useful life or term of lease | |||||||
Lease Accounting
Impairment of Long-Lived Assets
Long-lived assets, such as property and equipment and right-of-use assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Accounting guidance further requires that companies recognize an impairment loss only if the carrying amount of a long-lived asset is not recoverable based on its undiscounted future cash flows and measure an impairment loss as the difference between the carrying amount and fair value of the asset.
Grant Revenue
The Company's historical source of revenue was from its government research grants in which it serves as either the primary contractor or as a subcontractor. These grants are considered an ongoing major and central operation of the Company's business. Revenue was earned as research expenses related to the grants are incurred. Revenue earned on government grants, but not yet invoiced as of the balance sheet date, are recorded as unbilled receivables in the accompanying consolidated balance sheets for the year ended December 31, 2022. Funds received from government grants in advance of work being performed, if any, are recorded as deferred revenue until earned.
Research and Development
All costs associated with internal research and development are expensed as incurred. Research and development expenses include, among others, direct costs for salaries, employee benefits, subcontractors, crop trials, regulatory activities, facility related expenses, depreciation, and stock-based compensation. Costs incurred for seed multiplication and processing are included within research and development expense until the Company completes its transition to established commercial operations, at which time these costs are expected to be recorded within inventory. Costs incurred in connection with government research grants are recorded as research and development expense.
F- 12
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
During the year ended December 31, 2023, amounts paid to Yield10 for Camelina planting seed delivered to growers and from the Company's grain purchases delivered to its grain offtake partner have been recorded as an offset to research and development expense. The Company needs to more fully establish its commercial operations, including substantiation of the profitable economics of its Camelina product as a biofuel feedstock and validation of grower acceptance of the crop through larger acreage adoption before it begins to record payments for seed and grain deliveries as product revenue. Until then, the Company will consider its early, small-scale acreage production of Camelina, such as those completed during 2023, to be an initial proof of concept, or prototype, as defined under ASC 740, Research and Development. Yield10 will transition to commercialization and begin to record Camelina seed and grain inventory, cost of goods sold and product sales once the Company is satisfied the product has met these requirements.
General and Administrative Expenses
The Company's general and administrative expense includes costs for salaries, employee benefits, facilities expenses, consulting and professional service fees, travel expenses, depreciation and amortization expenses and office related expenses incurred to support the administrative and business development of the Company.
Intellectual Property Costs
The Company includes all costs associated with the prosecution and maintenance of patents within general and administrative expenses in the consolidated statements of operations.
Stock-Based Compensation
All stock-based payments to employees, members of the Board of Directors and non-employees are recognized within operating expense based on the straight-line recognition of their grant date fair value over the period during which the recipient is required to provide service in exchange for the award. See Note 10 for a description of the types of stock-based awards granted, the compensation expense related to such awards and detail of equity-based awards outstanding.
Basic and Diluted Net Loss per Share
Basic net loss per share is computed by dividing net loss available to common shareholders by the weighted-average number of common shares outstanding. Diluted net loss per share is computed by dividing net loss available to common shareholders by the weighted-average number of dilutive common shares outstanding during the period. Diluted shares outstanding is calculated by adding to the weighted shares outstanding any potential (unissued) shares of common stock from outstanding stock options and warrants based on the treasury stock method, as well as weighted shares outstanding of any potential (unissued) shares of common stock from restricted stock units and the conversion of preferred stock. In periods when a net loss is reported, all common stock equivalents are excluded from the calculation because they would have an anti-dilutive effect, meaning the loss per share would be reduced. Therefore, in periods when a loss is reported, basic and dilutive loss per share are the same. Common stock equivalents include stock options, restricted stock awards, convertible preferred stock and warrants.
The following potentially dilutive securities were excluded from the calculation of diluted net loss per share due to their antidilutive effect:
| Year Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Options | ||||||||||||||
| Restricted stock awards | ||||||||||||||
| Warrants | ||||||||||||||
| Total | ||||||||||||||
The table above disclosing potentially dilutive securities in the calculation of diluted net loss per share as of December 31, 2023, excludes certain transactions that were completed after December 31, 2023.
F- 13
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
On February 15, 2024, the Company's Board of Directors awarded 600,000 RSUs to certain officers, senior staff and outside consultants. These RSUs will vest in 50 % increments 6 and 12 months from the date the awards were granted.
On March 22, 2024, the Company entered into warrant exercise agreements with certain existing institutional investors, pursuant to which these investors agreed to exercise a portion of the warrants previously issued to them. In consideration for their immediate exercise of 3,191,140 total outstanding warrants for cash, the Company agreed to reduce the exercise price of the warrants held by these institutional investors, including any unexercised portion thereof, to $0.43 per share. The institutional investors also received in a private placement, new unregistered warrants to purchase up to an aggregate of 6,382,280 shares of common stock with an exercise price of $0.43 per share, which is equal to 200 % of the shares of common stock issued in connection with this current warrant exercise. See Note 17 - Subsequent Events.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company's tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided to reduce deferred tax assets to a level which, more likely than not, will be realized.
The Company accounts for uncertain tax positions using a "more-likely-than-not" threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors that include, but are not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. The provision for income taxes includes the effects of any resulting tax reserves or unrecognized tax benefits that are considered appropriate as well as the related net interest and penalties, if any. The Company evaluates uncertain tax positions on a quarterly basis and adjusts the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions.
See Note 13 for further discussion of income taxes. The Company had no amounts recorded for unrecognized tax expense or benefits as of December 31, 2023 and 2022.
Recent Accounting Standards Changes
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies that the Company adopts as of the specified effective date.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The FASB subsequently issued amendments to ASU 2016-13, which have the same effective date and transition date as the initial pronouncement. This standard requires entities to estimate an expected lifetime credit loss on financial assets ranging from short-term trade accounts receivable to long-term financings and report credit losses using an expected losses model rather than the incurred losses model that was previously used, and establishes additional disclosures related to credit risks. For available-for-sale debt securities with unrealized losses, this standard now requires allowances to be recorded instead of reducing the amortized cost of the investment. This standard limits the amount of credit losses to be recognized for available-for-sale debt securities to the amount by which carrying value exceeds fair value and requires the reversal of previously recognized credit losses if fair value increases. The guidance is effective for annual periods beginning after December 15, 2022 for SEC filers that are eligible to be smaller reporting companies and interim periods within those fiscal years. The adoption of this standard has not materially impacted the Company’s consolidated financial statements.
New pronouncements that are not yet effective but may impact the Company's financial statements in the future are described below.
In November 2023 the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosure. This standard requires disclosure of significant segment expenses that are regularly
F- 14
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
provided to a company's Chief Operating Decision Maker ("CODM") and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items to reconcile to segment profit or loss and the title and position of the entity's CODM. The amendments in this update also expand the interim segment disclosure requirements. All disclosure requirements under this standard are also required for public entities with a single reportable segment. This standard is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted and the amendments in this update are required to be applied on a retrospective basis. The Company is currently evaluating the potential impact that this new standard will have on its consolidated financial statements and related disclosures.
3. INVESTMENTS
The Company's investments consist of the following at December 31, 2022:
| Accumulated Cost at December 31, 2022 | Unrealized | Market Value at December 31, 2022 | |||||||||||||||||||||
| Gain | (Loss) | ||||||||||||||||||||||
| Short-term investments | |||||||||||||||||||||||
| U.S. government and agency securities | $ | $ | $ | ( | $ | ||||||||||||||||||
| Total | $ | $ | $ | ( | $ | ||||||||||||||||||
4. Fair Value Measurements
The Company has certain financial assets recorded at fair value which have been classified as Level 1 and Level 2 within the fair value hierarchy as described in the accounting standards for fair value measurements. Fair value is the price that would be received from the sale of an asset or the price paid to transfer a liability in an orderly transaction between independent market participants at the measurement date. Fair values determined by Level 1 inputs utilize observable data such as quoted prices in active markets for identical instruments. Fair values determined by Level 2 inputs utilize data points other than quoted prices in active markets that are observable either directly or indirectly. Fair values determined by Level 3 inputs utilize unobservable data points in which there is little or no market data, which require the reporting entity to develop its own assumptions. The fair value hierarchy level is determined by the lowest level of significant input.
The Company’s financial assets classified as Level 2 at December 31, 2023 and December 31, 2022 were initially valued at the transaction price and subsequently valued utilizing third-party pricing services. Because the Company’s investment portfolio may include securities that do not always trade on a daily basis, the pricing services use many observable market inputs to determine value including reportable trades, benchmark yields and benchmarking of like securities. The Company validates the prices provided by the third-party pricing services by reviewing their pricing methods and obtaining market values from other pricing sources. After completing the validation procedures, the Company did not adjust or override any fair value measurements provided by these pricing services as of December 31, 2023 and December 31, 2022.
The tables below present information about the Company’s assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2023 and December 31, 2022 and indicate the fair value hierarchy of the valuation techniques utilized to determine such fair value.
F- 15
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
| Fair value measurements at December 31, 2023 | |||||||||||||||||||||||
| Quoted prices in active markets for identical assets | Significant other observable inputs | Significant unobservable inputs | Balance as of | ||||||||||||||||||||
| Description | (Level 1) | (Level 2) | (Level 3) | December 31, 2023 | |||||||||||||||||||
| Assets | |||||||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
Money market funds | $ | $ | $ | $ | |||||||||||||||||||
| Total assets | $ | $ | $ | $ | |||||||||||||||||||
| Fair value measurements at December 31, 2022 | |||||||||||||||||||||||
| Quoted prices in active markets for identical assets | Significant other observable inputs | Significant unobservable inputs | Balance as of | ||||||||||||||||||||
| Description | (Level 1) | (Level 2) | (Level 3) | December 31, 2022 | |||||||||||||||||||
| Assets | |||||||||||||||||||||||
| Cash equivalents: | |||||||||||||||||||||||
Money market funds | $ | $ | $ | $ | |||||||||||||||||||
| Short-term investments: | |||||||||||||||||||||||
| U.S. government and agency securities | |||||||||||||||||||||||
| Total assets | $ | $ | $ | $ | |||||||||||||||||||
There were no transfers of financial assets or liabilities between category levels for the years ended December 31, 2023 and December 31, 2022.
5. Property and Equipment, Net
Property and equipment consist of the following:
| Year ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Equipment | $ | $ | ||||||||||||
| Furniture and fixtures | ||||||||||||||
| Leasehold improvements | ||||||||||||||
| Total property and equipment, at cost | ||||||||||||||
| Less: accumulated depreciation and amortization | ( | ( | ||||||||||||
| Property and equipment, net | $ | $ | ||||||||||||
F- 16
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
6. Accrued Expenses
Accrued expenses consist of the following:
| Year ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Employee compensation and benefits | $ | $ | |||||||||
| Leased facilities | |||||||||||
| Professional services | |||||||||||
| Field trials and related expenses | |||||||||||
| Other | |||||||||||
| Total accrued expenses | $ | $ | |||||||||
7. Commitments and Contingencies
Contractual Commitments
Exclusive Collaboration Agreement with Rothamsted Research Limited ("Rothamsted")
In November 2020, the Company signed an exclusive collaboration agreement with UK-based Rothamsted to support Rothamsted’s program to develop omega-3 oils in Camelina sativa. Under the agreement, Yield10 has provided Rothamsted with financial support for ongoing research including further EPA, DHA+EPA trait improvement, field testing and nutritional studies. The Company paid Rothamsted research funding and option fees totaling $219 , with a final payment of $31 remaining to be paid as of December 31, 2023, related to a final deliverable to be received from Rothamsted. Included within the agreement, the Company had an exclusive two-year option to sign a global, exclusive or non-exclusive license agreement to the technology. In November 2022, Yield10 and Rothamsted agreed to extend the collaboration agreement and in October 2023, the Company exercised its option to sign the exclusive license agreement for the technology with execution of the license agreement expected to be completed in the second quarter of 2024.
License Agreement with the University of Missouri ("UM")
Pursuant to a license agreement with UM dated as of May 17, 2018, Yield10 has an exclusive, worldwide license to two novel gene technologies to boost oil content in crops. Both technologies are based on significant new discoveries around the function and regulation of ACCase, a key rate-limiting enzyme involved in oil production. The UM license was expanded during May 2019 to include an exclusive worldwide license to a third gene in the ACCase complex, that the Company has designated C3012, that may complement the activity of C3007 to boost oil content in crops.
Pursuant to the UM license agreement, the Company is required to use diligent efforts to develop licensed products throughout the licensed field and to introduce licensed products into the commercial market. The Company's failure to achieve any milestone provided for under the license agreement would give UM the right to terminate the license agreement or render it nonexclusive, unless the Company is able to reach agreement with UM as to the potential adjustment of the applicable milestone.
The Company is obligated to pay UM a license execution payment, milestone payments relating to any regulatory filings and approvals covered by the license agreement, royalties on any sales of licensed products following regulatory approval, as well as a percentage of any sublicense royalties, if any, related to the licensed products. The Company or UM may terminate the license agreement in accordance with the terms of the agreement.
F- 17
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
Guaranteed Minimum Payments to Growers.
As an incentive for growers located in Canada and the U.S. to enter into Camelina commercial grain production contracts with the Company for the winter 2022/2023 and spring 2023 growing seasons, Yield10 offered minimum guaranteed payments per acre that reduce growers' risk of financial loss. The cost of these minimum payments was generally accrued on a straight-line basis over the expected growing season. Payment of minimum guarantees was conditional upon each grower fulfilling their contractual responsibilities and were offset by the purchase price of Yield10's Camelina planting seed provided to the growers and the contractual price that the Company pays for the quantity of grain that is harvested. During the year ended December 31, 2023, the Company incurred minimum guaranteed payments to growers amounting to approximately $72 , which represented the difference between the amounts contractually guaranteed and the actual amount of the delivered harvest. At December 31, 2023, remaining payments outstanding due to growers for the completed 2022/2023 winter and 2023 spring growing seasons totaled $204 , net of the growers' obligation to pay for the planting seed. Beginning with the winter 2023/2024 winter growing season, the Company discontinued the grower minimum payment incentive program.
Facility Leases
The Company leases facilities under non-cancelable leases expiring at various dates through November 30, 2026. See Note 11.
Litigation
From time to time, the Company may be subject to legal proceedings and claims in the ordinary course of business. The Company is not currently aware of any such proceedings or claims that it believes will have, individually or in the aggregate, a material adverse effect on the business, financial condition or the results of operations.
Guarantees
As of December 31, 2023 and December 31, 2022, the Company did not have significant liabilities recorded for guarantees.
The Company enters into indemnification provisions under various agreements with other companies in the ordinary course of business, typically with business partners, contractors, and customers. Under these provisions, the Company generally indemnifies and holds harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of its activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification provisions is unlimited. However, to date Yield10 Bioscience has not incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. As a result, the estimated fair value of these agreements is minimal. Accordingly, the Company has no liabilities recorded for these agreements as of December 31, 2023 and December 31, 2022.
8. License Agreements
In October 2019, the Company granted a non-exclusive license to J. R. Simplot ("Simplot"), to evaluate three of the Company's novel traits in potato. Under the license agreement, Simplot plans to conduct research with the yield traits C3003, C3004 and C4001 within its research and development program as a strategy to improve crop performance and sustainability.
In August 2020, the Company entered into a non-exclusive research agreement with GDM, a company specializing in plant genetics, to evaluate novel yield traits in soybean. Under the terms of the agreement, GDM is working with the Company's yield traits within its research and development program as a strategy to improve soybean yield performance and sustainability. The research agreement includes three novel yield traits in the first phase with the potential to expand the program to more traits in the future. In September 2023, the Company and GDM amended the research agreement to extend the term of the agreement through August 2025, in order to allow GDM more time to complete its evaluations.
F- 18
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
Neither of these research arrangements provides licensing revenue to the Company while Simplot and GDM perform trait evaluations.
9. Capital Stock and Warrants
Common Stock
Public Offering
On August 15, 2023, the Company closed on a public offering of 5,750,000 units at a public offering price of $0.65 per unit. Each unit consisted of one share of common stock and one warrant to purchase one share of common stock. The warrants, when issued, were immediately exercisable at an exercise price of $0.65 per share and expire five years from the date of issuance. The shares of common stock and accompanying warrants could only be purchased together during the offering, but were immediately separable upon issuance. The Company received cash proceeds of $3,125 from the offering, net of $613 in issuance costs.
Registered Direct Offering and Private Placement
On May 3, 2023, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor and an existing investor, pursuant to which the Company agreed to issue and sell (i) an aggregate of 931,600 shares (the “Shares”) of the Company’s common stock, par value $0.01 75,110 shares of common stock, and (iii) private placement warrants (the “Private Warrants”) to purchase an aggregate of 1,006,710 shares of common stock. The Shares, Pre-Funded Warrant and Private Warrants were sold on a combined basis for consideration equating to $2.98 for one Share and a Private Warrant to purchase one underlying share of common stock (or in lieu thereof, $2.9799 for a Pre-Funded Warrant to purchase one underlying share of common stock and a Private Warrant to purchase one underlying share of common stock). The exercise price of the Pre-Funded Warrant was $0.0001 per underlying share. The exercise price of the Private Warrant is $2.98 per underlying share.
The Shares and the Pre-Funded Warrant were offered pursuant to an effective registration statement on Form S-3 (File No. 333-254830), as initially filed with the SEC on March 29, 2021, and declared effective by the SEC on April 2, 2021. The Pre-Funded Warrant was fully exercised on May 12, 2023 and converted to 75,110 shares of the Company's common stock. The Private Warrant was sold in a concurrent private placement (the “Private Placement”), exempt from registration pursuant to Section 4(a)(2) and/or Rule 506 of the Securities Act of 1933, as amended (the “Securities Act”). The Private Warrants become exercisable beginning six months from the date of issuance, on November 6, 2023, and terminate on the fifth anniversary of that date.
Combined proceeds from the registered direct offering and private placement were $3,000 before issuance costs of $283 .
At-The-Market ("ATM") Program
On January 24, 2023, the Company entered into an Equity Distribution Agreement (the "Sales Agreement") with Maxim Group LLC ("Maxim") under which the Company could offer and sell shares of its common stock, $0.01 4,200 from time to time through Maxim, acting exclusively as the Company's sales agent. Maxim was entitled to compensation at a fixed commission rate of 2.75 % of the gross sales price for each share sold. Effective May 3, 2023, the Company terminated the Sales Agreement after issuing a total of 94,665 shares
F- 19
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
of common stock at per share prices between $3.03 and $4.08 , resulting in gross proceeds to the Company of $299 before offering costs and sales commissions totaling $196 .
Board of Director Stock Issuances
During the year ended December 31, 2023, certain members of the Company's Board of Directors elected to receive 99,237 shares of Yield10 common stock in lieu of receiving $49 in cash compensation payments for their services to the board and board committees.
Preferred Stock
The Company's certificate of incorporation, as amended and restated, authorizes it to issue up to 5,000,000 0.01 par value preferred stock.
Warrants
The following table summarizes information with regard to outstanding warrants to purchase common stock as of December 31, 2023:
| Issuance | Number of Common Shares Issuable Upon Exercise of Outstanding Warrants | Exercise Price | Expiration Date | |||||||||||||||||
| August 2023 Public Offering | $ | August 15, 2028 | ||||||||||||||||||
| May 2023 Registered Direct and Concurrent Private Placement | $ | November 6, 2028 | ||||||||||||||||||
| November 2019 Public Offering - Series B | $ | May 19, 2027 | ||||||||||||||||||
| November 2019 Private Placement - Series B | $ | May 19, 2027 | ||||||||||||||||||
| July 2017 Registered Direct Offering | $ | January 7, 2024 | ||||||||||||||||||
| Consultant | $ | September 11, 2024 | ||||||||||||||||||
| Total | ||||||||||||||||||||
Reserved Shares
The following shares of common stock were reserved for future issuance upon exercise of stock options, vesting of Restricted Stock Units ("RSUs") and conversion of outstanding warrants:
| December 31, 2023 | December 31, 2022 | |||||||||||||
| Stock Options | ||||||||||||||
| RSUs | ||||||||||||||
| Warrants | ||||||||||||||
| Total number of common shares reserved for future issuance | ||||||||||||||
10. Stock-Based Compensation
Stock Option Plans
F- 20
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
The Company adopted a stock plan in 2006 (the "2006 Plan"), which provided for the granting of incentive stock options, non-qualified stock options, stock appreciation rights, deferred stock awards, restricted stock awards, unrestricted stock awards, cash-based awards and dividend equivalent rights. In October 2014, the 2006 Plan was terminated, and the Company adopted a new plan (the "2014 Plan"). No further grants or awards were subsequently made under the 2006 Plan. A total of 3,662 options were awarded from the 2006 Plan and as of December 31, 2023, 79 of these options remain outstanding and eligible for future exercise.
The 2014 Plan provides for the granting of incentive stock options, non-qualified stock options, stock appreciation rights, deferred stock awards, restricted stock awards, unrestricted stock awards, cash-based awards and dividend equivalent rights. In May 2018, the 2014 Plan was terminated, and the Company adopted a new 2018 Stock Option and Incentive Plan, which was amended in May 2020 (the "2018 Stock Plan"). A total of 16,896 options were awarded from the 2014 Plan and as of December 31, 2023, 14,065 of these options remain outstanding and eligible for future exercise. A total of 3,619 restricted stock awards were awarded from the 2014 Plan and as of December 31, 2023, all of these restricted stock awards have vested. No further stock awards may be issued from the 2014 Plan.
The 2018 Stock Plan initially reserved for issuance 32,500 shares of the Company's common stock for grants of incentive stock options, non-qualified stock options, stock grants and other stock-based awards. In accordance with the terms of the 2018 Stock Plan, beginning on the first day in January 2019, the Company's Board of Directors has annually approved the addition of shares to the 2018 Stock Plan in amounts equal to 5 % of the outstanding shares of the Company's common stock on the day prior to the increase. Effective January 1, 2024 and January 1, 2023, Yield10's Board of Directors approved the addition of 601,621 and 247,210 shares, respectively. As of December 31, 2023, a total of 1,517,319 options and restricted stock awards have been issued from the 2018 Stock Plan, and as of that date, 1,324,777 options and restricted stock awards remain outstanding.
Expense Information for Stock Awards
The Company recognized stock-based compensation expense, related to employee stock awards, including awards to non-employees and members of the Board of Directors, of $1,592 and $1,903 for the years ended December 31, 2023 and 2022, respectively. At December 31, 2023, there was approximately $2,091 of stock-based compensation expense related to unvested awards not yet recognized which is expected to be recognized over a weighted average period of 2.52 years.
Stock Options
Options granted under the 2006 Plan, 2014 Plan and 2018 Stock Plan generally vest ratably over periods of to four years from the date of hire for new employees, the date of award for existing employees, or date of commencement of services with the Company for non-employees, and generally expire ten years from the date of issuance. The Company's policy is to issue new shares upon the exercise of stock options.
F- 21
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
A summary of the activity related to the shares of common stock covered by outstanding options is as follows:
| Number of Shares | Weighted Average Exercise Price | Remaining Contractual Term (in years) | Aggregate Intrinsic Value | ||||||||||||||||||||
| Balance at December 31, 2021 | $ | $ | |||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | |||||||||||||||||||||||
| Forfeited | ( | ||||||||||||||||||||||
| Expired | ( | ||||||||||||||||||||||
| Balance at December 31, 2022 | $ | ||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | |||||||||||||||||||||||
| Forfeited | ( | ||||||||||||||||||||||
| Expired | ( | ||||||||||||||||||||||
| Balance at December 31, 2023 | $ | $ | |||||||||||||||||||||
| Vested and expected to vest at December 31, 2023 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2023 | $ | $ | |||||||||||||||||||||
The weighted average grant date fair value per share of options granted during fiscal years 2023 and 2022, was $1.63 and $3.31 , respectively. No 7.46 years.
For the years ended December 31, 2023, and 2022, the Company determined the fair value of stock options using the Black-Scholes option-pricing model with the following assumptions for option grants, respectively:
| Year Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Expected dividend yield | ||||||||||||||
| Risk-free rate | ||||||||||||||
| Expected option term (in years) | ||||||||||||||
| Volatility | ||||||||||||||
The Company determined its volatility assumption based on actual market price fluctuations experienced during its trading history. The risk-free interest rate used for each grant is equal to the U.S. Treasury yield curve in effect at the time of grant for instruments with a term similar to the expected life of the related option. The expected term of the options is based upon evaluation of historical and expected future exercise behavior.
The stock price volatility and expected terms utilized in the calculation involve management's best estimates at that time, both of which impact the fair value of the option calculated under the Black-Scholes methodology and, ultimately, the expense that will be recognized over the life of the option. The accounting standard for stock-based compensation requires that the Company recognize compensation expense for only the portion of options that vest. The Company recognizes stock option forfeitures resulting from award terminations in the period in which the forfeiture occurs.
Restricted Stock Units ("RSUs")
The Company records stock compensation expense for RSUs on a straight-line basis over their requisite service period, which approximates the vesting period, based on each RSU's award date market value. As RSUs vests, the Company
F- 22
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
withholds a number of shares from its employees with an aggregate fair market value equal to the minimum tax withholding amount (unless the employee makes other arrangements for payment of the tax withholding) from the common stock issuable at the vest date. The Company then pays the minimum required income tax for the employees. During the years ended December 31, 2023 and December 31, 2022, the Company withheld vested shares with a fair value of $41 and $37 , respectively, to pay for minimum tax withholding associated with RSU vesting.
A summary of RSU activity for the year ended December 31, 2023 is as follows:
| Number of RSUs | Weighted Average Remaining Contractual Life (years) | ||||||||||
| Outstanding at December 31, 2022 | |||||||||||
| Awarded | |||||||||||
| Released | ( | ||||||||||
| Forfeited | |||||||||||
| Outstanding at December 31, 2023 | |||||||||||
| Weighted average remaining recognition period (years) | |||||||||||
Subsequent to year-end, on February 15, 2024, the Company's Board of Directors awarded 600,000 RSUs to certain officers, senior staff and outside consultants. These RSUs will vest in 50 % increments 6 and 12 months from the date the awards were granted.
11. LEASES
Maturity Analysis of Lease Liabilities
The Company's right-of-use assets and corresponding lease liabilities recorded under ASC 842 include its facility lease for its headquarters located in Woburn, Massachusetts, and a small number of automobile leases entered into under a fleet leasing program during the year ended December 31, 2023. At December 31, 2023, the Company's lease liability related to its Woburn facility and leased automobiles will mature as follows:
| Year ended December 31, | Undiscounted Cash Flows | ||||
| 2024 | $ | ||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| Thereafter | |||||
| Total undiscounted future lease payments | |||||
| Amount of lease payments representing interest | ( | ||||
| Total lease liabilities | $ | ||||
| Short-term lease liabilities | $ | ||||
| Long-term lease liabilities | $ | ||||
Quantitative Disclosure of Lease Costs
F- 23
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
| Year Ended December 31, | ||||||||||||||
| 2023 | 2022 | |||||||||||||
| Lease cost: | ||||||||||||||
| Operating lease cost | $ | $ | ||||||||||||
| Short-term lease cost | ||||||||||||||
| Sublease income | ( | ( | ||||||||||||
| Total lease cost, net | $ | $ | ||||||||||||
| Other information as of: | December 31, 2023 | December 31, 2022 | ||||||||||||
| Weighted-average remaining lease term (years) | ||||||||||||||
| Weighted-average discount rate | ||||||||||||||
Real Estate Leases
During 2016, the Company entered into a lease agreement, as amended, for its headquarters pursuant to which the Company leases 22,213 square feet of office and research and development space located at 19 Presidential Way, Woburn, Massachusetts. The lease agreement will terminate on November 30, 2026 and does not include options for an early termination or for an extension of the lease. Pursuant to the lease, the Company is required to pay certain pro rata taxes and operating costs associated with the premises throughout the term of the lease. During the initial buildout of the rented space, the landlord paid for certain tenant improvements that resulted in increased rental payments by the Company. As required by ASC 842, these improvements were recorded as a reduction in the valuation of the associated right-of-use asset. The Company provided the landlord with a security deposit in the form of an irrevocable letter of credit in the amount of $229 .
In December 2023, the Company notified the landlord that it was deferring monthly rental payments, beginning with the December 2023 rent, until such time that the Company is able to raise additional working capital. The landlord immediately notified the Company that it was in payment default under the terms of the lease and has subsequently withdrawn funds held under the irrevocable letter of credit to cover rent for the months of December 2023 through February 2024, leaving a remaining balance in the letter of credit of $12 . The Company reinitiated payment of its monthly rent beginning with the month of March 2024 and intends to return the letter of credit back to its $229 balance as available funding permits.
In October 2016, the Company entered into a sublease agreement with a subsidiary of CJ CheilJedang Corporation ("CJ") with respect to CJ's sublease of 9,874 square feet of its leased facility located in Woburn, Massachusetts. The sublease space was determined to be in excess of the Company's needs. The CJ sublease is coterminous with the Company's master lease and CJ will pay pro rata rent and operating expenses proportionate to the amounts payable to the landlord by the Company, as adjusted from time to time in accordance with the terms of the master lease. Future CJ sublease payments have not been presented as an offset to total undiscounted future lease payments of $2,458 shown in the lease maturity analysis table above. CJ provided the Company with a security deposit of $103 in the form of an irrevocable letter of credit. As a result of Yield10's payment default, CJ now pays the landlord directly for its sublease.
The Company's wholly-owned subsidiary, YOI, located in Saskatoon, Saskatchewan, Canada, leases approximately 9,600 square feet of office, laboratory and greenhouse space located within Innovation Place at 410 Downey Road and within the research facility of National Research Council Canada located at 110 Gymnasium Place. None of these leases contains renewal or early termination options. YOI's leases for these facilities expire on various dates through September 30, 2024.
F- 24
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
12. CONVERTIBLE NOTE PAYABLE, NET
On April 27, 2023, the Company signed a non-binding letter of intent (“LOI”) with Marathon Petroleum Corporation for a potential investment in Yield10 by Marathon and for an offtake agreement for low-carbon intensity Camelina feedstock oil to be used in renewable fuels production. In connection with signing the LOI, the Company sold and issued to MPC Investment LLC, an affiliate of Marathon, a senior unsecured convertible note in the original principal amount of $1,000 (the “Convertible Note”) which is convertible into shares of the Company’s common stock at a conversion price equal to $3.07 per share, subject to any mandatory adjustments and certain conditions and limitations set forth in the Convertible Note. If not converted or terminated earlier, all outstanding principal and accrued and unpaid interest on the Convertible Note will be due and payable in full in cash on the Convertible Note's expected maturity date of August 24, 2024. Yield10 used the net proceeds of $967 , after debt issuance costs of $33 , from the Convertible Note for working capital and general corporate purposes.
The Convertible Note contains customary events of default for such an instrument, accrues interest at 8.0 % per annum, payable semi-annually in arrears, and is expected to mature on August 24, 2024, unless earlier repaid or converted prior to such date in accordance with its terms. The Company may, at its option prior to any interest payment date, pay the interest due on such interest payment date in kind ("PIK Interest"), in which case such PIK Interest will be capitalized and added to the unpaid principal amount of the Convertible Note. Interest expense accrued on the Convertible Note through December 31, 2023 is included in other income (expense), net, in the Company's condensed consolidated statements of operations included herein.
The issuance costs of $33 are being amortized as interest expense, using the effective interest rate method, through the expected maturity date of August 24, 2024, resulting in an effective interest rate of 10.7 %. At December 31, 2023, $17 in issuance costs remain to be amortized through the Maturity Date. If Yield10 and Marathon enter into a definitive offtake agreement or similar transaction that qualifies as a Qualified Financing (as defined in the Convertible Note) prior to the Maturity Date, the Convertible Note will convert into the securities issued in respect of such Qualified Financing and the Company will recognize any remaining unamortized issuance costs.
13. Income Taxes
Income Taxes and Deferred Tax Assets and Liabilities
The components of loss from operations before provision for income taxes consist of the following:
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Domestic | $ | ( | $ | ( | |||||||
| Foreign | ( | ||||||||||
| Net loss from operations before income tax provision | $ | ( | $ | ( | |||||||
F- 25
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Current Tax Provision: | |||||||||||
| Federal | $ | $ | |||||||||
| State | |||||||||||
| Foreign | |||||||||||
| Total current | |||||||||||
| Deferred Tax Benefit: | |||||||||||
| Federal | |||||||||||
| State | |||||||||||
| Foreign | |||||||||||
| Total deferred | |||||||||||
| Total tax provision | $ | $ | |||||||||
Significant components of the Company's deferred tax assets are as follows:
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Deferred Tax Assets: | |||||||||||
| Net operating loss carryforward | $ | $ | |||||||||
| Capitalization of research and development expense | |||||||||||
| Credit carryforwards | |||||||||||
| Stock compensation | |||||||||||
| Lease liability | |||||||||||
| Other temporary differences | |||||||||||
| Total deferred tax assets | |||||||||||
| Valuation allowance | ( | ( | |||||||||
| Net deferred tax assets | |||||||||||
| Deferred Tax Liabilities: | |||||||||||
| Depreciation | ( | ( | |||||||||
| Right-of-use asset | ( | ( | |||||||||
| Net deferred taxes | $ | $ | |||||||||
F- 26
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
Tax Rate
The items accounting for the difference between the income tax (provision) benefit computed at the federal statutory rate of 21% and the provision for income taxes were as follows:
| Year Ended December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| Federal income tax at statutory federal rate | % | % | |||||||||
| State taxes | % | % | |||||||||
| Permanent differences | ( | % | ( | % | |||||||
| Tax credits | % | % | |||||||||
| Foreign rate differential | % | ( | % | ||||||||
| Stock compensation | ( | % | ( | % | |||||||
| Other | ( | % | % | ||||||||
| Change in valuation allowance | ( | % | ( | % | |||||||
| Total | % | ( | % | ||||||||
Tax Attributes
At December 31, 2023, the Company had U.S. net operating loss carryforwards ("NOLs") for federal and state income tax purposes of approximately $25,130 and $25,124 , respectively. All of the $25,130 of federal NOLs will carry forward indefinitely. The Company's state NOL carryforwards will begin to expire on various dates through 2043. The Company also had available research and development and investment tax credits for federal and state income tax purposes of approximately $569 and $412 , respectively. These federal and state credits will begin to expire on various dates through 2043. In Canada, the Company has cumulative research tax credits totaling $123 that will begin to expire on various dates through 2037.
Management of the Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets ("DTA"), which is comprised principally of NOL carryforwards. Under the applicable accounting standards, management has considered the Company's history of losses and concluded that it is more likely than not that the Company will not recognize the benefits of U.S. federal and state DTAs. Accordingly, a full valuation allowance has been established against the U.S. net DTAs. The Company has also concluded, based on its financial projections, that it is more likely than not the $123 of DTAs of its wholly-owned Canadian subsidiary, YOI, may not be recognized in the future, resulting in the Company's recording of a full valuation allowance against the assets.
Utilization of the NOL and research and development credit ("R&D Credit") carryforwards may be subject to a substantial annual limitation under Section 382 of the U.S. Internal Revenue Code of 1986 (the "Code") due to ownership change limitations, as defined by the Code, that have occurred previously or that could occur in the future. These ownership changes may limit the amount of NOL and R&D Credit carryforwards that can be utilized annually to offset future U.S. taxable income and tax, respectively. The Company evaluated its Section 382 ownership changes through May 31, 2021 and has determined that the most recent change that occurred in November 2019 resulted in all NOL and R&D Credit carryforwards outstanding as of that date becoming fully limited. The Company has reduced its associated deferred tax assets accordingly. To the extent an ownership change occurred in the future, the NOL, R&D Credit carryforwards and other deferred tax assets recorded after the November 2019 ownership change may also be subject to limitations.
Other
The tax years 2020 through 2023 remain open to examination by major taxing jurisdictions to which the Company is subject, which are primarily in the U.S. The statute of limitations for NOLs utilized in future years will remain open beginning in the year of utilization.
F- 27
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
The Company's policy is to record estimated interest and penalties related to uncertain tax positions as income tax expense. As of December 31, 2023 and 2022, the Company had no
14. Employee Benefits
The Company maintains a 401(k) savings plan in which substantially all of its regular U.S. employees are eligible to participate. Participants may contribute up to 60 % of their annual compensation to the plan, subject to eligibility requirements and annual IRS limitations. The Company's plan provides for a matching contribution in common stock of up to 4.5 % of a participant's total compensation dependent upon the level of participant contributions made during the plan year. Pursuant to this plan, the Company issued 119,971 , and 36,706 shares of common stock during the years ended December 31, 2023, and December 31, 2022, respectively, and recorded $108 , and $133 , respectively, of related expense. Company contributions are fully vested upon issuance.
15. Government Research Grants
During 2018, the Company entered into a sub-award with Michigan State University ("MSU") to support a Department of Energy ("DOE") funded grant entitled "A Systems Approach to Increasing Carbon Flux to Seed Oil." The Company's participation under this five-year grant has been awarded incrementally on an annual basis with the first year commencing September 15, 2017. Funding for this sub-award in the amount of $2,957 was appropriated by the U.S. Congress through the final contractual year ending in September 2022. During the years ended December 31, 2023 and December 31, 2022, Yield10 recognized grant revenue of $60 and $450 , respectively, from this sub-award and as of December 31, 2023, the DOE sub-award has been completed with no further amounts to be recognized.
16. Geographic Information
The geographic distribution of the Company's revenues and long-lived assets are summarized in the table below. Foreign revenue is based on the country in which the Company's subsidiary that earned the revenue is domiciled.
| U.S. | Canada | Total | ||||||||||||||||||
| Year Ended December 31, 2023 | ||||||||||||||||||||
| Revenue | $ | $ | $ | |||||||||||||||||
| Identifiable long-lived assets | $ | $ | $ | |||||||||||||||||
| Year Ended December 31, 2022 | ||||||||||||||||||||
| Revenue | $ | $ | $ | |||||||||||||||||
| Identifiable long-lived assets | $ | $ | $ | |||||||||||||||||
F- 28
YIELD10 BIOSCIENCE, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(In thousands, except for share and per share amounts)
17. Subsequent Events
Vision Bioenergy Oilseeds, LLC ("Vision") License
On February 14, 2024, the Company granted Vision Bioenergy Oilseeds, LLC a global license to certain proprietary spring and winter Camelina varieties, including varieties exhibiting herbicide tolerance. Under the license, Vision has a -year period of exclusivity to commercialize the licensed traits and varieties for use in biofuels. Once that period elapses, the license granted to Vision will convert to a non-exclusive status worldwide. In consideration for the license and the Company’s completion of certain near-term deliverables, Vision will make cash payments to the Company totaling $3,000 . The Company retained the right to sublicense these Camelina traits and plant varieties, as well as continue to utilize and develop the varieties to produce omega-3 oil and other Camelina oil and meal products.
Warrant Exercise Agreements
On March 22, 2024, the Company entered into warrant exercise agreements with certain existing institutional investors, pursuant to which these investors agreed to exercise (i) a portion of the warrants issued to them in May 2023, which were exercisable for 671,140 shares of the Company’s common stock and had an exercise price of $2.98 per share, and (ii) a portion of the warrants issued to them in August 2023, which were exercisable for 2,520,000 shares of common stock and had an exercise price of $0.65 per share. In consideration for their immediate exercise of these 3,191,140 total warrants for cash, the Company agreed to reduce the exercise price of the May 2023 and August 2023 warrants held by these institutional investors, including any unexercised portion thereof, to $0.43 per share, which was equal to the closing price of the Company’s common stock on The Nasdaq Stock Market prior to the execution of the agreements. The institutional investors also received in a private placement, new unregistered warrants to purchase up to an aggregate of 6,382,280 shares of common stock with an exercise price of $0.43 per share, which is equal to 200 % of the shares of common stock issued in connection with this current warrant exercise. The Company is expected to receive $1,372 in cash proceeds from the current exercise of the warrants, before financial advisory and other expenses incurred in completing the arrangement.
The Company has agreed to hold an annual or special meeting of shareholders on or prior to the date that is ninety days following the closing date of the warrant exercise for the purpose of obtaining shareholder approval, as may be required by the applicable rules and regulations of the Nasdaq Stock Market, with respect to issuance of the new warrants and the shares issuable upon the exercise thereof.
F- 29